Abstract
Sarcopenia is common in patients with liver cirrhosis and related to higher mortality. Implantation of a transjugular intrahepatic portosystemic shunt (TIPS) is a feasible method for reducing cirrhosis-related portal hypertension, but also possible improvement of the patient`s muscle status. We aimed to analyze changes in muscle quantity and prevalence of sarcopenia after TIPS. We retrospectively surveyed the muscle status in 52 patients (mean age 54.2 years) before and after TIPS by evaluating skeletal (SMI) and psoas muscle indices (PMI) in CT and MR images. Model for End-Stage Liver Disease (MELD), Freiburg index of post-TIPS survival (FIPS), and their underlying laboratory parameters (e.g., Albumin) were analyzed. Prevalence of sarcopenia was 84.6%. After a median follow-up of 16.5 months after TIPS, SMI (0.020) and PMI (p < 0.001) increased, and sarcopenia decreased by 14.8% (0.109). MELD and PMI after TIPS were negatively correlated (r = − 0.536, p < 0.001). Albumin levels increased in patients with increased SMI after TIPS (p = 0.022). Confirming the positive impact of TIPS implantation on muscle indices in patients with liver cirrhosis, we found indications for improved survival and possible indications for altered metabolism with increased albumin levels in patients with increased muscle quantity.
Similar content being viewed by others
Introduction
Regardless of the etiology, liver cirrhosis is the common end stage of chronic liver diseases1, including alcoholic liver disease (ALD), non-alcoholic liver disease (NAFLD), chronic hepatitis B and C, autoimmune liver disease, hemochromatosis, and Wilson’s disease. The prevalence of etiologies differs between world regions. In Asia and Africa, liver cirrhosis develops predominately after infection with hepatitis B or C. In contrast, the leading causes in Europe and America are NAFLD and ALD2,3.
The prognosis of liver cirrhosis depends on the severity of hepatic dysfunction4. In daily practice, the Child–Pugh and the model for end-stage liver disease (MELD) score are generally applied as the main prognostic tools5. However, both scoring systems lack important parameters regarding the nutritional status of patients, as liver cirrhosis is strongly related to the development of malnutrition and sarcopenia6.
Sarcopenia and malnutrition are known to have an impact not only on the morbidity and quality of life6,7 but on survival in patients with liver cirrhosis8. According to the European Working Group on Sarcopenia in Older People (EWGSOP2), sarcopenia is defined as a generalized and progressive skeletal muscle disorder9 and is associated with malnutrition10. The diagnosis is made by evaluating low muscle strength and low muscle quantity or quality. The development of malnutrition and sarcopenia in patients with liver cirrhosis is caused by altered protein metabolism, which leads to increased muscle depletion. Notably, the prevalence of sarcopenia is different among different entities of liver cirrhosis. Patients with alcoholic liver disease tend to have a higher prevalence of sarcopenia than patients with other causes of liver cirrhosis11.
According to the American Association for the Study of Liver Diseases, the gold standard for assessing sarcopenia in patients with liver cirrhosis is CT imaging with the skeletal muscle index derived from measuring the height-normalized skeletal muscle area at L3. Hence, low skeletal muscle mass and sarcopenia are usually used synonymously, although muscle strength is rarely measured in studies on sarcopenia in patients with liver cirrhosis6.
Tissue inflammation within the liver parenchyma leads to the remodeling of regular liver parenchyma into fibrotic tissue and, subsequently, the development of portal hypertension2. In decompensated cirrhosis, this drives the formation of therapy-refractory ascites and variceal hemorrhage due to excessive varicosis. In these cases, a transjugular intrahepatic portosystemic shunt (TIPS) is a feasible treatment option12 to lower portal hypertension and, thus, ascites and the risk for variceal hemorrhage. The latest data suggest that the implantation of a TIPS not only lowers portal hypertension but may also improve body composition, specifically muscle status, and thus reduce morbidity and mortality10,13,14,15,16,17,18,19.
We conducted this study to evaluate changes in skeletal muscle mass (SMM) after TIPS implantation using the PMI and SMI and compared subgroups of cirrhotic patients regarding their etiology of cirrhosis. We also compared the psoas muscle index (PMI) and skeletal muscle index (SMI) and analyzed the correlation with the MELD (Model for End-Stage Liver Disease) and FIPS (The Freiburg index of post-TIPS survival).
Materials and methods
Patient selection
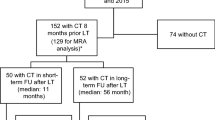
In this retrospective study, we included patients with liver cirrhosis who underwent a TIPS procedure at the Department of Radiology and Nuclear Medicine, Otto-von-Guericke University Magdeburg, between April 2010 and April 2021. The majority of cases (94.2%) comprised elective procedures due to therapy-refractory ascites. A few patients underwent an emergency procedure due to severe, otherwise not manageable bleeding. All patients gave verbal and written consent for the procedure. In addition, it was essential for the patients to undergo a minimum of one computed tomography (CT scan) or magnetic resonance imaging (MRI), both prior to and following the TIPS procedure, to assess the PMI and SMI. We only included patients with imaging at least six months after TIPS.
Exemplary indications for the performance of a CT or MRI were planning of the TIPS procedure, search for hepatocellular carcinoma, evaluation of liver cirrhosis, portal vein thrombosis, and evaluation for liver transplantation.
The institutional review board approved the retrospective data analysis and waived specific further consent from patients (Number: 145/21, Ethics Committee, University of Magdeburg, Magdeburg, Germany). All methods were performed in accordance with the relevant guidelines and regulations.
TIPS procedure
The TIPS procedure was performed under local anesthesia with an ultrasound-guided puncture of the right internal jugular vein, followed by the insertion of a 10F catheter sheath. This was followed by the catheterization of the right hepatic vein. Simultaneously, an assistant conducted an ultrasound of the liver with the imaging of the right hepatic and central portal veins. After confirmation of the correct position, the portal vein was directly punctured through the liver parenchyma. Next, a guidewire and pigtail catheter were inserted to stabilize and confirm the intraportovenous position. After administration of 7.5 mg Piritramid i.v., a balloon catheter was inserted to dilate the intervascular parenchyma. Finally, a Luminexx® stent with 8 or 10 mm diameter or GORE® Viatorr® TIPS endoprosthesis with 10 mm diameter was implanted. The stents were 40–100 mm long. After stent or stent-graft implantation, the portal pressure gradient was measured via a pigtail catheter in the central portal vein and the right atrium. A Shaldon catheter was inserted in the right internal jugular vein for easy access and possible further dilation of the stent during the following days.
Image acquisition and analysis
For the evaluation of muscle area and indices, we analyzed (contrast-enhanced) computed tomography (CT, 128-section scanner, Somatom, Siemens® or 80-section scanner, Toshiba®) or magnetic resonance imaging (MRI, 1.5 or 3.0 T, Philips®) from one to three months before the TIPS procedure. CT or MRI images were usually obtained for the evaluation of complications arising from cirrhosis or for the exclusion of hepatocellular carcinoma. After TIPS, CT and MR images from at least six months post-procedure were analyzed. First, the areas of the right and left psoas major muscle at the L3 inferior endplate level in the CT and MR images were delineated by hand using Infinitt® PACS Viewer. In the second step, we used AsanJ-Morphometry software (Asan Image Metrics, Seoul, Korea) for semi-automated adipose and muscle tissue segmentation at the cross-sectional area at the L3 inferior endplate level. The segmentation comprises an estimation of total fat area (TFA), skeletal fat area (SFA), visceral fat area (VFA), total muscle area (TMA), total muscle fat area (TMFA), and area of right and left psoas major muscle (PMA). For further analysis, we focused on the psoas muscle area (PMA) and total muscle area (TMA), which comprises the complete muscle area of the cross-sectional L3 endplate level. For semi-automated segmentation, we used predefined thresholds for Hounsfield units (HU) on CT (> − 30 HU and ≤ 150 HU) and the signal intensity (SI) on pre-contrast T1-weighted MRI (> 350 SI and ≤ 750 SI) for muscle and adipose area (≤ − 30 HU in CT and > 100 SI and ≤ 350 SI in MR images)20.
Two readers with five years of experience in abdominal imaging, respectively, conducted the delineation and segmentation.
Analysis of clinical parameters
For the calculation of the MELD21 and FIPS22 score, we gathered laboratory parameters (creatinine, bilirubin, INR, albumin) from the period of the pre-interventional and post-interventional imaging. The MELD score includes creatinine, bilirubin, and INR to evaluate the severity of liver disease. It was initially developed to predict the survival of patients undergoing TIPS21. The FIPS was developed to identify patients with a high risk of post-interventional mortality and includes creatinine, bilirubin, albumin, and the patient’s age. A FIPS score of ≥ 0.92 (85th percentile) is associated with a significantly decreased survival rate after TIPS implantation22. A FIPS of ≥ 0.64 was also confirmed as a relevant prognostic cut-off value23. We also separately analyzed changes in creatinine, albumin, and bilirubin levels.
Statistical analysis
We used IBM SPSS Statistics 26.0® (IBM Corp., Armonk, NY, USA) and Excel (Microsoft®, Office16) for statistical analysis. Patients’ characteristics were evaluated using descriptive statistics. We used the Mann–Whitney U test to evaluate muscle area and indices between groups and performed Pearson’s correlation analysis with PMI, SMI, MELD, and FIPS. Categorical variables were tested with the Chi-squared test. For the evaluation of muscle area changes over time, we used the McNemars test.
All tests were carried out two-sided, and a p-value ≤ 0.05 was considered statistically significant.
Results
Patient characteristics
In this retrospective study, we included 52 patients (42 males, 10 females) with a mean age of 54.2 years (± 10.3) who had undergone TIPS procedures for complications of liver cirrhosis between 2010 and 2021. Furthermore, all of them had received a CT or MR imaging before and after the intervention (Table 1).
The majority of patients (69.2%) suffered from ALD. The second most common cause of liver cirrhosis was NASH, followed by autoimmune hepatitis. Most patients were classified as Child–Pugh B and suffered from ascites and relevant esophageal or gastral varicosis. Encephalopathy was less prevalent, with 9.6%.
Therapeutic results of the TIPS procedure
The TIPS procedures were performed between April 2010 and April 2021.
In two-thirds of cases, TIPS was performed due to therapy-refractory ascites. The second most common cause was secondary prevention of variceal bleeding. Overall, the majority of procedures (n = 49) were elective.
After TIPS implantation, the pressure gradients dropped from 21.9 ± 3.8 to 9.8 ± 3.1 mmHg, meeting the recommended target pressure gradient of < 12 mmHg24. In nine patients (17.3%), a revision had to be performed to dilate the stent or stent graft further. In follow-up imaging, ascites was resolved in 57.7% of cases.
Due to a lack of imaging, 35 patients also treated in our department could not be included in this retrospective analysis. Of these, only four patients were lost to follow-up within a year but at least 6 months after the TIPS procedure. Baseline data on these patients is provided in Supplementary Material Table 1.
Increase of muscle area and decrease of sarcopenia after TIPS
In the majority of patients (74.0%), we analyzed CT images obtained at baseline and after a follow-up of at least six months and a median follow-up of 16.5 months. In a quarter of patients (26.0%), we analyzed MR images.
There was a positive correlation between the psoas muscle area delineated by hand and the semi-automated segmentation of the psoas muscle area (r = 0.773, p < 0.001).
There was no correlation between SMI and PMI before TIPS (r = 0.26; p = 0.11); however, we found a positive correlation after TIPS (r = 0.564; p < 0.001).
After TIPS, a mean increase of 9.7 cm2 in TMA (p = 0.016) and of 4.1 cm2 in PMA (p < 0.001) was observed (Table 2 and Figs. 1a,b and 2). This resulted in significant increases of SMI (0.020) and PMI (p < 0.001).
For the classification of possible sarcopenia, we applied previously used cut-off values for the SMI (39.5 cm2/m2 for women, ≤ 52 cm2/m2 for men)25 and the PMI (≤ 6.36 cm2/m2 in men ≤ 3.92 cm2/m2 in women)26. According to these cut-off values, most patients were classified sarcopenic (84.6%, 92.3%) at baseline. After the TIPS procedure, the proportion of patients with sarcopenia declined to 69.8% (SMI, p = 0.109) and 68.1% (PMI, p = 0.004). In a subgroup analysis of male patients, we found a significant decrease in sarcopenia according to the SMI (p = 0.039) and PMI (p = 0.008). There were no significant changes in muscle areas/indices in patients not classified as sarcopenic before the TIPS procedure (e.g., PMA, p = 0.50).
We did not evaluate weight and BMI since the majority of patients had relevant ascites, and weight information after drainage of ascites was often not available.
Analyzing patients with ascites after TIPS based on follow-up imaging, we found a significantly lower psoas muscle area (15.5 vs. 12.7 cm2, p = 0.037) and lower PMI (5.1 vs. 4.2 cm2/m2, p = 0.045) compared to patients without ascites.
Patients with ascites still showed some increase of muscle area after TIPS (TMA 135.4 vs. 138.7 cm2, p = 0.281; PMA 11.9 vs. 13.0 cm2, p = 0.184), but these results were not statistically significant.
MELD and FIPS
On average, the MELD score before TIPS was 12.8. There was a slight increase to 13.4 after TIPS (Table 2). The average FIPS was − 0.263 prior to the TIPS procedure. According to the FIPS, overall survival one month after TIPS was estimated at 95.0%, 85.6% after three months, and 80.1% after six months. Before TIPS, there were three high-risk patients according to the cut-off value of ≥ 0.92 and nine high-risk patients with a FIPS higher than ≥ 0.64. In our study cohort, ascertained overall survival six months after TIPS was 100.0%.
As MELD and FIPS are based on similar parameters, there was a positive correlation between both scores (r = 0.513; p < 0.001), and the MELD in high-risk patients was significantly elevated (p = 0.001).
The MELD after TIPS was also inversely correlated to PMA (r = − 0.296, p = 0.039) and PMI after TIPS (r = − 0.536, p < 0.001).
However, we found no correlation between FIPS and muscle area/indices or significant differences between patients with a high and average risk of reduced post-TIPS survival (Suppl. Mat. Table S1).
Analyzing parameters for calculations of MELD and FIPS separately, we found no significant differences between patients classified as sarcopenic and non-sarcopenic. There was a slight, but not significant increase in albumin levels comparing all patients before and after TIPS (p = 0.091) (Table 2).
Interestingly, we found significantly higher serum albumin levels in patients with increased SMI compared to patients with no increase after TIPS (36.0 vs. 28.7; p = 0.022).
Analysis according to the origin of liver cirrhosis
We analyzed differences in muscle area and indices between subgroups of patients according to the origin of the liver cirrhosis (Suppl. Mat. Table S2 and Fig. S1). For computational reasons, we formed four groups: ALD, NASH, autoimmune hepatitis, and Other. The group `Other` comprises different entities with one patient each: primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), Budd-Chiari syndrome, and unknown.
Comparing patients with alcoholic liver disease to all other patients, we found significant differences in the PMA (16.5 vs. 9.7; p = 0.006) and PMI (5.3 vs. 3.3; p = 0.016) after TIPS with no significant differences before the TIPS procedure (Table 3 and Fig. 3). Additionally, there was a significantly greater change in PMA in patients with alcoholic liver disease than in all other patients (5.7 vs. 1.1; p = 0.041).
Discussion
According to current studies, the prevalence of sarcopenia in patients with liver cirrhosis is estimated to be 25–45%8, depending on the method applied. At baseline, most patients in our study cohort were classified sarcopenic, with 85–92% depending on the used index system. As sarcopenia and malnutrition are associated with higher morbidity and mortality6,8, there is a need for therapeutic approaches to increase muscle quantity and quality.
Several studies have reported a positive correlation between TIPS implantation and an increase in muscle quantity13,14,15,16,17,18,19, along with indications for enhanced overall survival10. We evaluated muscle area and muscle indices in patients with liver cirrhosis by analyzing PMA and TMA in CT and MR images before and at least six months after the TIPS procedure.
Patients in our study cohort showed significant increases in TMA, PMA, SMI, and PMI. However, comparing patients with and without ascites after TIPS in follow-up imaging, patients with remaining ascites demonstrated significantly lower PMA and PMI, but increases in these parameters were still present, albeit not significant.
After TIPS, significantly fewer patients, including three patients with remaining ascites, were classified sarcopenic according to the PMI, and male patients were classified significantly less sarcopenic according to the SMI after the TIPS procedure.
It is suggested that the positive effect of a TIPS procedure on muscle quantity might be due to the deceleration of a catabolic metabolism, which is caused by proinflammatory cytokines, leading to loss of muscle mass27, the reversal of portal hypertension and aftereffects such as ascites13. This is also indicated by our results, as patients with resolved ascites after TIPS showed the greatest improvement regarding muscle quantity and reduction of sarcopenia.
As the function of hepatocytes in liver cirrhosis is impaired, so is the synthesis of albumin28. Albumin is solely produced in the liver parenchyma and provides an anti-inflammatory effect by transporting toxic metabolites, such as bilirubin and acids. It also serves as an antioxidant. Long-term administration of albumin reduces complications and improves survival29. We analyzed changes in albumin, bilirubin, and creatinine and found a slight, albeit not significant, increase in albumin levels after the TIPS procedure. However, we found a significant elevation in albumin levels in patients with increased SMI after TIPS compared to patients without an increase after the TIPS procedure. Our findings might indicate a metabolic shift in patients with increased muscle mass after TIPS implantation. Further studies are needed to evaluate the possible effects of albumin on or as a biomarker for muscle quantity.
We found no positive effect of TIPS on muscle area and indices in patients not classified as sarcopenic before the TIPS procedure. This is in line with findings of Liu et al.10.
ALD was the most common etiology of liver cirrhosis in our study cohort. Patients with ALD seem to exhibit the lowest muscle area compared to other etiologies of liver cirrhosis and also show a higher rate of muscle depletion11. We evaluated muscle area and indices between subgroups of patients with different etiologies of liver cirrhosis. Before the TIPS procedure, we found no significant differences between patients with ALD and all other patients. However, after the TIPS procedure, we found a significantly higher PMA and PMI in patients with ALD than in all other patients. To our knowledge, this has not been reported before. A possible explanation might be additional lifestyle changes in patients with ALD with the abstain from alcohol.
We retrospectively applied the FIPS to analyze the likelihood of post-TIPS survival. We identified three high-risk patients with FIPS ≥ 0.92 and nine high-risk patients with FIPS ≥ 0.64. As expected, the MELD in these patients was significantly elevated, as there was a strong correlation between MELD and FIPS. Ascertained survival six months after TIPS was 100.0%.
We found no significant differences between high-risk and average-risk patients regarding muscle area and indices before and after TIPS. A possible explanation could be the relatively small number of high-risk patients. However, there was a strong negative correlation between MELD after TIPS and PMI after TIPS, implicating a higher likelihood of survival in patients with higher psoas muscle index after TIPS.
Limitations
This is a retrospective study in an uncontrolled study design, analyzing patients over a period of 11 years. Changes in the therapeutic management of liver cirrhosis are likely, which makes for a heterogenic study group. As the majority of patients undergoing TIPS procedures at our Department did not receive CT or MR imaging, the sample size is limited.
Using CT as well as MR images could hamper the data quality or comparability, as it is not undisputed if measurements in CT and MR images are interchangeable and most studies on muscle quantity are solely based on CT images. However, this seems not likely, as the majority of analysed images were CTs and two different imaging analysis methods with a strong correlation of results were used.
The use of cut-off values for sarcopenia in existing studies is very heterogeneous. We decided to apply cut-off values, which have been used on a predominantly European study group25, reflecting our cohort's patient demography.
Most importantly, as with most studies on sarcopenia in liver cirrhosis, we only evaluated muscle quantity, not quality. Further studies are needed to evaluate not only muscle quantity but also muscle function.
Conclusions
In summary, our study confirms previous studies, demonstrating a positive impact of a TIPS procedure on muscle area and indices in patients with liver cirrhosis who were classified as sarcopenic. Patients with resolved ascites and alcoholic liver disease seem to profit the most. In addition, we found higher albumin levels in patients with increased SMI after TIPS compared to patients without an increase. A possible association between albumin and muscle quantity needs further evaluation.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
06 November 2023
The original online version of this Article was revised: In the original version of this Article, the author name Felix Barajas Ordonez was incorrectly indexed, which has been corrected.
Abbreviations
- ALD:
-
Alcoholic liver disease
- CT:
-
Computed-tomography
- EWGSOP:
-
European Working Group on Sarcopenia in Older People
- FIPS:
-
Freiburg index of post-TIPS survival
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HBV:
-
Hepatitis B virus
- HU:
-
Hounsfield units
- LSMM:
-
Low skeletal muscle mass
- NAFLD:
-
Non-alcoholic liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- MELD:
-
Model for end-stage liver disease
- MRI:
-
Magnetic resonance imaging
- PBC:
-
Primary biliary cirrhosis
- PMA:
-
Area of right and left M. psoas
- PMI:
-
Psoas muscle index
- PSC:
-
Primary sclerosing cirrhosis
- SMI:
-
Skeletal muscle index
- SMM:
-
Skeletal muscle mass
- SFA:
-
Skeletal fat area
- TFA:
-
Total fat area
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
- TMA:
-
Total muscle area
- TMFA:
-
Total muscle fat area
- VFA:
-
Visceral fat area
References
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. Burden of liver diseases in the world. J. Hepatol. 70, 151–171 (2019).
Ginès, P. et al. Liver cirrhosis. Lancet 398, 1359–1376 (2021).
Galle, P. R. et al. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
D’Amico, G. et al. Competing risks and prognostic stages of cirrhosis: A 25-year inception cohort study of 494 patients. Aliment Pharmacol. Ther. 39, 1180–1193 (2014).
Cholongitas, E., Germani, G. & Burroughs, A. K. Prioritization for liver transplantation. Nat. Rev. Gastroenterol. Hepatol. 7, 659–668 (2010).
Lai, J. C. et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 Practice guidance by the American Association for the Study of Liver Diseases. Hepatology 74, 1611–1644 (2021).
Norman, K., Kirchner, H., Lochs, H. & Pirlich, M. Malnutrition affects quality of life in gastroenterology patients. World J. Gastroenterol. WJG 12, 3380 (2006).
Tantai, X. et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 76, 588–599 (2022).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 39, 412–423 (2010).
Liu, J. et al. Sarcopenia in patients with cirrhosis after transjugular intrahepatic portosystemic shunt placement. Radiology 303, 711–719 (2022).
Welch, N. et al. Continued muscle loss increases mortality in cirrhosis: Impact of aetiology of liver disease. Liver Int. 40, 1178–1188 (2020).
Rössle, M. TIPS: 25 years later. J. Hepatol. 59, 1081–1093 (2013).
Dasarathy, J., Alkhouri, N. & Dasarathy, S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: A critical review of literature. Liver Int. 31, 1250–1258 (2011).
Artru, F. et al. Consequences of TIPSS placement on the body composition of patients with cirrhosis and severe portal hypertension: A large retrospective CT-based surveillance. Aliment Pharmacol. Ther. 52, 1516–1526 (2020).
Shoreibah, M. G. et al. Psoas muscle density in combination with model for end-stage liver disease score can improve survival predictability in transjugular intrahepatic portosystemic shunts. J. Vasc. Interv. Radiol. 30, 154–161 (2019).
Plauth, M. et al. Weight gain after transjugular intrahepatic portosystemic shunt is associated with improvement in body composition in malnourished patients with cirrhosis and hypermetabolism. J. Hepatol. 40, 228–233 (2004).
Montomoli, J. et al. Body composition changes after transjugular intrahepatic portosystemic shunt in patients with cirrhosis. World J. Gastroenterol. 16, 348–353 (2010).
Tsien, C., Shah, S. N., Mccullough, A. J. & Dasarathy, S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur. J. Gastroenterol. Hepatol. 25, 85–93 (2013).
Praktiknjo, M. et al. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology 67, 1014–1026 (2018).
Park, J. et al. Reliable and robust method for abdominal muscle mass quantification using CT/MRI: An explorative study in healthy subjects. PLOS ONE 14, e0222042 (2019).
Malinchoc, M. et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 31, 864–871 (2000).
Bettinger, D. et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J. Hepatol. 74, 1362–1372 (2021).
Stockhoff, L., Schneider, H., Tergast, T. L., Cornberg, M. & Maasoumy, B. Freiburg index of post-TIPS survival (FIPS) a valid prognostic score in patients with cirrhosis but also an advisor against TIPS?. J. Hepatol. 75, 487–489 (2021).
Wong, F. The use of TIPS in chronic liver disease. Ann. Hepatol. 5, 5–15 (2006).
Levolger, S. et al. Sarcopenia impairs survival in patients with potentially curable hepatocellular carcinoma. J. Surg. Oncol. 112, 208–213 (2015).
Lee, C. M., Kang, B. K. & Kim, M. Radiologic definition of sarcopenia in chronic liver disease. Life 11, 86 (2021).
Argilés, J. M., Campos, N., Lopez-Pedrosa, J. M., Rueda, R. & Rodriguez-Mañas, L. Skeletal muscle regulates metabolism via interorgan crosstalk: Roles in health and disease. J. Am. Med. Dir. Assoc. 17, 789–796. https://doi.org/10.1016/j.jamda.2016.04.019 (2016).
Carvalho, J. R. & Machado, M. V. New insights about albumin and liver disease. Ann. Hepatol. 17, 547–560 (2018).
Bernardi, M. et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut 69, 1127 (2020).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
C.M.: Writing—original draft, image analysis, formal analysis, revision. M.T.: Writing—review and editing. S.G.: Data curation. J.S.: Writing—review and editing. F.B.: Data curation and image analysis. M.P.: Supervision. J.O.: Conceptualization, methodology. P.L.: Conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
March, C., Thormann, M., Geipel, S. et al. Increase of radiologically determined muscle area in patients with liver cirrhosis after transjugular intrahepatic portosystemic shunt. Sci Rep 13, 17092 (2023). https://doi.org/10.1038/s41598-023-43938-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43938-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.