Abstract
Patients with transfusion-dependent thalassemia (TDT) have an increased risk of osteoporosis and fractures. They also have several potential factors associated with sarcopenia. There has been currently no study on sarcopenia and its association with falls and fractures in TDT. This study aims to determine the prevalence of and factors associated with osteoporosis, fragility fractures, and sarcopenia in adults with TDT. A cross-sectional study was conducted at the hematologic clinic at King Chulalongkorn Memorial Hospital, Bangkok, Thailand. Clinical data and laboratory testing were collected. Bone mineral density and morphometric vertebral fracture were assessed. Sarcopenia was defined using the 2014 and 2019 Asian Working Group for Sarcopenia (AWGS) criteria. We included 112 TDT patients aged 35.1 ± 12.5 years. The prevalence of osteoporosis was 38.4%. Fragility fractures were found in 20.5% of patients. Lower BMI (OR 0.29; 95% CI 0.12–0.72, P = 0.007) and hypogonadal state (OR 3.72; 95% CI 1.09–12.74, P = 0.036) were independently associated with osteoporosis. According to the 2014 AWGS criteria, the prevalence of overall sarcopenia and severe sarcopenia was 44.6% and 13.4%, respectively. Severe sarcopenia was strongly associated with fragility fractures (OR 4.59, 95% CI 1.21–17.46, P = 0.025). In conclusion, osteoporosis, fragility fractures, and sarcopenia were prevalent in adults with TDT. Severe sarcopenia was associated with fragility fractures. Early osteoporosis and sarcopenia screening and prevention may reduce fracture risk and its complications in these patients.
Similar content being viewed by others
Introduction
Thalassemia is one of the most common genetic disorders in the world characterized by ineffective erythropoiesis, hemolysis, and anemia due to defective globin production1,2. In the most severe form of thalassemia, transfusion-dependent thalassemia (TDT), patients require regular lifelong blood transfusions to survive3,4. Despite advances in transfusion and chelation therapy, high rates of morbidity and mortality from the disease itself as well as multiple end-organ dysfunctions caused by iron excess persist2,3,4.
Thalassemia bone disease (TBD) is characterized by bone pain, deformity, decreased bone mineral density (BMD), and fragility fractures. It has emerged as the major challenge in managing the growing morbidity burden associated with severe thalassemia phenotype5. Patients with TDT may experience pain as a result of fractures, which can develop over time6. In comparison to the general population, pain-related quality of life declines significantly with age7. Patients with more pain locations reported higher levels of anxiety and depression8.
The pathogenesis of TBD is incompletely understood but believed to be multifactorial, including childhood growth retardation, bone marrow expansion, iron and chelator toxicity, physical inactivity, low body weight, nutritional deficiencies, and hormonal deficiencies4,9. Depending on the study population, osteoporosis definition, and co-morbidities, the prevalence of osteoporosis in adults with TDT has been reported to range from 17.6 to 74.6%10,11,12,13. Compared to controls, adolescents and young adults with TDT had lower trabecular volumetric BMD, cortical area, cortical thickness, and periosteal circumference14. A recent meta-analysis found a higher prevalence of fractures in TDT (18%, 95% confidence interval (CI) 16–19%) than in non-TDT (7%, 95% CI 4–10%)15. Although osteoporosis was strongly associated with increased risk of fracture in postmenopausal women and aging men, it was unknown whether decreasing BMD predicted fracture in TDT14,16,17,18,19.
Sarcopenia is a progressive clinical syndrome characterized by general loss of skeletal muscle mass and impaired muscle function20,21. Muscle and bone are anatomically and functionally connected. The evidence associating sarcopenia, low BMD, and fracture with an elevated risk of mortality and morbidity has been widely characterized in the general population22. TDT patients presented various potential risk factors for sarcopenia, including low body weight, poor nutrition, limited physical activity, and chronic inflammatory stage2,4,9. As a result, it is critical to investigate sarcopenia in these patients.
Currently, there have been no studies examining the prevalence of sarcopenia and its associated negative health outcomes in TDT patients. It is also unclear whether sarcopenia is associated with TDT-related falls and fractures. Therefore, the current study aimed to determine the prevalence of osteoporosis and sarcopenia in adults with TDT and examine their association with fracture risk. Our findings will contribute to a greater comprehension of the pathogenesis of bone abnormalities in TDT, which may aid in the development of fracture prevention strategies in order to decrease fracture risks and their complications.
Results
Characteristics of the study population
A total of 112 patients were included in the final analysis. Study recruitment flow is summarized in Supplementary Fig. 1. There were 66 women and 46 men with a ratio of 1.4 to 1. The mean age and BMI were 35.1 ± 12.5 years and 19.9 ± 2.7 kg/m2 respectively. One-third of them (9 men and 27 women) had hypogonadism. All of the men and 14 of the 27 women had secondary hypogonadism, and 13 women reached natural menopause. All of the patients received regular blood transfusions and iron chelation therapy, and 41.1% underwent splenectomy. Seven patients (6.3%) had a history of falls in the past year. Vitamin D deficiency was prevalent among women (50.9%). Endocrinopathies included low IGF-1 levels (33.9%), subclinical/secondary hypothyroidism (32.2%), and secondary hypogonadism (21.4%). Men and women with hypogonadism had significantly lower IGF-1 levels than eugonadal patients. The demographic, clinical, and laboratory characteristics of the study population are shown in Table 1.
Prevalence of osteoporosis, fragility fracture, and sarcopenia in adults with TDT
Fifty-eight percent of the patients had BMD below the expected range for their age. Based on the BMD T-score, 55.4% of the patients had osteopenia and 38.4% had osteoporosis. The majority of patients had either osteoporosis at both the lumbar spine and the hip (48.8%) or only at the lumbar spine (37.2%), while a minority had isolated hip osteoporosis (14%). At all sites, osteoporosis was prevalent in hypogonadal patients of both sexes. The prevalence of fragility fracture and morphometric VF were 20.5% (23/112) and 8.0% (9/112), respectively. The majority of patients had a single fracture with the lumbar spine having the highest prevalence, followed by the forearms, humerus, femurs, and clavicle. Women tended to have fractures more than men, and although not statistically significant, hypogonadal women had more fractures than eugonadal women (Table 2).
In this study, 98.2% of the patients had low skeletal muscle mass. According to the 2014 AWGS criteria, 22.3% and 17.9% had low gait speed and low handgrip strength, respectively. The prevalence of overall sarcopenia was 44.6% (sarcopenia 31.2%, severe sarcopenia 13.4%). Using the 2019 revised AWGS criteria, the prevalence of overall sarcopenia and severe sarcopenia increased to 61.6% and 24.1%, respectively (Table 3). About half of sarcopenic patients had osteoporosis and vice versa (Supplementary Fig. 2).
Factors associated with osteoporosis, fragility fractures, and sarcopenia
Results from the univariate analysis revealed that age, BMI, a history of falls in the past year, serum ferritin level, hypogonadal state, CTX, P1NP, IGF-1 level, and severe sarcopenia were associated with osteoporosis. In multivariate analysis, however, only BMI (OR 0.29; 95% CI 0.12–0.72, P = 0.007) and hypogonadal state (OR 3.72; 95% CI 1.09–12.74, P = 0.036) were independently associated with osteoporosis (Supplementary Table S1).
According to the 2014 AWGS criteria, a history of falls in the past year, IGF-1 levels, osteoporosis, and severe sarcopenia were associated with fragility fracture. In multivariate analysis, severe sarcopenia (OR 4.59; 95% CI 1.21, 17.46, P = 0.025) and IGF-1 levels (OR 0.98; 95% CI 0.96, 0.99, P = 0.023) were independently associated with fragility fracture (Table 4). In addition, age (P = 0.032) and deferasirox usage (P = 0.031) were significantly associated with overall sarcopenia, while a history of falls in the past year (P = 0.032) was associated with severe sarcopenia. However, these associations were not found significant in multivariate analyses (Table 5).
Discussion
The present study evaluated the prevalence of osteoporosis and fragility fractures, focusing on the prevalence of sarcopenia and its associated risk factors in TDT. Half of the patients in our study had low bone mass for their age, and 38.4% had osteoporosis as defined by BMD T-score ≤ − 2.5. The overall prevalence of fragility fractures was 20.5%. Furthermore, we demonstrated that nearly all patients had low skeletal muscle mass with almost half having sarcopenia. We also found that severe sarcopenia was strongly associated with fragility fractures.
The prevalence of osteoporosis and fracture in TDT patients was highly heterogeneous, depending on patient characteristics, comorbidities, diagnostic criteria of osteoporosis, assessment method, definition of fracture, and sample size. Our findings were consistent with previous reports10,12, which found that approximately half of adults with TDT had BMD values below the expected range for their age. In a recent study conducted in Iran13, the prevalence of low BMD was found to be 74.6%, with half of the participants having hypogonadism, compared to 32.1% in our study. In contrast, a 17.6% rate of osteoporosis was reported in a study that omitted TDT patients with hypogonadism or menopause11. All studies, including our study, demonstrated a higher prevalence of osteoporosis at the lumbar spine than the hip region, regardless of gender or gonadal status.
The prevalence of fracture among adults with TDT was also highly variable, ranging from 11.623 to 55%19. However, most fracture data were collected via a self-reported questionnaire and/or medical record with each method having different types of possible bias. Some studies included traumatic fractures in reported total fractures, which could overestimate the prevalence of fragility fractures. In our study, fracture data was confirmed and accurately reported through an in-person interview following a review of medical records. Asymptomatic VFs were also evaluated with spinal X-rays, and hands, finger, foot, ankle, skull and facial bone fractures were excluded. We feel our study more accurately reflected the occurrence of fragility fractures among these patients compared to most previous studies.
The underlying mechanisms that lead to decreased bone mass and increased fracture risk in TDT remain unknown, but it is believed that they are complex and involve both traditional and thalassemia-specific risk factors9. TDT patients are likely to have several traditional risk factors for low BMD/fracture, including physical inactivity, low body weight, and nutritional deficiencies4,9,24. Several nontraditional factors have been investigated, including genetic factors25,26, marrow expansion27, iron toxicity28,29, and hormonal deficiencies30,31. The present study has confirmed the association between hypogonadism and osteoporosis in TDT patients, which has been reported in several studies30,32,33,34. We found that the IGF-1 level was associated with osteoporosis, however, the association did not attain statistical significance in a multivariate analysis. The study findings indicated that individuals with hypogonadism had significantly lower IGF-1 levels than those with eugonadism. A negative correlation was also observed between IGF-1 levels and fracture. It is noteworthy to mention that the inability to attain maximum bone mass19,31 leading to potential fractures in TDT patients can be attributed to both hypogonadism and GH deficiency.
In postmenopausal osteoporosis, the relationship between BMD measured by DXA and fracture is well-established35,36. The effect of BMD on fracture in TDT was controversial23,37. We found no association between BMD and fracture, which could be explained by the fact that TBD is a composite of several factors, many of which cause significant bone microarchitecture deterioration, but are not entirely reflected by BMD.
Vitamin D plays an important role in calcium and skeletal homeostasis, and vitamin D deficiency results in secondary hyperparathyroidism, bone loss, and mineralization defects38. Despite the high prevalence of low vitamin D status among TDT patients39,40,41,42, the contribution of vitamin D to TBD has not been shown. We found that half of our patients had vitamin D deficiency, a rate comparable to that of the general Thai population43. However, no association was found between vitamin D status and fracture. The majority of our patients had 25(OH)D levels greater than 12 ng/dL, which was unlikely to result in osteomalacia. In addition, there was no correlation between low 25(OH)D and high intact parathyroid hormone, suggesting that they may have relative hypoparathyroidism. These factors may partially explain why vitamin D deficiency was not associated with bone loss and/or fracture.
Sarcopenia is an age-related disease, characterized by a general loss of skeletal muscle mass and impaired muscle function, and is associated with a wide range of adverse health outcomes, including falls and fractures44. In addition to elderly populations, sarcopenia was substantially more common in various patient categories with the prevalence ranging from 18% in diabetic individuals to 66% in patients with unresectable esophageal cancer. A high prevalence of sarcopenia has also been found in patients with renal and liver disease who require surgery, as well as in individuals with various site-specific malignancies45. According to the 2014 AWGS definition, we found that sarcopenia was prevalent in young to middle-aged adults with TDT (44.6%), and that 30% of these individuals had severe sarcopenia. Even though there have been no comparative studies of sarcopenia in patients with thalassemia, the above prevalence was much higher than reported among community-dwelling older Thai people (ranging from 10 to 30%)46,47,48, indicating that TDT accelerated the development of sarcopenia. We suggested that physicians should be aware of the risk of sarcopenia in TDT patients, not just the elderly, but also the middle-aged.
Several mechanisms, including aging, oxidative stress, inflammation, mitochondrial dysfunction, and apoptosis may be involved in the pathogenesis of sarcopenia44. Underlying mechanisms leading to sarcopenia in TDT have not been adequately studied. We believe that they are multifactorial, including both risk factors found in the general elderly population and risk factors specific to thalassemia.
Age-related iron accumulation in skeletal muscles causes muscle atrophy and is likely associated with oxidative damage and mitochondrial dysfunction, according to animal studies49. Recent clinical data have consistently suggested a correlation between iron accumulation and sarcopenia. Kim et al.50 demonstrated that serum ferritin levels were substantially associated with sarcopenia in middle-aged and elderly Korean women. Patients with elevated serum ferritin levels had a twofold increased risk of sarcopenia compared to those with normal serum ferritin levels. Another study by Perna et al.51 also found that elderly patients with sarcopenia had significantly elevated serum ferritin levels and serum inflammatory markers. These findings suggested that excess iron may play a role in the development of sarcopenia in TDT. The presence of chronic, severe anemia may contribute to sarcopenia in TDT patients. In a previous study involving middle-aged to elderly individuals52,53,54, a negative association between hemoglobin level and sarcopenia was observed. In one report, a 1 g/dL increase in hemoglobin was associated with a 5% reduction in the risk of sarcopenia (OR 0.95, 95% CI 0.90–0.98)53. In the general population, an association between hormonal deficiencies, such as vitamin D deficiency, hypogonadism, growth hormone deficiency, and sarcopenia has been described55. However, there have been few studies investigating the effect of these factors on muscle mass and/or function in TDT patients56.
In our study, we found no significant association between sarcopenia (based on either the 2014 or 2019 AWGS criteria) and age, BMI, vitamin D level, IGF-1 level, hypogonadism, pre-transfusion hemoglobin level, or serum ferritin level. A relatively small sample size and distinctions in the study population may have resulted in insufficient power to detect any associations in the multivariate analyses.
In the general population, the effect of decreased muscle mass and strength on fall risk, BMD, and fractures has been well described57,58,59. In one retrospective cohort of adults with TDT, there was a positive correlation between skeletal muscle mass and BMD60. No association between sarcopenia and BMD was demonstrated in our study, however, we found that a significant proportion of TDT patients with osteoporosis had sarcopenia and vice versa. Therefore, sarcopenia should be routinely assessed in TDT patients, especially for those with osteoporosis.
A recent meta-analysis of prospective studies in older adults found that sarcopenic individuals had a significantly higher risk of falls (OR 1.89; 95% CI 1.33–2.68, P < 0.001) and fractures (OR 1.71; 95% CI 1.44–2.03, P = 0.011) than non‐sarcopenic individuals61. Although we found no association between sarcopenia and falls in TDT, patients with severe sarcopenia, according to the 2014 AWGS criteria had a 4.6-fold increased risk of fragility fracture (OR 4.59, 95% CI 1.21–17.46, P = 0.025).
According to our knowledge, this is the first study to report the prevalence and risk factors of sarcopenia in adults with TDT. Our findings highlighted the significance of sarcopenia in TDT, which was not only common and occurred at a younger age, but was also associated with fracture. Although there is limited evidence to support sarcopenia as a predictor of osteoporotic fractures, it is widely accepted that sarcopenia and osteoporosis/fracture are strongly connected. We consider that assessing sarcopenia should be incorporated of any fracture prevention strategy in TDT patients, particularly those with osteoporosis. In the absence of specific sarcopenia medications, interventions known to improve sarcopenia in the general population, such as exercise, protein supplements, and adequate vitamin D levels, may have an effect on bone mass and should be encouraged in these individuals. A longitudinal investigation on the outcome of sarcopenia in TDT patients might be beneficial.
This study contains some limitations. First, the participants were recruited from a hematology clinic at a university hospital in Thailand, where the majority of patients had received early and regular blood transfusions and chelation therapy and were extensively monitored and regularly followed up. Findings cannot necessarily be generalized to other settings where resources are more limited. Second, most participants in the study were young to middle-aged adults, so the study results also should not be generalized to children, adolescents or older adults until further study. Third, the prevalence of osteoporosis may be exaggerated if the T-score is used to classify BMD for all participants, as some of them may not have reached their peak bone mass. However, the proportion of patients with low BMD by Z-score was comparable between adults under and over 30 years of age, indicating that low BMD is likely to persist in younger adults as they age. Fourth, we lacked information on dietary intake, physical activity, and inflammatory markers, which could be additional risk factors for sarcopenia. In addition, our study did not assess other potential consequences of sarcopenia, such as physical disabilities, depression, and decreased quality of life. Last, as a cross-sectional investigation, we were unable to provide support for a causal relationship between the associated factors and the outcomes.
Conclusion
Osteoporosis, fragility fractures, and sarcopenia were prevalent among adult patients with TDT. Severe sarcopenia was strongly associated with fragility fracture. Screening, prevention, and treatment strategies for osteoporosis and sarcopenia may reduce fracture risk and its complications.
Materials and methods
Study design and participants
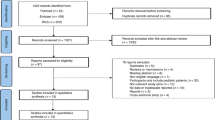
A cross-sectional study was conducted between August 2020 and November 2020. One hundred and fifty-nine TDT patients over 18 years of age who attended the hematology clinic of the King Chulalongkorn Memorial Hospital, Bangkok, Thailand, were invited to participate in this study. Participants with a known history of bone marrow transplantation, cancer, inflammatory bowel disease, being on anti-osteoporotic drugs or medications affecting bone metabolism, and/or being hospitalized were excluded. A total of 112 patients who met the inclusion criteria were enrolled.
The study was reviewed and approved by the Chulalongkorn University Faculty of Medicine’s Institutional Review Board in Bangkok, Thailand (IRB number 126/63). All study participants provided written informed consent before any procedures were performed. All procedures were conducted following applicable rules and guidelines. The trial registration number was TCTR 20201008006.
Clinical data collection
At the time of study enrollment, the demographic and clinical information of all participants were collected from an electronic medical record system and interviewed by trained study personnel. A structured questionnaire was used to collect data, including a history of thalassemia, reproduction, and traditional osteoporosis and fracture risk history. We measured anthropometric variables, including weight and current height. The data collection was performed at the same visit as the blood sample collection and BMD measurement or within a month.
After at least 8 h of fasting, whole blood samples were collected, and serum and plasma were stored at − 80 °C until analysis. Complete blood count, ferritin, calcium, phosphorus, albumin, creatinine, liver function tests, fasting plasma glucose, insulin-like growth factor-1 (IGF-1), intact parathyroid hormone (ICMA; intact PTH assays Roche Elecsys®), serum 25-hydroxyvitamin D (25(OH)D) levels (CLIA; DiaSorin LIAISON®), free thyroxin (T4) and thyroid stimulating hormone (TSH) (ECLIA; Cobas e601 analyzer, Roche Diagnostics) were measured in all participants. A fasting plasma glucose level of ≥ 126 mg/dL was used to define diabetes. Subclinical hypothyroidism was defined as a TSH level above the upper limit of the reference range and a normal level of free T4, and overt hypothyroidism was defined as a free T4 level below the lower limit of the reference range with any TSH concentration. In this study, low IGF-1 was defined as IGF-1 less than the lower limit of normal IGF-1 level of that age. Age-specific reference ranges of IGF-1 levels were provided by SIEMENS (IMMULITE® 2000 systems). For the 25(OH)D assay, the intra- and interassay coefficients of variation were less than 6%. Vitamin D deficiency was defined as serum 25(OH)D levels of < 20 ng/mL62. Bone turnover markers including serum procollagen type 1 N-terminal propeptide and C-terminal cross-linking telopeptide of type I collagen were measured using a chemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany) in an Elecsys 2010 machine.
Serum FSH, LH, total testosterone, and sex hormone binding globulin levels were measured in all men. Men with calculated free testosterone concentration of < 0.245 nmol/L based on Vermeulen’s formula were defined as hypogonadism63. In women with irregular menstruation, FH, LH, and estradiol levels were measured. Secondary hypogonadism was diagnosed in women with irregular or absent menstruation for at least 12 months with serum estradiol concentrations < 50 pg/mL and inappropriately low or normal serum FSH concentrations. Woman over the age of 45 years who had not had a menstrual period for a year and had FSH levels > 30 mIU/mL was diagnosed as menopause64.
Bone mineral density measurement
BMD at the lumbar spine, femoral neck, and total hip were measured using dual energy X-ray absorptiometry (DXA) on a QDR 4500 bone densitometer (Hologic, Inc., Bedford, MA). BMD Z- and T-scores were calculated using the manufacturer-supplied reference databases for Asian populations. According to the IOF Committee of Scientific Advisors Working Group on Osteoporosis Pathophysiology 201265, they recommended using BMD T-score according to the WHO operational definition of osteoporosis in young adults with a chronic disorder or taking medications known to affect bone metabolism, unless it appears that he/she is still growing. In this study, osteoporosis was defined as a T-score of ≤ − 2.5 standard deviation (SD), whereas osteopenia was defined as a T-score between − 1 and − 2.5 SD below the young adult mean value. BMD Z-scores were also calculated. Individuals with a Z-score ≤ − 2.0 SD were defined as having low bone mass compared to a healthy person of the same age and gender66.
Fragility fracture and morphometric vertebral fracture assessment
A fragility fracture is defined as a fracture sustained from force comparable to a fall from a standing position or less, excluding fractures of the skull, face, fingers, and toes. For morphometric VF assessment, in accordance with standard protocol, a lateral thoraco-lumbar X-ray radiograph was obtained with a tube-to-film distance of 101.6 cm, which included information regarding the positioning of the participants and the radiographic technique used. The radiographs were obtained from the left lateral position, centered at the L1 level. Using Genant’s semi-quantitative method67, morphometric vertebral fracture (VF) was diagnosed by three independent radiologists. Disputes regarding the value of a joint were settled by consensus. The VF was determined by evaluating vertebral bodies from the T4 to L4 levels.
Diagnosis of sarcopenia
The Asian Working Group for Sarcopenia (AWGS) criteria were used to define sarcopenia. The updated 2019 consensus, when compared to the 2014 AWGS criteria, may increase the estimated prevalence of sarcopenia due to an increase in cutoff values for handgrip strength and gait speed. Liu et al.68 observed that the significantly associated factors with sarcopenia were not the same when sarcopenia was classified using different criteria. Most factors associated with the 2014 AWGS criteria showed no consistent pattern with the 2019 AWGS, and the validity of the AWGS 2019 consensus requires more research. Since there is no current study on sarcopenia in TDT patients, we decided to investigate its prevalence and associated variables using both the 201421 and 201969 revised AWGS criteria. A DXA whole-body scan was used to determine skeletal muscle mass (Hologic QDR 4500, Inc., Bedford, MA). A skeletal muscle mass index of < 7.0 kg/m2 for men and < 5.4 kg/m2 for women was used to define muscle mass loss. Handgrip strength was measured using a grip dynamometer (TKK5401®; Takei Scientific Instruments, Tokyo, Japan). The participant was instructed to squeeze the dynamometer with their dominant hand, adducted arm beside the body, and elbow flexed to a 90° angle for 3 s at their maximum force. Handgrip strength was measured twice, with the highest values being recorded. For the 2014 AWGS criteria, low handgrip strength was defined as < 26 kg for men and < 18 kg for women, whereas the 2019 AWGS criteria used < 28 kg for men and < 18 kg for women. Physical performance was determined by the usual 6-m gait speed. Low physical performance was defined as a gait speed cut-off of < 0.8 (the 2014 AWGS criteria) and < 1.0 (the 2019 AWGS criteria) meter per second. Sarcopenia was diagnosed in patients who met the criteria for low muscle mass and either low handgrip strength or low gait speed. Participants with low muscle mass, low handgrip strength, and low gait speed were diagnosed with severe sarcopenia. In this study, overall sarcopenia was defined as the combination of sarcopenia and severe sarcopenia.
Sample size calculation
Based on a prevalence estimate of the prevalence of osteoporosis in Thai adult with TDT (17.6%)11, with a sampling variability of 7%, it was estimated that a sample size of at least 114 was needed for statistical adequacy to estimate the true prevalence of osteoporosis.
Statistical analyses
Data analysis was performed using SPSS, version 26 for Windows Evaluation Software (SPSS Inc, Chicago, USA). Descriptive statistics were used to categorize and summarize demographic data. Numbers and percentages were used to present gender-specific category data. The Kolmogorov–Smirnov test was used to determine the normality of a continuous data distribution. Data having a normal distribution were presented as means with standard deviations (SD), whereas data with a non-normal distribution were presented as a median with interquartile range (IQR). Comparisons of continuous data between study groups were performed using the unpaired t-test or non-parametric equivalents (Mann–Whitney U test); categorical comparisons were performed using Chi-square or Fisher’s exact test as appropriate. All P values were two-sided and considered significant if they were < 0.05.
Logistic regression analysis was performed to calculate the odds ratio (OR) and 95% CI between predictive variables and osteoporosis, sarcopenia, and fragility fracture. The variance inflation factor (VIF) was used to quantify multicollinearity among independent variables. If VIF is > 10, multicollinearity was evaluated. We considered that there was no multicollinearity concern because none of the VIF values exceeded 10. In the multivariable logistic regression (Enter method, SPSS Statistics), all variables with P values < 0.20 in the univariate models and known potential confounders were entered. A P value < 0.05 was considered statistically significant.
Data availability
The datasets generated during and/or analyses during the current study are available from the corresponding author upon reasonable request.
References
Modell, B. & Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 86, 480–487 (2008).
Taher, A. T. & Saliba, A. N. Iron overload in thalassemia: Different organs at different rates. Hematol. Am. Soc. Hematol. Educ. Progr. 2017(1), 265–271 (2017).
Porter, J. B. Practical management of iron overload. Br. J. Haematol. 115, 239–252 (2001).
Farmakis, D. et al. 2021 Thalassaemia International Federation Guidelines for the management of transfusion-dependent thalassemia. Hemasphere 6(8), e732. https://doi.org/10.1097/HS9.0000000000000732 (2022).
Vichinsky, E. P. The morbidity of bone disease in thalassemia. Ann. N. Y. Acad. Sci. 850, 344–348 (1998).
Piga, A. Impact of bone disease and pain in thalassemia. Hematol. Am. Soc. Hematol. Educ. Progr. 2017(1), 272–277 (2017).
Trachtenberg, F. et al. Pain as an emergent issue in thalassemia. Am. J. Hematol. 85(5), 367–370 (2010).
Oliveros, O. et al. Pain over time and its effects on life in thalassemia. Am. J. Hematol. 88(11), 939–943 (2013).
Wong, P., Fuller, P. J., Gillespie, M. T. & Milat, F. Bone disease in thalassemia: A molecular and clinical overview. Endocr. Rev. 37(4), 320–346 (2016).
Tzoulis, P. et al. Prevalence of low bone mass and vitamin D deficiency in beta-thalassemia major. Hemoglobin 38(3), 173–178 (2014).
Nakhakes, C., Jaruluxananan, S., Saengsuda, Y. & Saengsuda, S. Risk factors for osteoporosis and the relationship between osteoporosis and hemoglobin level in adult patients with thalassemia in Rajavithi hospital. Asian Biomed. 9(2), 169–174 (2015).
Teawtrakul, N., Chukanhom, S., Charoensri, S., Somboonporn, C. & Pongchaiyakul, C. The trabecular bone score as a predictor for thalassemia-induced vertebral fractures in Northeastern Thailand. Anemia 2020, 4634709. https://doi.org/10.1155/2020/4634709 (2020).
Bordbar, M. et al. Bone mineral density in transfusion-dependent thalassemia patients and its associated factors in Southern Iran. Arch. Osteoporos. 15(1), 148. https://doi.org/10.1007/s11657-020-00811-7 (2020).
Fung, E. B. et al. Characterization of low bone mass in young patients with thalassemia by DXA, pQCT and markers of bone turnover. Bone 48(6), 1305–1312 (2011).
Charoenngam, N., Rittiphairoj, T. & Ponvilawan, B. Fracture prevalence in thalassemia: A systematic review and meta-analysis. Arch. Osteoporos. 16(1), 171. https://doi.org/10.1007/s11657-021-01026-0 (2021).
Ekbote, V. et al. Increased prevalence of fractures in inadequately transfused and chelated Indian children and young adults with beta thalassemia major. Bone 143, 115649. https://doi.org/10.1016/j.bone.2020.115649 (2021).
Engkakul, P. et al. Unrecognized vertebral fractures in adolescents and young adults with thalassemia syndromes. J. Pediatr. Hematol. Oncol. 35(3), 212–217 (2013).
Sutipornpalangkul, W., Janechetsadatham, Y., Siritanaratkul, N. & Harnroongroj, T. Prevalence of fractures among Thais with thalassaemia syndromes. Singap. Med. J. 51(10), 817–821 (2010).
Vogiatzi, M. G. et al. Bone disease in thalassemia: A frequent and still unresolved problem. J. Bone Miner. Res. 24(3), 543–557 (2009).
Chen, L. K. et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 15(2), 95–101 (2014).
Morley, J. E., Anker, S. D. & von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology-update 2014. J. Cachexia Sarcopenia Muscle 5, 253–259 (2014).
Lo, J. H., Yiu, T., Ong, M. T. & Lee, W. Y. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Transl. 23, 38–52 (2020).
Wong, P. et al. Thalassemia bone disease: A 19-year longitudinal analysis. J. Bone Miner. Res. 29(11), 2468–2473 (2014).
Fung, E. B. et al. Relationship between chronic transfusion therapy and body composition in subjects with thalassemia. J. Pediatr. 157(4), 641–647 (2010).
Grant, S. F. et al. Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type Iα1 gene. Nat. Genet. 14, 203–205 (1996).
Uitterlinden, A. G. et al. The association between common vitamin D receptor gene variations and osteoporosis: A participant-level meta-analysis. Ann. Intern. Med. 145(4), 255–264 (2006).
Steer, K., Stavnichuk, M., Morris, M. & Komarova, S. V. Bone health in patients with hematopoietic disorders of bone marrow origin: Systematic review and meta-analysis. J. Bone Miner. Res. 32(4), 731–742 (2017).
Rossi, F. et al. Iron overload causes osteoporosis in thalassemia major patients through interaction with transient receptor potential vanilloid type 1 (TRPV1) channels. Haematologica 99(12), 1876–1884 (2014).
Voskaridou, E. & Terpos, E. Pathogenesis and management of osteoporosis in thalassemia. Pediatr. Endocrinol. Rev. 6, 86–93 (2008).
Carmina, E. et al. Hypogonadism and hormone replacement therapy on bone mass of adult women with thalassemia major. Calcif. Tissue Int. 74(1), 68–71 (2004).
Vogiatzi, M. G. et al. Differences in the prevalence of growth, endocrine and vitamin D abnormalities among the various thalassaemia syndromes in North America. Br. J. Haematol. 146(5), 546–556 (2009).
Anapliotou, M. L. et al. The contribution of hypogonadism to the development of osteoporosis in thalassaemia major: New therapeutic approaches. Clin. Endocrinol. (Oxf.) 42, 279–287 (1995).
Chern, J. P. et al. Hypogonadotropic hypogonadism and hematologic phenotype in patients with transfusion-dependent beta-thalassemia. J. Pediatr. Hematol. Oncol. 25(11), 880–884 (2003).
Gamberini, M. R., De Sanctis, V. & Gilli, G. Hypogonadism, diabetes mellitus, hypothyroidism, hypoparathyroidism: Incidence and prevalence related to iron overload and chelation therapy in patients with thalassaemia major followed from 1980 to 2007 in the Ferrara centre. Pediatr. Endocrinol. Rev. 6, 158–169 (2008).
Kanis, J. A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359, 1929–1936 (2002).
Johnell, O. et al. Predictive value of BMD for hip and other fractures. J. Bone Miner. Res. 20(7), 1185–1194 (2005).
Lee, S. L. K., Wong, R. S. M., Li, C. K. & Leung, W. K. Prevalence and risk factors of fractures in transfusion dependent thalassemia—A Hong Kong Chinese population cohort. Endocrinol. Diabetes Metab. 5(4), e340. https://doi.org/10.1002/edm2.340 (2022).
Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 22(4), 477–501 (2001).
Tzoulis, P. et al. Prevalence of low bone mass and vitamin D deficiency in β-thalassemia major. Hemoglobin 38(3), 173–178 (2014).
Fung, E. B., Aguilar, C., Micaily, I., Haines, D. & Lal, A. Treatment of vitamin D deficiency in transfusion-dependent thalassemia. Am. J. Hematol. 86, 871–873 (2011).
Dresner Pollack, R., Rachmilewitz, E., Blumenfeld, A., Idelson, M. & Goldfarb, A. W. Bone mineral metabolism in adults with beta-thalassaemia major and intermedia. Br. J. Haematol. 111(3), 902–907 (2000).
Voskaridou, E. & Terpos, E. New insights into the pathophysiology and management of osteoporosis in patients with beta thalassaemia. Br. J. Haematol. 127(2), 127–139 (2014).
Chailurkit, L. O., Aekplakorn, W. & Ongphiphadhanakul, B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health 11, 853. https://doi.org/10.1186/1471-2458-11-853 (2011).
Marzetti, E. et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 29, 11–17 (2017).
Yuan, S. & Larsson, S. C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 144, 155533. https://doi.org/10.1016/j.metabol.2023.155533 (2023).
Therakomen, V., Petchlorlian, A. & Lakananurak, N. Prevalence and risk factors of primary sarcopenia in community-dwelling outpatient elderly: A cross-sectional study. Sci. Rep. 10(1), 19551. https://doi.org/10.1038/s41598-020-75250-y (2020).
Khongsri, N., Tongsuntud, S., Limampai, P. & Kuptniratsaikul, V. The prevalence of sarcopenia and related factors in a community-dwelling elders Thai population. Osteoporos. Sarcopenia 2(2), 110–115 (2016).
Yuenyongchaiwat, K. & Boonsinsukh, R. Sarcopenia and its relationships with depression, cognition, and physical activity in Thai community-dwelling older adults. Curr. Gerontol. Geriatr. Res. 2020, 8041489. https://doi.org/10.1155/2020/8041489 (2020).
Zhao, G. Is iron accumulation a possible risk factor for sarcopenia? Biol. Trace Elem. Res. 186(2), 379–383 (2018).
Kim, T. H., Hwang, H. J. & Kim, S. H. Relationship between serum ferritin levels and sarcopenia in Korean females aged 60 years and older using the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. PLoS ONE 9(2), e90105. https://doi.org/10.1371/journal.pone.0090105 (2014).
Perna, S. et al. Sarcopenia and sarcopenic obesity in comparison: Prevalence, metabolic profile, and key differences. A cross-sectional study in Italian hospitalized elderly. Aging Clin. Exp. Res. 29(6), 1249–1258 (2017).
Tseng, S. H., Lee, W. J., Peng, L. N., Lin, M. H. & Chen, L. K. Associations between hemoglobin levels and sarcopenia and its components: Results from the I-Lan longitudinal study. Exp. Gerontol. 150, 111379. https://doi.org/10.1016/j.exger.2021.111379 (2021).
Liu, Q. et al. Hemoglobin level is negatively associated with sarcopenia and its components in Chinese aged 60 and above. Front. Public Health 11, 1081843. https://doi.org/10.3389/fpubh.2023.1081843 (2023).
Hirani, V. et al. Low hemoglobin concentrations are associated with sarcopenia, physical performance, and disability in older Australian men in cross-sectional and longitudinal analysis: The Concord Health and Ageing in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 71(12), 1667–1675 (2016).
Cruz-Jentoft, A. J., Landi, F., Topinková, E. & Michel, J. P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 13(1), 1–7 (2010).
Soliman, A. et al. Growth hormone deficiency in adults with thalassemia: An overview and the I-CET recommendations. Georgian Med. News 222, 79–88 (2013).
Marcell, T. J. Sarcopenia: Causes, consequences, and preventions. J. Gerontol. A Biol. Sci. Med. Sci. 58, 911–916 (2003).
Dixon, W. G. et al. Low grip strength is associated with bone mineral density and vertebral fracture in women. Rheumatology (Oxford) 44(5), 642–646 (2005).
Szulc, P., Feyt, C. & Chapurlat, R. High risk of fall, poor physical function, and low grip strength in men with fracture-the STRAMBO study. J. Cachexia Sarcopenia Muscle 7(3), 299–311 (2016).
Wong, P. et al. The effect of gonadal status on body composition and bone mineral density in transfusion-dependent thalassemia. Osteoporos. Int. 25(2), 597–604 (2014).
Yeung, S. S. Y. et al. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 10(3), 485–500 (2019).
Holick, M. F. et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96(7), 1911–1930 (2011).
Ho, C. K., Stoddart, M., Walton, M., Anderson, R. A. & Beckett, G. J. Calculated free testosterone in men: Comparison of four equations and with free androgen index. Ann. Clin. Biochem. 43(Pt 5), 389–397 (2006).
Gordon, C. M. et al. Functional hypothalamic amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 102(5), 1413–1439 (2017).
Ferrari, S. et al. Osteoporosis in young adults: Pathophysiology, diagnosis, and management. Osteoporos. Int. 23(12), 2735–2748 (2012).
Lewiecki, E. M. et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 43(6), 1115–1121 (2008).
Genant, H. K., Wu, C. Y., van Kuijk, C. & Nevitt, M. C. Vertebral fracture assessment using a semiquantitative technique. J. Bone Miner. Res. 8, 1137–1148 (1993).
Liu, X. et al. The comparison of sarcopenia diagnostic criteria using AWGS 2019 with the other five criteria in West China. Gerontology 67(4), 386–396 (2021).
Chen, L. K. et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21(3), 300–307 (2020).
Acknowledgements
Authors would like to thank the participants for volunteering in this study and the research team of the Department of Medicine, Faculty of Medicine, Chulalongkorn University for assistance with the English-language presentation.
Funding
This work was supported by Chulalongkorn University (CU_GR_63_106_30_13), The Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University (RA-MF-43/63), The Thai Endocrine Society Research Grants (2563/04), and The Royal College of Physicians of Thailand Grand (5/2563).
Author information
Authors and Affiliations
Contributions
S.T.: Conceptualization, Data analysis, Data curation, Investigation, Methodology, Validation, Writing; N.H., K.K., N.N., P.B., P.S.: Investigation, Writing—review & editing; C.P., P.S.: Conceptualization, Writing—review & editing; L.W.: Conceptualization, Funding acquisition, Data curation, Investigation, Methodology, Validation, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thavonlun, S., Houngngam, N., Kingpetch, K. et al. Association of osteoporosis and sarcopenia with fracture risk in transfusion-dependent thalassemia. Sci Rep 13, 16413 (2023). https://doi.org/10.1038/s41598-023-43633-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43633-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.