Abstract
The purpose of this study was to identify the effect of antihypertensive medication on risks of open-angle glaucoma (OAG) among patients diagnosed with hypertension (HTN). A total of 5,195 patients, who were diagnosed with HTN between January 1, 2006 and December 31, 2015, and subsequently diagnosed with OAG, were selected for analysis. For each OAG patient, 5 non-glaucomatous, hypertensive controls were matched (n = 25,975) in hypertension diagnosis date, residential area, insurance type and economic status. Antihypertensive medications were stratified into 5 types: angiotensin converting enzyme inhibitor (ACEi), angiotensin receptor blockers (ARB), calcium channel blockers (CCB), β-blockers and diuretics. Relative risks were calculated. After adjusting for age, sex, body mass index, lifestyle, comorbidities, blood pressure (BP), follow-up duration, and use of other types of antihypertensive drugs, ARB and CCB were found to slightly increase OAG risks (RR 1.1087 (95% CI 1.0293–1.1942); 1.0694 (1.0077–1.1349), respectively). Combinations of ARB with diuretics (1.0893 (1.0349–1.1466)) and CCB (1.0548 (1.0122–1.0991)) also increased OAG risks. The risks for OAG were found to increase by antihypertensive medication use, but the effects appeared to be small. Further studies are necessary to identify the associations of increased BP, medication and therapeutic effect with OAG.
Similar content being viewed by others
Introduction
Open-angle glaucoma (OAG) is characterized by chronic and progressive degeneration of retinal ganglion cells and associated morphologic changes to the optic nerve and retinal nerve fiber layer (RNFL)1. Intraocular pressure (IOP) has been identified as the single most important risk factor in development and progression of the disease, and the current treatment strategy involves reduction of IOP2,3. Studies have shown that IOP reduction, however, does not always slow down glaucomatous progression, particularly in normal-tension glaucoma (NTG) patients whose IOP is already low4. Vascular factors, such as systemic blood pressure (BP), have been implicated in the pathogenesis of NTG, instead5,6. However, the exact nature of the relationship between BP and OAG has remained controversial, for conflicting results have repeatedly been reported by both clinic-based and population-based studies5,7,8.
The relationship is further complicated by antihypertensive treatment. Some have argued that antihypertensive medications are more relevant to the pathogenesis of OAG over BP, either through overtreatment and resultant low BP9 or the mechanism of the drug itself10,11. As antihypertensive medications may potentially be modifiable risk factors for OAG, and given that each class of antihypertensive medications has its own unique mechanism, their effects on OAG risks need to be separately identified. However, previous studies have been limited by differences in the duration and severity of hypertension (HTN) to determine associations11,12. In the present study, using Korean National Health Insurance database, we sought to investigate OAG risks associated with individual classes of antihypertensive medication while controlling for BP, duration and concomitant use of other antihypertensive drugs.
Results
Baseline characteristics
Out of 31,170 subjects included in the study, 5,195 subjects were diagnosed with OAG following HTN diagnosis (Table 1). They were 62.4 ± 12.7 years old and 50.8% were males. Each OAG subject was matched to 5 controls, making up 25,975 control subjects. The glaucoma subjects consisted of older individuals (P < 0.001), and were on greater numbers of antihypertensive medications (P < 0.001). SBP was comparable between glaucoma and non-glaucoma subjects (P = 0.859), but DBP was significantly lower in glaucoma subjects (P < 0.001). Total cholesterol levels were lower, but fasting glucose levels were higher in glaucoma subjects. Follow-up durations were comparable between glaucoma and non-glaucoma subjects (P = 0.052).
History of antihypertensive medication use
Each type of medication was stratified into current, recent and past use depending on the date of most recent prescription relative to the index date (Table 2). The use of CCB and ARB either within 30 days or at the time of OAG diagnosis was associated with slightly increased OAG risks (RR 1.109 (95% CI 1.029–1.194) for ARB; 1.069 (1.008–1.135) for CCB). According to the results, past use (defined as use of a specific type of medication more than 365 days before the index date) of diuretics, CCB, ARB and ACEi was associated with decreased risks of OAG (0.931 (0.873–0.992) for diuretics, 0.878 (0.824–0.937) for CCB, 0.880 (0.798–0.971) for ACEi, and 0.874 (0.805–0.949) for ARB).
Cumulative duration of antihypertensive medication use
The cumulative durations of antihypertensive medication use, defined as the sum of consecutive prescriptions, were divided into 2 years or less, between 2 and 4 years, and more than 4 years, as shown in Table 3. Mild increase in relative risks were noted when diuretics (1.098 (1.022–1.181)) or β-blockers (1.086 (1.014–1.164)) were used for 2 years or less. The use of diuretics for longer than 4 years was associated with decreased risks of OAG (0.874 (0.769–0.994)) relative to no use at all. Other types of antihypertensive medications did not show significant associations between cumulative duration of drug use and OAG risks.
Combination of antihypertensive medications
The effect of antihypertensive medications on OAG risks when used in combinations with other types of antihypertensive drugs was examined (Table 4). Diuretics were found to further increase OAG risks when used in combination with either β-blockers (1.094 (1.005–1.096) relative to those not on β-blockers) or ARB (1.089 (1.035–1.147) relative to those not on ARB). The OAG risks were also found to increase when CCB was used in combination with ARB (1.055 (1.012–1.099) in comparison to those not on ARB).
Discussion
The present study investigated the relationship between systemic antihypertensive medication and the risk of OAG by comparing the risks of OAG among HTN patients on medical therapy. According to the results, the use of CCB and ARB was associated with increased risks of OAG diagnosis, but the risks were not affected by the time length of the drug use. The effect of CCB on OAG risks were further increased if the drug was used in combination with ARB. Although the results were statistically significant, the risks mediated by antihypertensive medications in HTN patients were relatively small as indicated by low odds ratios.
Previous studies that assessed the association between antihypertensive treatment and OAG have put forth conflicting results. For instance, the Barbados Eye Study found that those who were treated for HTN had lower risks of developing OAG in comparison to those with untreated HTN13. Its 9-year incidence phase failed to find a significant association between antihypertensive treatment and OAG14, similar to the Singapore Malay Study15. In contrast, the Rotterdam Eye Study concluded that the low diastolic perfusion pressure increased OAG risks only in subjects receiving antihypertensive therapy16. Similarly, the Thessaloniki Eye Study reported that increased cupping and decreased rim area were noted in patients whose DBP fell below 90 mmHg from antihypertensive treatment, while the association was not noted in patients with similar DBP without treatment17. Previous studies have also failed to agree on which type of antihypertensive medications is associated with increased OAG risks. The Rotterdam Eye Study found a 1.8-fold increased risks of OAG incidence in non-glaucomatous subjects on CCB18. A retrospective, case–control study found significantly elevated risks of OAG diagnosis for those on CCB as well19. A recent meta-analysis of 10 studies on the topic also found a higher odds of glaucoma with the use of CCB20. Other studies, however, have shown significant associations for other types of antihypertensive medications. The same meta-analysis found β-blockers associated with lower odds of glaucoma21. The European Glaucoma Prevention Study noted an association between OAG development and the use of diuretics22, while a population-based study out of the United Kingdom found increased risks regarding the use of ACEi19. Another population-based, cross-sectional study by Chong et al. found increased loss of retinal ganglion cells in patients on ACEi and diuretics23. In contrast, a retrospective review of the Groningen Longitudinal Glaucoma Study cohort found that ARB delayed glaucoma progression in older patients and lower risk of suspect conversion in those on ACEi or ARB24. Many of the studies mentioned above, however, were limited in their ability to control for the duration and severity of hypertension, which may affect OAG itself. Therefore, the present study attempted to identify the effect of medications independently of HTN by matching OAG patients to those who were diagnosed with HTN at the same time. According to our analysis, the risks of OAG increased for HTN patients on CCB and ARB, after adjusting for the effect of other types of antihypertensive medications. The combinations of CCB with ARB increased the risks further in comparison to those on CCB and not on ARB.
Our results regarding CCB and ARB were in somewhat disagreement with previous cellular and animal studies on the same drugs. For instance, the components of the Renin–Angiotensin–Aldosterone (RAA) are responsible for aqueous humor formation and secretion according to studies on cultured human non-pigmented ciliary epithelial cells25,26. In theory, inhibition of the pathway must have beneficial effects in glaucoma patients by suppressing IOP. ARB was also found to suppress retinal ganglion cell death in an animal study27,28. As for calcium blockade, it is so far believed that DHP-sensitive, voltage-gated calcium channels are present on ciliary epithelial cells to regulate gap junctions between the pigmented and non-pigmented ciliary epithelial cells29. This way, CCB in eyes may regulate aqueous humor production and secretion by decreasing intracellular calcium influx30,31. Vascular smooth muscles of the choroid are also found to be regulated at least in part by calcium32. In a study on rabbits, oral CCB (nicardipine) enhanced retinal and choroidal blood flow33. Studies involving human subjects, however, have failed to yield consistent results on the ability of CCB to improve ocular circulation in glaucoma patients29.
More recent studies in human subjects have rather pointed the impact of CCB on glaucoma in the other direction. For instance, a meta-analysis of 11 population-based cohort studies of the European Eye Epidemiology Consortium found increased risks of glaucoma with the use of CCB34. This result was echoed by a recent meta-analysis that showed increased odds of glaucoma in association with CCB20. Both studies went on to suggest that CCBs elevate risks via mechanisms that are independent of IOP. The authors of the former study explained their finding (even though CCB is expected to reduce calcium ion levels and induce vasodilation to restore blood flow to local ischemic tissues) by suggesting that CCB-induced vasodilation may inadvertently result in diversion of blood flow from ischemic tissues to non-ischemic tissues because vasodilation is already maximized and autoregulation impaired in ischemic tissues29,34. If true, this mechanism may play a critical role especially in the population of the present study, which is known for its high prevalence of low-tension glaucoma. This speculation is further supported by our analysis that the combination of CCB and ARB as well as the combination of diuretics and ARB increased risks for glaucoma in comparison to those not on ARB. A previous study of BP control involving 17,187 HTN patients showed that the combination of RAS blockers with CCB or RAS blockers with CCB and diuretics resulted in better 24-h BP control, but more pronounced BP dip35. However, the exact mechanisms by which the medications affect glaucoma pathogenesis need to be further studied individually.
Our analysis on the association between glaucoma risks and the time lengths of antihypertensive medication yielded interesting results. When diuretics (1.098 (1.022–1.181)) or β-blockers (1.086 (1.014–1.164)) were used for 2 years or less, the risks for glaucoma increased. However, when diuretics were used for longer than 4 years, the risks of OAG (0.874 (0.769–0.994)) decreased relative to no use at all. β-blockers, in contrast, did not demonstrate significant associations with glaucoma risks when used long-term. Both β-blockers and diuretics have been associated with lower IOP when systemically administered34. It is possible that the such reduced IOP were not sufficient to lower glaucoma risks but delayed early detection of glaucoma instead, given that elevated IOP often draws attention to possible glaucoma patients34. In addition, reduction in systemic BP may have impaired perfusion of the optic nerve, causing local ischemia, thereby increasing risks in the short-term. The difference in the degree of association with glaucoma risks in the long-term between the two drugs may be related to the different patterns with which they lower BP. According to a previous study, while diuretics and β-blockers reduce systolic BP to a similar extent, β-blockers reduce diastolic BP to a greater degree36. The significance of diastolic BP in glaucoma has been highlighted by a number of studies in the past37,38,39. The differences between the 2 drugs to affect cardiovascular complications may have also resulted in risk differences in the long-term. For instance, diuretics have shown a more favourable outcome in preventing cardiovascular morbidity compared to beta-blockers in the past40,41. Furthermore, it is possible that the use of β-blockers or diuretics may be an indication of the presence of other cardiovascular comorbidities or uncontrolled hypertension, both of which may have increased risks of glaucoma42. We understand that this remains a possibility despite our attempt to control for most of cardiovascular comorbidities, BP levels and the severity of HTN in regression analyses. Lastly, we speculate along with the authors of a previous study that there may be a ceiling effect for some antihypertensive medications where no further increase in risks for glaucoma damage is noted once a “ceiling” has been reached24.
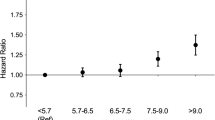
It is important to also consider that the relative inconsistency among study results, including those of the present study, may be an indication that no specific effect exists for any particular type of antihypertensive medication. Rather, HTN is a crucial risk factor and the use of antihypertensive medication is merely an indication of a history of abnormally elevated BP. HTN has consistently been suggested as a risk factor for OAG. The Blue Mountains Eye Study found that the risk of OAG increased by more than 50% if HTN is present43. The Baltimore Eye Survey also supported the relationship between increased OAG risks and HTN44. A meta-analysis from our group also demonstrated that systemic HTN increased OAG risks by approximately 20%6. Our previous study that investigated the association between OAG and untreated high BP demonstrated that elevated BP alone increased risks for OAG, indicating that a significant relationship exists between HTN and OAG independent of antihypertensive treatment42. Furthermore, CCB, which showed significant associations with OAG in multiple studies including our own, is generally not the first line treatment and is often prescribed in refractory hypertension45,46. Also, when adjusting for the duration of hypertension, SBP and DBP, the relative risks of those types of antihypertensive medications showing statistically significant P-values are quite small in our analysis. Their effect on OAG risks, if present, were not dose-dependent. It is likely that if there is to be any causal relationship between antihypertensive medication and OAG, the OAG risks conferred by medications are generally small and not affected by ongoing, long-term use.
The deleterious effect of overtreatment of HTN and resultant hypotension needs to be carefully assessed in the future. A number of previous studies have raised concerns about the impact of medically induced hypotension on OAG. The study as a follow-up to the Thessaloniki Eye Study found that ACE inhibitors and ARB significantly increased the likelihood of having larger cup size and higher C/D ratio17. However, when the analysis was repeated by separating the study population by BP levels, all classes of antihypertensive medications were associated with larger cup size and higher CD ratios for those whose DBP was below 90 mmHg while on antihypertensive treatment. The associations were not present for any of antihypertensive medications in those with DBP lower than 90 mmHg without antihypertensive medications, leading the investigators to conclude that it was the medical reduction of DBP, rather than any specific type of antihypertensive medication, that was associated with increased OAG risks. Similarly, a prescription-based study from Denmark reported that all classes of antihypertensive medications significantly increased the likelihood of a later onset of glaucoma47. The relationship between OAG, BP and treatment is complex and randomized clinical trials are necessary to generate strong evidence on whether and if so, what classes of antihypertensive medications are associated with OAG.
The main strengths of the present study include a large population dataset that contains a comprehensive list of potential confounding factors. Furthermore, our analysis was limited to those who do not have a history of prior antihypertensive or IOP-lowering medication use, which allowed evaluation of the incidence of glaucoma among patients with hypertension. The limitations of the study are as follows. First, the study population consists of Korean adults under a universal health insurance and screening program, and NTG is more prevalent among Korean adults, so the results may not be generalizable to other ethnic groups or populations under different health care systems. Second, the OAG cases might have been underestimated because of the asymptomatic nature in early stages, and possible omission of medical treatment due to low IOP. Third, the diagnosis and subsequent management of both OAG and HTN may not be uniform across clinicians. Fourth, prescription patterns for HTN may be complicated by concurrent systemic diseases, and the effects of other drugs or diseases may not have been completely eliminated. The number of drug types and the use of multiple drugs for the treatment of systemic HTN may be an indication for a more severe underlying condition. Fifth, prescriptions do not always reflect the actual medication use. Lastly, ocular factors such as IOP, perimetry and disc imaging were not available in this administrative claim database.
In conclusion, the use of CCB and ARB for HTN treatment was found to slightly increase OAG risks, but the risks mediated by antihypertensive medications were relatively small. Further studies are necessary to identify whether OAG risks are affected by specific mechanisms of different types of antihypertensive medications.
Methods
Data source
The data for the present study are derived from the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC), a population-based cohort established by the National Health Insurance Service (NHIS). The NHIS is the sole provider of healthcare in South Korea48. The cohort data are comprised of 1,137,861 participants, who were randomly selected from the total of approximately 46,000,000 Korean population on January 1, 2002 and followed up for 14 years until December 31, 2015. The data include disease diagnosis, drug prescriptions, interventions, and procedures as well as sociodemographic variables. The study adhered to the Declaration of Helsinki and all federal laws in the country. Informed consent was waived by the Institutional Review Board of Yonsei University Severance Hospital due to the retrospective study design and de-identified, routinely collected nature of the data. The study protocol was approved by the Institutional Review Board of Yonsei University Severance Hospital (approval number 4–2021-0689).
Study population
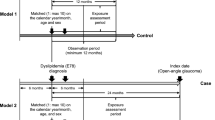
The study population was selected as shown in Fig. 1, using the data of 1,137,861 adults who represent approximately 2% of Korean population. Subjects with any previous hospital claims of any form of glaucoma (n = 15,992) according to the International Classification of Diseases, 10th Revision (ICD-10) coding (H40.x, H42.x, and Q15.0) between January 2002 and December 2005 were excluded. Subjects with prior prescriptions of topical glaucoma medication (n = 8,514) during this period were also excluded. Those with any previous hospital claims of any form of HTN (n = 35,952) (I10.x, I11.x, I12.x, I13.x, and I15.x) or antihypertensive medications (n = 98,522) between January 2002 and December 2005 were excluded as well.
A flowchart of study population selection. From 1,137,861 subjects, those with previous glaucoma diagnosis (n = 15,992), previous glaucoma medication use (n = 8,514), previous HTN diagnosis (n = 35,952) and previous antihypertensive medication use (n = 98,522) were excluded. Subjects with HTN diagnosis were identified, and those with subsequent diagnosis of OAG were matched to 5 controls to obtain a final sample of 31,170 subjects for analysis.
Subjects who were diagnosed with HTN between January 2006 and December 2015, and subsequently diagnosed with OAG were considered the cohort of the study. HTN was considered to be present if (1) hospital claims of HTN according to ICD-10 (I10.x, I11.x, I12.x, I13.x and I15.x), and (2) prescription of 1 or more antihypertensive medication for at least 30 days were both noted49. OAG diagnosis was defined as satisfying the following 2 criteria: (1) at least 2 outpatient/ambulatory visits between 2006 and 2015 containing ICD-10 codes of OAG (H40.10x, H40.13x, and H40.19x), and (2) prescriptions of topical glaucoma medication at least once during the same period. The first date on which OAG diagnosis criteria were satisfied was counted as the index date. One cohort subject was frequency-matched on household income, residential area and insurance type to 5 control subjects, who also satisfied HTN diagnosis criteria on the same date, leaving a final sample of 31,170 subjects for analysis. The index date for the controls was set as the index date of their matched cohort. Subjects were followed until the following endpoints: diagnosis of OAG, death, or end of the study period, whichever came first.
Antihypertensive medication classification
Antihypertensive medications were identified in the claims data according to the protocol developed by the Korean Society of Hypertension50. The medications were categorized into the following 5 classes: angiotensin II receptor blockers (ARB), angiotensin converting enzyme inhibitor (ACEi), β-blockers, calcium channel blockers (CCB), and diuretics. The history of antihypertensive medication prescription was examined using the following definitions: current use (the most recent prescription either lasted until the index date or 30 days before that date), recent use (the most recent prescription ended between 31 and 365 days before the index date), and past use (the most recent prescription ended more than 365 days before the index date) and never use (no recorded use)51. The cumulative duration of drug use was considered only for the currently used drug at the time of the index date, and the duration was calculated by adding the time of consecutive prescriptions.
Covariate adjustment
Individuals’ lifestyle and comorbidities were adjusted for calculation of relative risks. Subjects’ status on tobacco smoking (never, past, or current), alcohol consumption (none, 1–2 times/week, or ≥ 3 times/week), and physical exercise (none, 1–2 times/week, or ≥ 3times/week) were collected from self-reports at the time of HTN diagnosis. Body mass index (BMI), serum fasting glucose and serum total cholesterol levels were obtained during the examinations at the same time. The presence of comorbidities between the diagnosis of HTN and the endpoint, such as ischemic heart disease, myocardial infraction (MI), coronary artery obstructive disease (CAOD) and chronic kidney disease (CKD), was identified using hospital claims according to ICD-10 and considered present when at least 2 claims were made. The date of the first claim was taken as the date of diagnosis.
Statistical analyses
Continuous variables are presented as mean ± standard deviation (SD)52, and categorical variables as frequency and percentage. Cox proportional hazards analyses were performed to obtain relative risks (RR) and 95% confidence intervals (CI) for OAG events for each class of antihypertensive medications. Subjects not using the antihypertensive medication in question served as the reference. The RRs were adjusted for age, sex, calendar year of HTN diagnosis, BMI, alcohol intake, smoking, total cholesterol, fasting glucose, systolic blood pressure (SBP), diastolic blood pressure (DBP), MI, CAOD, CKD, follow-up duration and use of other types of antihypertensive drug. Of note, SBP and DBP recorded at the time of HTN diagnosis were used for analyses. The variables for adjustment were selected a priori on the basis of their known associations with BP and OAG53,54. Statistical analyses were performed using SAS version 7.4 (SAS Institute Inc.).
Data availability
The data that support the findings of this study are available from the Korean National Health Insurance Service (NHIS), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Korean NHIS.
References
Weinreb, R. N., Aung, T. & Medeiros, F. A. The pathophysiology and treatment of glaucoma: A review. JAMA 311, 1901–1911. https://doi.org/10.1001/jama.2014.3192 (2014).
Coleman, A. L. Glaucoma. Lancet 354, 1803–1810. https://doi.org/10.1016/S0140-6736(99)04240-3 (1999).
Heijl, A. et al. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 120, 1268–1279. https://doi.org/10.1001/archopht.120.10.1268 (2002).
Lee, J. S., Bae, H. W., Park, S., Kim, C. Y. & Lee, S. Y. Systemic arterial stiffness is associated with structural progression in early open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 63, 28. https://doi.org/10.1167/iovs.63.3.28 (2022).
Deokule, S. & Weinreb, R. N. Relationships among systemic blood pressure, intraocular pressure, and open-angle glaucoma. Can. J. Ophthalmol. 43, 302–307. https://doi.org/10.3129/i08-061 (2008).
Bae, H. W. et al. Systemic hypertension as a risk factor for open-angle glaucoma: A meta-analysis of population-based studies. PLoS One 9, e108226. https://doi.org/10.1371/journal.pone.0108226 (2014).
Jammal, A. A. et al. Blood pressure and glaucomatous progression in a large clinical population. Ophthalmology 129, 161–170. https://doi.org/10.1016/j.ophtha.2021.08.021 (2022).
Boland, M. V. & Quigley, H. A. Risk factors and open-angle glaucoma: Classification and application. J. Glaucoma 16, 406–418. https://doi.org/10.1097/IJG.0b013e31806540a1 (2007).
Harris, A. et al. Association of the optic disc structure with the use of antihypertensive medications: The thessaloniki eye study. J. Glaucoma 22, 526–531. https://doi.org/10.1097/IJG.0b013e31824d1e12 (2013).
Funk, R. O., Hodge, D. O., Kohli, D. & Roddy, G. W. Multiple systemic vascular risk factors are associated with low-tension glaucoma. J. Glaucoma 31, 15–22. https://doi.org/10.1097/IJG.0000000000001964 (2022).
Zheng, W. et al. Systemic medication associations with presumed advanced or uncontrolled primary open-angle glaucoma. Ophthalmology 125, 984–993. https://doi.org/10.1016/j.ophtha.2018.01.007 (2018).
Wang, S. V. et al. Using healthcare databases to refine understanding of exploratory associations between drugs and progression of open-angle glaucoma. Clin. Pharmacol. Ther. 106, 874–883. https://doi.org/10.1002/cpt.1490 (2019).
Leske, M. C., Wu, S. Y., Nemesure, B. & Hennis, A. Incident open-angle glaucoma and blood pressure. Arch. Ophthalmol. 120, 954–959. https://doi.org/10.1001/archopht.120.7.954 (2002).
Leske, M. C. et al. Risk factors for incident open-angle glaucoma: The Barbados Eye Studies. Ophthalmology 115, 85–93. https://doi.org/10.1016/j.ophtha.2007.03.017 (2008).
Zheng, Y. et al. Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: The singapore malay eye study. Invest. Ophthalmol. Vis. Sci. 51, 3399–3404. https://doi.org/10.1167/iovs.09-4867 (2010).
Hulsman, C. A., Vingerling, J. R., Hofman, A., Witteman, J. C. & de Jong, P. T. Blood pressure, arterial stiffness, and open-angle glaucoma: The Rotterdam study. Arch. Ophthalmol. 125, 805–812. https://doi.org/10.1001/archopht.125.6.805 (2007).
Topouzis, F. et al. Association of blood pressure status with the optic disk structure in non-glaucoma subjects: The Thessaloniki eye study. Am. J. Ophthalmol. 142, 60–67. https://doi.org/10.1016/j.ajo.2006.02.055 (2006).
Muskens, R. P. et al. Systemic antihypertensive medication and incident open-angle glaucoma. Ophthalmology 114, 2221–2226. https://doi.org/10.1016/j.ophtha.2007.03.047 (2007).
Langman, M. J., Lancashire, R. J., Cheng, K. K. & Stewart, P. M. Systemic hypertension and glaucoma: Mechanisms in common and co-occurrence. Br. J. Ophthalmol. 89, 960–963. https://doi.org/10.1136/bjo.2004.053397 (2005).
Leung, G., Grant, A., Garas, A. N., Li, G. & Freeman, E. E. A systematic review and meta-analysis of systemic antihypertensive medications with intraocular pressure and glaucoma. Am. J. Ophthalmol. https://doi.org/10.1016/j.ajo.2023.03.014 (2023).
Duan, N., Cui, K., Zhu, C. & Jin, S. Study on phase evolution and promoting the pozzolanic activity of electrolytic manganese residue during calcination. Environ. Res. 227, 115774. https://doi.org/10.1016/j.envres.2023.115774 (2023).
Miglior, S. et al. Intercurrent factors associated with the development of open-angle glaucoma in the European glaucoma prevention study. Am. J. Ophthalmol. 144, 266–275. https://doi.org/10.1016/j.ajo.2007.04.040 (2007).
Chong, R. S. et al. Association of antihypertensive medication with retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness. Ophthalmology 128, 393–400. https://doi.org/10.1016/j.ophtha.2020.07.051 (2021).
Pappelis, K., Loiselle, A. R., Visser, S. & Jansonius, N. M. Association of systemic medication exposure with glaucoma progression and glaucoma suspect conversion in the groningen longitudinal glaucoma study. Invest. Ophthalmol. Vis. Sci. 60, 4548–4555. https://doi.org/10.1167/iovs.19-27984 (2019).
Choudhary, R., Kapoor, M. S., Singh, A. & Bodakhe, S. H. Therapeutic targets of renin-angiotensin system in ocular disorders. J. Curr. Ophthalmol. 29, 7–16. https://doi.org/10.1016/j.joco.2016.09.009 (2017).
Cullinane, A. B., Leung, P. S., Ortego, J., Coca-Prados, M. & Harvey, B. J. Renin-angiotensin system expression and secretory function in cultured human ciliary body non-pigmented epithelium. Br. J. Ophthalmol. 86, 676–683. https://doi.org/10.1136/bjo.86.6.676 (2002).
Quigley, H. A. et al. Losartan treatment protects retinal ganglion cells and alters scleral remodeling in experimental glaucoma. PLoS One 10, e0141137. https://doi.org/10.1371/journal.pone.0141137 (2015).
Yang, H., Hirooka, K., Fukuda, K. & Shiraga, F. Neuroprotective effects of angiotensin II type 1 receptor blocker in a rat model of chronic glaucoma. Invest. Ophthalmol. Vis. Sci. 50, 5800–5804. https://doi.org/10.1167/iovs.09-3678 (2009).
Araie, M. & Mayama, C. Use of calcium channel blockers for glaucoma. Prog. Retin. Eye Res. 30, 54–71. https://doi.org/10.1016/j.preteyeres.2010.09.002 (2011).
Abelson, M. B., Gilbert, C. M. & Smith, L. M. Sustained reduction of intraocular pressure in humans with the calcium channel blocker verapamil. Am. J. Ophthalmol. 105, 155–159. https://doi.org/10.1016/0002-9394(88)90179-1 (1988).
Green, K., Bountra, C., Georgiou, P. & House, C. R. An electrophysiologic study of rabbit ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 26, 371–381 (1985).
Abernethy, D. R. & Schwartz, J. B. Calcium-antagonist drugs. N. Engl. J. Med. 341, 1447–1457. https://doi.org/10.1056/NEJM199911043411907 (1999).
Tamaki, Y., Araie, M., Tomita, K. & Tomidokoro, A. Time change of nicardipine effect on choroidal circulation in rabbit eyes. Curr. Eye Res. 15, 543–548. https://doi.org/10.3109/02713689609000765 (1996).
Vergroesen, J. E. et al. Association of systemic medication use with glaucoma and intraocular pressure: The European eye epidemiology consortium. Ophthalmology https://doi.org/10.1016/j.ophtha.2023.05.001 (2023).
de la Sierra, A. et al. Office and ambulatory blood pressure control in hypertensive patients treated with different two-drug and three-drug combinations. Clin. Exp. Hypertens 38, 409–414. https://doi.org/10.3109/10641963.2016.1148160 (2016).
Chen, J. M., Heran, B. S., Perez, M. I. & Wright, J. M. Blood pressure lowering efficacy of beta-blockers as second-line therapy for primary hypertension. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD007185.pub2 (2010).
Asefa, N. G., Neustaeter, A., Jansonius, N. M. & Snieder, H. Autonomic dysfunction and blood pressure in glaucoma patients: The lifelines cohort study. Invest. Ophthalmol. Vis. Sci. 61, 25. https://doi.org/10.1167/iovs.61.11.25 (2020).
Okumura, Y., Yuki, K. & Tsubota, K. Low diastolic blood pressure is associated with the progression of normal-tension glaucoma. Ophthalmologica 228, 36–41. https://doi.org/10.1159/000335978 (2012).
Kwon, J., Jo, Y. H., Jeong, D., Shon, K. & Kook, M. S. Baseline systolic versus diastolic blood pressure dip and subsequent visual field progression in normal-tension glaucoma. Ophthalmology 126, 967–979. https://doi.org/10.1016/j.ophtha.2019.03.001 (2019).
Wright, J. M. & Musini, V. M. First-line drugs for hypertension. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD001841.pub2 (2009).
Wiysonge, C. S. et al. Beta-blockers for hypertension. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD002003.pub2 (2007).
Lee, J. S. et al. Increased risks of open-angle glaucoma in untreated hypertension. Am. J. Ophthalmol. 252, 111–120. https://doi.org/10.1016/j.ajo.2023.03.032 (2023).
Mitchell, P., Lee, A. J., Rochtchina, E. & Wang, J. J. Open-angle glaucoma and systemic hypertension: The blue mountains eye study. J. Glaucoma 13, 319–326. https://doi.org/10.1097/00061198-200408000-00010 (2004).
Sommer, A. Glaucoma risk factors observed in the Baltimore Eye Survey. Curr. Opin. Ophthalmol. 7, 93–98. https://doi.org/10.1097/00055735-199604000-00016 (1996).
Acelajado, M. C., Hughes, Z. H., Oparil, S. & Calhoun, D. A. Treatment of resistant and refractory hypertension. Circ. Res. 124, 1061–1070. https://doi.org/10.1161/CIRCRESAHA.118.312156 (2019).
Wu, A., Khawaja, A. P., Pasquale, L. R. & Stein, J. D. A review of systemic medications that may modulate the risk of glaucoma. Eye (Lond) 34, 12–28. https://doi.org/10.1038/s41433-019-0603-z (2020).
Kolko, M., Horwitz, A., Thygesen, J., Jeppesen, J. & Torp-Pedersen, C. The prevalence and incidence of glaucoma in denmark in a fifteen year period: A nationwide study. PLoS One 10, e0132048. https://doi.org/10.1371/journal.pone.0132048 (2015).
Seong, S. C. et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 7, e016640. https://doi.org/10.1136/bmjopen-2017-016640 (2017).
Kuang, T. M., Xirasagar, S., Kao, Y. W., Shia, B. C. & Lin, H. C. Association of systemic hypertension with primary open-angle glaucoma: A population-based case-control study. Am. J. Ophthalmol. 218, 99–104. https://doi.org/10.1016/j.ajo.2020.04.020 (2020).
Korean Society, H., Hypertension Epidemiology Research Working, G., Kim, H. C. & Cho, M. C. Korea hypertension fact sheet 2018. Clin. Hypertens 24, 13, https://doi.org/10.1186/s40885-018-0098-0 (2018).
Choi, H. K., Soriano, L. C., Zhang, Y. & Rodriguez, L. A. Antihypertensive drugs and risk of incident gout among patients with hypertension: Population based case-control study. BMJ 344, d8190. https://doi.org/10.1136/bmj.d8190 (2012).
Lumley, T., Diehr, P., Emerson, S. & Chen, L. The importance of the normality assumption in large public health data sets. Annu. Rev. Public Health 23, 151–169. https://doi.org/10.1146/annurev.publhealth.23.100901.140546 (2002).
D’Agostino, R. B. Sr. et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 117, 743–753. https://doi.org/10.1161/CIRCULATIONAHA.107.699579 (2008).
Kim, K. E. et al. Prevalence, awareness, and risk factors of primary open-angle glaucoma: Korea National Health and Nutrition Examination Survey 2008–2011. Ophthalmology 123, 532–541. https://doi.org/10.1016/j.ophtha.2015.11.004 (2016).
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (No.NRF-2019 R1A2C1091089). The funding organization had no role in the design or conducting of this research. None of the authors have any commercial or proprietary interests in any of the instruments or materials described in this study.
Author information
Authors and Affiliations
Contributions
Conception and design: C.Y.K. Analysis and interpretation: J.S.L., H.R.C., H.W.B., S.Y.L., W.C., and S.W.L. Data collection: J.S.L., H.R.C. Obtained funding: C.Y.K. Overall responsibility: J.S.L., C.Y.K. and S.W.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J.S., Cha, H.R., Bae, H.W. et al. Effect of antihypertensive medications on the risk of open-angle glaucoma. Sci Rep 13, 16224 (2023). https://doi.org/10.1038/s41598-023-43420-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43420-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.