Abstract
MIS-C is a systemic inflammation disorder with poorly characterised immunopathological mechanisms. We compared changes in the systemic immune response in children with MIS-C (n = 12, 5–13 years) to healthy controls (n = 14, 5–15 years). Analysis was done in whole blood treated with LPS. Expression of CD11b and Toll-like receptor-4 (TLR4) in neutrophils and monocytes were analysed by flow cytometry. Serum cytokines (IL-1β, IL-2, IL-6, IL-8, IL-10, IL-Ira, TNF-α, TNF-β, IFN-Υ, VEGF, EPO and GM-CSF) and mRNA levels of inflammasome molecules (NLRP3, ASC and IL-1β) were evaluated. Subpopulations of lymphocytes (CD3+, CD19+, CD56+, CD4+, CD8+, TCR Vδ1+, TCR Vδ2+) were assessed at basal levels. Absolute counts of neutrophils and NLR were high in children with MIS-C while absolute counts of lymphocytes were low. Children with MIS-C had increased levels of IL-6, IL-10, TNF-β and VEGF serum cytokines at the basal level, and significantly increased TNF-β post-LPS, compared to controls. IL-1RA and EPO decreased at baseline and post-LPS in MIS-C patients compared to controls. The percentage of CD3+ cells, NK cells and Vδ1 was lower while B cells were higher in children with MIS-C than in controls. Dysregulated immune response in children with MIS-C was evident and may be amenable to immunomodulation.
Similar content being viewed by others
Introduction
Multisystem inflammatory disease in Children (MIS-C) or Paediatric Multisystem Inflammatory Syndrome Temporally Associated with Coronavirus disease 2019 (COVID-19) (PIMS-TS) is a post-infectious complication of SARS-CoV-2, first reported between April and May 20201,2. According to The World Health Organization (WHO), and The Centres for Disease Prevention and Control in Europe and the US (CDC), MIS-C presents 4–6 weeks after primary infection with SARS-Cov-2 with high fever, multi-organ dysfunction and systemic inflammation1,2,3,4,5. This presentation of hyperinflammatory shock in children has similarities to Kawasaki disease (KD), toxic shock syndrome (TSS), bacterial sepsis, and macrophage-activation syndrome (MAS)1,2,4,5,6,7,8. Over half of the patients require admission to an intensive care unit, and some will require extracorporeal membrane oxygenation (ECMO)9,10 but overall mortality has remained at < 2%9. Early recognition and treatment of children and adolescents with MIS-C, and a better understating of the pathological mechanism are essential to prevent serious complications, such as coronary artery aneurysms10,11,12.
Multiple alterations in inflammatory markers have been described in MIS-C with high neutrophil count, high neutrophil/lymphocyte ratios (NLR) and low absolute lymphocyte count similar to adults with acute COVID-194,7,8,9,13,14,15,16. Recommended treatments include intravenous immunoglobulin (IVIG) and corticosteroids, with resistant cases responding to targeted immunomodulators, including anti-TNF and anti-IL1 agents7,11. Furthermore, SARS-CoV-2 proteome-wide immuno-profiling of children with MIS-C, children with COVID-19 and healthy controls revealed differential cytokine responses, IgM/IgG/IgA epitope diversity, as well as differences in antibody binding and avidity17. The presence of several IgG and IgA autoantigens, some of which are associated with autoimmune diseases like systemic lupus erythematosus (SLE), Sjogren's disease, polymyositis and dermatomyositis were also reported in children with MIS-C6. This suggests a strong immune response mediating the pathogenesis of MIS-C. This study aims to further explore the acute phase immune response in children with MIS-C/ PIMS-TS compared to healthy controls. In addition, innate immune response to endotoxin will be assessed ex-vivo as a measurement of ‘endotoxin tolerance’ or trained immunity, a form of innate immune memory that might indicate reduced capacity of response to an inflammatory stimulus.
Results
Demographics
Thirty-two children were recruited for this study, 18 clinically diagnosed with MIS-C (age range 5–13 years) and 14 healthy paediatric controls (age range 1–15 years). Clinical data was analysed from 18 children with MIS-C and all cases included in the study met the criteria for diagnosis of MIS-C as per WHO guidelines (Table 1). Four patients were COVID-19 positive at the time of diagnosis and treatment for MIS-C, and no blood samples were collected as per the biosafety restrictions of the research centre. Whole blood was collected from 26 children, including 12 children with MIS-C and 14 paediatric controls. The median age of the MIS-C cohort was 8.5 ± 3 years and 61% were males. In the healthy control group, the median age was 7.8 ± 4 years and 50% were males. The most common presenting symptoms for children with MIS-C were fever (100%), gastrointestinal affectations (89%), which usually included vomiting and/or diarrhoea and abdominal pain, a blanching polymorphous rash (72%) and cardiac symptoms/tachycardic (72%). Interestingly, 1 case (5%) did not have any known history of COVID or confirmed/suspected close contact status but otherwise met the criteria for MIS-C. In clinically collected samples, the average counts of neutrophils and lymphocytes in the MIS-C cohort were 9.43 ± 4.83 and 1.37 ± 1.07 × 109 cells per litre respectively, with an average NLR of 10.05 ± 6.86 (Table S1).
Analysis of granulocytes and lymphocyte populations in healthy controls and children with MIS-C
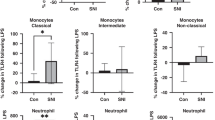
Activation of neutrophils (CD66b+) and monocytes (CD66b− CD14+/CD16+) was evaluated by expression of cell activation and migration marker CD11b, and recognition of lipopolysaccharide (LPS) by Toll-like receptor-4 (TLR4), following LPS treatment for 1 h at 37 °C and analysed by flow cytometry. Neutrophil CD11b expression increased significantly post-LPS stimulation in controls (p < 0.0001) compared to the control untreated sample, but not in children with MIS-C when the post-LPS sample was compared to its corresponding untreated sample (Fig. 1A). No significant difference was observed when comparing control and children with MIS-C. Neutrophil TLR4 expression was no different following LPS stimulation between healthy controls and MIS-C patients (Fig. 1B). CD11b expression in monocytes was not statistically different when compared between controls and children with MIS-C at basal levels or after stimulation with LPS (Fig. 1C). Monocyte CD11b expression was not different between healthy controls and children with MIS-C at baseline or after LPS stimulation (Fig. 1C). Similar to neutrophils, TLR4 expression in the total monocyte population was not significantly different following LPS stimulation in children with MIS-C or when comparing controls to children with MIS-C (Fig. 1D).
Expression of CD11b and TLR4 in neutrophils and monocytes in healthy controls and Multisystem inflammatory syndrome in children (MISC) patients: TLR4 expression in neutrophils corresponds to healthy controls (n = 11) and children with MISC (n = 11). Whole blood from both groups was stimulated with LPS (10 ng/mL) for 1 h at 37 °C, and leukocyte markers were analysed by flow cytometry. Neutrophils and monocytes were identified based on their size and granularity followed by labelling with CD66b + for granulocytes, and CD66b − and CD14 + for monocytes. (A, C) CD11b and (B, D) TLR4 expression in neutrophils and monocytes, respectively, are shown as Geometric Mean Channel Fluorescence (MFI) with median and interquartile range (IQR); “veh” refers to unstimulated (PBS). Comparisons were done between two independent cohorts and both conditions (‘Veh’ and ‘LPS-treated’). ****p < 0.0001 using Kruskal–Wallis test.
To further characterise the role of monocytes in the immune response of children with MIS-C, expression of CD11b and TLR4 were determined in the classical (CD14high CD16neg/low), intermediate (CD14high CD16high), and non-classical (CD14lowCD16high) subtypes of monocytes. CD11b expression significantly increased in classical monocytes following LPS stimulation in controls (p = 0.0001) but not in children with MIS-C (Fig. 2A). Expression of TLR4 in classical monocytes was not different when comparing LPS treatment in both groups. No differences in the expression of CD11b or TLR4 were found in classical, intermediate and non-classical monocytes following LPS treatment or when comparing children with MIS-C to controls (Fig. 2B). Children with MIS-C showed a tendency to LPS hyporesponsiveness for neutrophil and classical monocytes CD11b expression.
Expression of CD11b and TLR4 in subpopulations of monocytes in healthy controls and Multisystem inflammatory syndrome in children (MISC) patients: Whole blood from controls was stimulated with LPS (10 ng/mL) for 1 h at 37 °C, and monocyte markers were analysed by flow cytometry. Subpopulations were differentiated based on the expression of CD14 and CD16 into classical (CD14high CD16neg/low), intermediate (CD14high CD16high), and non-classical (CD14lowCD16high) (Fig. 1). (A) CD11b and (B) TLR4 expression in subpopulations of monocytes are shown as Geometric Mean Channel Fluorescence (MFI) with median and interquartile range (IQR); “veh” refers to unstimulated (PBS). Comparisons were done between two independent cohorts and both conditions (‘Veh’ and ‘LPS-treated’). ***p < 0.001, using Kruskal–Wallis test.
Frequencies of lymphocytes were determined by staining whole blood with antibodies specific for CD3, CD19 and CD56. Total T, B, and NK cells were enumerated as percentages of total lymphocytes (Figure S2). Figure 3A shows lower frequencies of T and NK cells in children with MIS-C compared to healthy controls (p = 0.0008 and p = 0.0333 respectively). In contrast, B cell frequencies increased in children with MIS-C (p < 0.0001). These results show that T, B and NK cell frequencies in peripheral circulation reflected systemic alterations of the immune response in children with MIS-C.
Frequencies of lymphocytes in healthy controls (n = 14) and Multisystem inflammatory syndrome in children (MISC) patients (n = 11). (A) Frequencies of T cell, B cell and NK cells. (B) Frequencies of CD4+, CD8+, CD4+CD8+ (double positive), CD4−CD8− (double negative), innate T cells—Vδ1 T cells and Vδ2 T cells. Results are presented as percentages with median and interquartile range (IQR). Comparisons were made between two independent cohorts. *p < 0.05, ***p < 0.001, ****p < 0.0001 using Mann Whitney test.
Further analysis of T cells subpopulations based on the expression of CD4 and CD8 positivity, allowed the identification of helper (CD4+ CD8-), cytotoxic (CD8+ CD4-) and unconventional (CD4+ CD8+ and CD4− CD8−) T cells in both groups. The frequency of these subpopulations was determined as percentages of total CD3+ cells (Figure S2). No difference in the proportions of CD4+, CD8+, CD4+ CD8+ or CD4− CD8− T cells was observed when comparing children with MIS-C with healthy controls (Fig. 3B, left). Gamma-delta (γδ) T cells subsets, Vδ1 and Vδ2, were also evaluated as percentages of total CD3+ cells (Fig. 3B, right). Vδ1 T cells were significantly reduced in MIS-C patients compared to healthy controls (p = 0.0005), while no difference in Vδ2 subsets was observed between the children with MIS-C and the healthy control group. There was no change in T cell subtypes of helper, cytotoxic and unconventional Vδ2 T cells in the circulating blood of children with MIS-C.
NLRP3 inflammasome activation and serum cytokine levels in healthy controls and children with MIS-C
Whole blood mRNA levels of two main components of the NLR family pyrin domain containing 3 (NLRP3) inflammasome, NLRP3 and Apoptosis-associated speck-like protein containing a CARD (ASC), and of one of its pro-inflammatory products, interleukin-1 β (IL-1β) were evaluated (Fig. 4A). NLRP3 mRNA levels increased after LPS stimulation in controls (p = 0.0011) and children with MIS-C (p = 0.0025) when compared to their corresponding untreated sample, but no difference was observed when groups were compared. No statistically significant difference in the levels of ASC mRNA was observed between untreated samples and LPS or between groups. mRNA expression of IL-1β was seen to increase following stimulation with LPS in both controls (p < 0.0001) and children with MIS-C (p = 0.0290).
NLRP3 inflammasome activation was evaluated by analysing the (A) messenger RNA levels of NLPR3, ASC and IL-1β, and (B) protein expression of IL-1β and IL-1RA. Whole blood of healthy controls and Multisystem inflammatory syndrome in children (MISC) patients were stimulated with LPS (10 ng/mL) for 1 h at 37 °C. Samples were then processed for RNA analysis by PCR and cytokines expression in serum. (A) NLPR3, ASC and IL-1β were measured in total mRNA extracted from whole blood of healthy controls (Veh n = 14; LPS n = 11) and Multisystem inflammatory syndrome in children (MISC) patients (n = 8). (B) Cytokine concentrations of IL-1β (left) and IL-1RA (right) were measured by multiplex enzyme-linked immunosorbent assay (ELISA) in serum of healthy controls (n = 14) and Multisystem inflammatory syndrome in children (MISC) patients (n = 11). Results are presented as (A) fold change expression of NLRP3, ASC and IL-1β with median and interquartile range (IQR), and (B) pg/mL concentration with median and IQR; “veh” refers to unstimulated (PBS). Comparisons were done between two independent cohorts and both conditions (‘Veh’ and ‘LPS-treated’). *p < 0.05, **p < 0.01, ****p < 0.0001 using Kruskal–Wallis test.
As an indirect measurement of NLRP3 inflammasome activation, protein expression of the pro-inflammatory NLRP3 activated cytokine IL-1β, and anti-inflammatory IL-1 regulator cytokine, IL-1RA, were measured in serum samples from both groups by multiplex immunoassay (Fig. 4B). A significant increase in mature IL-1β expression following LPS stimulation was observed in controls (p = 0.0088) but not in children with MIS-C when compared to the corresponding untreated samples for each group (Fig. 4B, left). No significant difference was observed in IL-1β expression between children with MIS-C and controls when both treatments were evaluated. However, children with MIS-C seem to have impaired pro-inflammatory responses to LPS compared to healthy controls. In contrast, the anti-inflammatory response, measured by IL-1RA levels, was significantly reduced in children with MIS-C at both basal and LPS-treated samples when compared to controls (p = 0.0013 and p < 0.0001 respectively).
The levels of interleukin-2 (IL-2), IL-6, IL-8, IL-10, tumour necrosis factor-α (TNF-α), TNF-β, human interferon-γ (IFN-γ), granulocyte macrophage-colony stimulating factor (GM-CSF), vascular endothelial growth factor (VEGF) and erythropoietin (EPO) in serum samples controls were measured by multiplex immunoassay in both groups. Figure 5 shows the serum levels of these ten soluble molecules, which mediate the immune response, at basal level and after stimulation with LPS in the control group and children with MIS-C. Increased levels of IL-2 (p = 0.0009), IL-6 (p = 0.0153), IL-8 (p = 0.0004), TNF-α (p < 0.0001) and VEGF (p = 0.0003) were seen in healthy controls following LPS treatment. Increases in these cytokines were not observed in children with MIS-C after treatment with LPS.
Cytokine response in healthy controls and Multisystem inflammatory syndrome in children (MISC) patients at baseline and on Lipopolysaccharides (LPS) stimulation: IL-2, IL-6, IL-8, IL-10, IFNɣ, TNFα, TNFβ, EPO, GM-CSF and VEGF. Whole blood was stimulated with LPS (10 ng/mL) for 1 h at 37 °C. Cytokine concentrations were measured by multiplex enzyme-linked immunosorbent assay (ELISA) in serum of healthy controls (IL-6, IL-8, IL-10, IFNɣ, TNFα, EPO, GM-CSF and VEGF n = 11; IL-2, n = 14; TNFβ, n = 10) and children with MISC (n = 12). Results are presented as pg/mL concentration with median and interquartile range (IQR); “veh” refers to unstimulated (PBS). Comparisons were done between two independent cohorts and both conditions (‘Veh’ and ‘LPS-treated’). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 using Kruskal–Wallis test.
Basal levels of IL-6 (p = 0.0468), IL-10 (p = 0.0029), TNF-β (p = 0.0020) and VEGF (p = 0.0056) were significantly higher in children with MIS-C when compared to basal levels in healthy controls. TNF-β levels (p = 0.0001) were also observed to be significantly increased in MIS-C patients compared to healthy controls after LPS treatment. In contrast, EPO was significantly reduced at basal level (p = 0.0406) and with LPS treatment (p = 0.0037) in children with MIS-C compared to controls. No differences were observed in IFN-γ and GM-CSF following LPS treatment or when comparing the groups.
Discussion
Neutrophils are key players in both KD and COVID-1918,19,20,21. Neutrophil counts and neutrophil/lymphocyte ratio (NLR) are higher in children with MIS-C compared to children with COVID-19, and neutrophil counts have been suggested as a predictor of responsiveness to intravenous immunoglobulin therapy in patients with KD4,7,9,13,14,15,16,19,20. Our findings are in line with previous reports indicating high absolute counts of neutrophils and NLR, as well as low counts of absolute lymphocytes in children with MIS-C compared to normal ranges. Earlier studies have described significant increases of CD11b in MIS-C when compared to children with mild and severe COVID-19, but reduced expression of the marker when comparing children with COVID-19 and healthy controls22. Similarly, we observed a tendency to reduce CD11b in children with MIS-C after a secondary challenge with endotoxin, however, this did not reach significance, likely due to our sample size. Our results contrast those of García-Salido et al. (2022), who reported high baseline expression of CD11b in monocytes and neutrophils in children with MIS-C compared to healthy controls and in children with KD23. These differences could be due to the number of patients included in the studies and the different methodological approaches employed when defining neutrophil and monocyte populations by flow cytometry. Furthermore, contradictory results indicating decreased or increased expression of CD11b in neutrophils in KD compared to controls could suggest heterogeneity in the expression of this marker in the population and the need for a larger cohort of studies23,24,25.
Several studies have reported distinguishing features of uncontrolled neutrophil activation in children with MIS-C5,6,26,27,28,29,30. Through transcriptional profiling, Boribong et al. (2021) identified a clear signature of neutrophil activation, degranulation and spontaneous neutrophil extracellular trap (NET) formation26. Other activation markers such as Fc-γ receptor I (FcγRI; CD64) and CD54 (ICAM1) were upregulated in neutrophils, total monocytes and CD16 + non-classical monocytes in children with MIS-C when compared to controls6,28,31. Interestingly, we found CD11b expression in MIS-C did not increase after stimulation with LPS, as it did in controls. To our knowledge, no other study has described changes in neutrophil response in children with MIS-C following a secondary stimulation. However, evaluation of LPS levels in the plasma of children with MIS-C shows increased levels of this microbial marker compared to children with COVID-19 and controls32. Low-grade endotoxemia (presence of LPS) was also detectable in patients with SARS-CoV-2 infection and neutrophils of early onset KD patients33,34,35. Based on our results and these reports of LPS levels, it is difficult to conclude whether the response observed in our study is due to an altered secondary response by trained immunity or the presence of co-infection after SARS-CoV-2 infection. Further analyses are required to fully understand this interaction.
LPS receptor TLR-4 was proposed as one of the pathogenic mechanisms triggered by SARS-CoV-2 infection36,37,38. It has been suggested that TLR4 can directly bind the SARS-CoV-2 spike glycoprotein or can be activated by lung viral infection-derived subproducts such as oxidized phospholipids and alarmin S100A936,37,38. We measured the cell surface expression of TLR4 in neutrophils and monocytes and found no statistical differences in the expression of TLR4 in any cell type, or when comparing the MIS-C patient group to the control group. To our knowledge, no other study has evaluated TLR4 in children with MIS-C to date. TLR4 and some of its mediated inflammatory signalling molecules are reported to be upregulated in severe COVID-19 patients38,39. Root-Bernstein et al. (2021) suggest TLR4's role in severe COVID-19 is due to synergistic immune activation induced by SARS-CoV-2-bacterium/fungus coinfection39. TLR4 may have a pathological role in COVID-19 inflammation, our data and other data published to date do not indicate a similar role in MIS-C.
Our data suggests an alteration in lymphocyte frequencies in children with MIS-C. Our total T cell and NK cell findings are in line with previous reports and could indicate migration to inflamed tissues5,6,8,14,40,41. Gruber et al. 2020 reported similar results when immunophenotyping peripheral blood of MIS-C patients and healthy controls by mass cytometry6. A transcriptome-wide analysis using RNA sequencing of peripheral blood of MIS-C patients found depletion of NK cells when compared to controls42. Increased frequencies of B cells have also been reported by others in acute MIS-C patients14,29. Ramaswamy et al. (2021) also reported more mutated IgG clones and lower B cell clonal diversity in these patients29. However, contrasting findings have been described, where total B cell counts were reduced with higher class-switched memory B cell counts in the MIS-C resolution phase compared with the acute phase28.
When analysing the frequencies of helper (CD4+ CD8−), cytotoxic (CD8+ CD4−) and unconventional CD4+ CD8+, CD4− CD8− and gamma-delta (γδ: Vδ1 and Vδ2) T cells subsets, we saw reduced Vδ1 T cells in the MIS-C patients when compared to controls. Reduced helper (CD4+), cytotoxic (CD8+) and γδ T subsets have been described in the acute phase of MIS-C5,6,8,28,40. Carter et al. (2020) reported high frequencies of CD4+CCR7+ T cells and lower γδ T cells in the acute phase of MIS-C patients as well as more regulatory T cell counts in the convalescence phase28. Ramaswamy et al. 2021 also described high proportions of regulatory T cells and decreased helper (CD4+) T cells in the peripheral blood of MIS-C patients compared to controls29. The expansion of the Vβ21.3 T cell receptor β chain in both CD4 and CD8 T cell subsets indicates an activated phenotype in children with MIS-C43. While memory CD8+ T are reported to be increased in patients, RNA sequencing analysis of blood from MIS-C patients indicates exhaustion of effector CD8+ T cells and potentially impaired cytotoxicity and pathogen-killing capacity42. Most of these changes in the adaptive immune response seem to normalize in the resolution and convalescence phases of MIS-C28,43.
NLRP3 inflammasome activation is a crucial mechanism of the innate immune system against invading pathogens. Canonical activation of NLRP3 requires priming by Toll-like receptors (TLR) ligands such as LPS, and assembly of a multiprotein complex (NLRP3, ASC and Caspase 1) which in turn mediates proteolytic processing of pro-interleukin-1β (IL-1β) and pro-IL-18, inducing inflammation. Wang et al. (2021) used whole blood microarray mRNA analysis to show a significant increase of NLRP3, Caspase 1, and IL-1β, as well as some noncanonical inflammasome markers (Caspase 4 and Caspase 5) in acute KD patients and MIS-C when compared to convalescent controls (healthy children with previous history of KD)44. They suggest a strong tendency towards caspase-4/5-dependent noncanonical inflammasome activation in MIS-C patients which could eventually trigger caspase-1-dependent NLRP3 inflammasome activation44. Our results show an increase in NLRP3 mRNA after LPS, but not in ASC mRNA, in both healthy children and children with MIS-C, but no difference between groups. Increased protein expression of cytokine IL-1β and IL-18 is an indirect indication of NLRP3 inflammasome activation, while anti-inflammatory IL-1 (IL-1RA) provides information about its regulation. In this study, an increase in mature IL-1β and IL-1RA was observed in healthy controls after LPS stimulation, as expected in a healthy pro-inflammatory response. This was not observed in children with MIS-C, which indicates an alteration in NLRP3 inflammasome activation in these patients. No statistical differences in the expression of mature IL-1β or IL-1β mRNA were observed in our cohort of MIS-C patients when compared to controls, however, a tendency towards reduction was seen. IL-1β protein expression in our study contrasts with reports from other research groups showing not only an increase in IL-1β but also of IL-18, and a strong association between IL-1β levels and a more severe disease course27,28,43,45. Furthermore, our data indicates the downregulation of anti-inflammatory IL-1RA in MIS-C children at both basal level and in LPS-treated samples compared to healthy controls. The opposite results have been previously described by other studies, where IL-1RA was increased in serum or plasma of children with MIS-C14,43,45.
Soluble molecules are important mediators of immune response and can trigger the activation or inactivation of specific cell types or molecular responses. We measured the levels of a panel of cytokines associated with pro- and anti-inflammatory signalling pathways. A significant increase in basal levels of IL-6, IL-10, TNF-β and VEGF in the serum of children with MIS-C compared to healthy controls was observed. These findings are in line with previous reports of an increase in IL-6 and IL-10 in MIS-C patients6,14,27,28,40,43,45,46,47,48. Our data contrasts with others in the expression of IL-8, TNF-α, and IFNγ. We found no difference while an increased level in MIS-C children was seen in these studies27,28,43,45,47,48,49. Syrimi et al. found no differences in the levels of these cytokines/chemokines when comparing patients to healthy controls14. To date, no other reports are showing an increase in TNF-β and VEGF. Many other soluble markers are consistently high in MIS-C patients when compared to healthy controls or COVID-19 patients, including cytokines IL-2, IL-2RA, IL-15, IL-and 17A, cytokine receptors sIL-2R, Tumor necrosis factor receptor 1 (sTNF-R1) and sTNF-R2, chemokines CCL2, CCL3, CCL4, CCL20, CCL28, CDCP1, CXCL9 and CXCL10, and soluble markers sCD40L, plasminogen activator inhibitor 1 (PAI-1), pentraxin-3 (PTX3), myeloperoxidase (MPO), monocyte chemoattractant protein (MCP)-1, MCP-2, macrophage-inflammatory protein (MIP)-1α and MIP-1β6,14,40,43,45,46. Discrepancies in cytokines expression profiles are likely due to the number of patients recruited, characteristics of the cohorts, methodological approaches, and the timing of sample collection. A clear profile of cytokines would allow clinicians to discriminate between patients with MIS-C and COVID-19 patients, or between different grades of severity in MIS-C patients5,46,47. Caldarale et al. (2021) suggest that the pattern of cytokines/chemokines in MIS-C leans toward a Th1-type immune response48. Altogether, the MIS-C soluble markers profile seems to indicate inflammation, favour lymphocytic and myeloid activation, and mucosal immune dysregulation5,6,49.
Interestingly, when we exposed whole blood of MIS-C patients to endotoxin challenge we saw no increase in levels of IL-2, IL-6, IL-8, TNF-α and VEGF as was observed in healthy controls. There is a marked hyporeactivity in MIS-C patients to LPS. Increased TNF-β and reduced EPO were the only significant variations in children with MIS-C following LPS treatment compared to controls. TNF-β levels have not been reported to be increased in a MIS-C cohort so far, especially with such a considerable difference after LPS treatment. However, other members of the TNF superfamily of molecules such as TNF-α and sCD40L, and receptors sTNF-R1 and sTNF-R2 were found to be increased at basal levels in a MIS-C14,27,43,45,47. In KD, TNF-α and TNF-β levels have been reported to be high intracellularly in monocytes and lymphocytes of patients in the acute stage, but TNF-β reduced in serum50,51. In contrast to TNF-β, EPO expression was low in children with MIS-C compared to controls for both basal levels and after LPS. So far, changes in EPO levels have not been described in this cohort by other studies. Considering its anti-inflammatory, anti-apoptotic and protective function, EPO has been suggested as a potential treatment for COVID-1952,53. However, no clear results of its therapeutic efficacy for COVID-19 were found. Further studies are required to fully understand the role of TNF-β and EPO in the pathogenesis of MIS-C.
This study identified a decreased activation of neutrophils and monocytes when evaluated by CD11b expression, and reduced levels of pro-inflammatory cytokines in response to endotoxin challenge in MIS-C. Further studies are required to fully understand the pathological immune responses in MIS-C and to identify potential immunomodulatory therapies.
Methods
Ethical approval and study participants
Ethical committee approval for the study of blood samples from children was granted by the National Research Ethics Committee for COVID-19-related Research (NREC COVID-19; 20-NREC-COV-032). All experiments were performed following relevant guidelines and regulations. Informed written consent was obtained from all parents of children who took part in the study.
Blood and serum samples were obtained from healthy school-age children (aged 5–15 years) undergoing phlebotomy as part of a day case procedure and children with MISC (aged 5–13 years) admitted to CHI. Healthy controls were having minor surgical procedures. Children with medical co-morbidities or chronic conditions were excluded from being healthy controls. Case definition for MIS-C was described as per recommendations of Centers for Disease Control and Prevention (CDC): individual aged < 21 years, presenting with fever (> 38.0 °C for ≥ 24 h), laboratory evidence of inflammation, clinically severe illness requiring hospitalization, multisystem (> 2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic or neurological), no alternative plausible diagnoses. A recent positive history of SARS-CoV-2 infection or exposure to a suspected or confirmed COVID-19 case 4 weeks before the onset of symptoms was included but not necessarily used as a diagnostic criterion.
Blood sampling
Whole-blood sampling was performed following informed parental consent Sampling was performed using an aseptic technique via central and peripheral arterial lines and venous sampling at times of routine patient phlebotomy. The blood (1–3 mL) was collected in sodium citrate tubes and processed within 2 h of collection.
Granulocytes cell surface antigen expression
100µL of whole blood was incubated at 37 °C for 1 h (5% CO2) in the presence or absence of Lipopolysaccharides (LPS) at a final concentration of 10 ng/ml (SIGMA Life Science, Ireland). Phosphate-buffered saline (PBS) was used in unstimulated samples (defined as “vehicle”) at an equal volume to LPS. Cells were then stained for 10 min at room temperature with monoclonal antibodies (mAb) specific for CD66b-Pacific Blue (6 µg/mL; G10F5; BioLegend, USA), CD14-PerCP (6 µg/mL; HCD14; BioLegend, USA), CD15-PECy7 (4 µg/mL; W6D3; BioLegend, USA), CD16-FITC (8 µg/mL; B73.1; BioLegend), USA, TLR4-APC (4 µg/mL; HTA125; BioLegend, USA), and CD11b-PE (1 µg/mL; D12; BD Biosciences, UK). After staining, cells were lysed in 1 mL FACS lysis buffer (BD Biosciences, Oxford, UK). Finally, the cells were washed with PBA buffer (PBS containing 0.1% fetal calf serum and 0.13% sodium azide), fixed with 1% paraformaldehyde (PFA) and analysed on a BD FACSCanto II flow cytometer and analysed with FlowJo software v10.8.1 (Tree Star, Ashland, USA). BD FACSCanto II performance check is done once a week using BD FACSDiva™ CS&T Research Beads (655,050; BD Biosciences, UK), and internal control of compensation is performed every time samples were acquired, using BD™ CompBeads Anti-Mouse Ig, κ/Negative Control Compensation Particles Set (552,843; BD Biosciences, UK). Samples were processed individually based on the time of collection. Neutrophils were defined as FSC-A and CD66b+ in single-cell gate and total granulocytes. Monocytes based on FSC-A, CD66b− , CD16+ and CD14+ . Monocytes were subdivided into classical (CD14high CD16neg/low), intermediate (CD14high CD16high), and non-classical (CD14lowCD16high) subtypes. CD11b and TLR4 were used as markers of activation. A minimum of 30,000 events were collected, and the relative expression of antigens was expressed as Geometric Mean Channel Fluorescence (MFI). The gating strategy used is shown in Figure S1.
Lymphocyte subset phenotyping
100µL of whole blood were stained for 10 min at room temperature with monoclonal antibodies specific for CD3-Pacific Blue (4 µg/mL; HIT3a; BioLegend, USA), CD4-PE-Cy7 (4 µg/mL; SK3/Leu3a; BD, USA), CD8-PerCP (4 µg/mL; SK1; BioLegend, USA), CD19-APC (1 µg/mL; SJ25C1; BioLegend, USA), CD56-APC-Cy7 (2 µg/mL; 5.1H11; BioLegend, USA), Vδ1-FITC (3 µg/mL; TS8.2; Invitrogen, USA) and Vδ2-PE (1 µg/mL; B6; BioLegend, USA) T cell receptors (TCR). MAbs were diluted to pre-determined concentrations in PBA buffer (PBS containing 0.1% fetal calf serum and 0.13% sodium azide). After staining, cells were lysed in 1 mL FACS lysis buffer (BD Biosciences, Oxford, UK). Finally, the cells were washed with PBA buffer (PBS containing 0.1% fetal calf serum and 0.13% sodium azide), fixed with 1% paraformaldehyde (PFA) and analysed on a BD FACSCanto II flow cytometer and analysed with FlowJo software v10.8.1 (Tree Star, Ashland, USA). BD FACSCanto II performance check is done once a week using BD FACSDiva™ CS&T Research Beads (655,050; BD Biosciences, UK), and internal control of compensation is performed every time samples are acquired, using BD™ CompBeads Anti-Mouse Ig, κ/Negative Control Compensation Particles Set (552,843; BD Biosciences, UK). Samples were processed individually based on the time of collection. T, B and NK cell percentages were presented as their corresponding proportions in Lymphocytes only (100% of lymphocytes calculated based on the number of T cells+ , B cells+ and NK cells). NK cells were defined as CD3− CD56+ cells, and B cells were defined as CD3- CD19+ cells. T cells were defined as CD3+ cells. Within the CD3+, fours subpopulations were defined based on the expression of CD4 and CD8: CD4 T cells (CD4+ CD8−), CD8 T cells (CD4− CD8+), Double positive T cells (CD4+ CD8+) and Double negative T cells (CD4− CD8−). Vδ1 and Vδ2 T cells were defined as cells expressing CD3 and the Vδ1 or Vδ2 TCRs, respectively. The gating strategy used for enumeration of the different cell populations is shown in Figure S2.
Quantitative real-time polymerase chain reaction (qRT–PCR) and analysis
300µL of whole blood was incubated at 37 °C for 1 h in the presence or absence of LPS at a final concentration of 10 ng/ml. Phosphate-buffered saline (PBS) was used in unstimulated samples (defined as “vehicle”) at an equal volume to LPS. After incubation, 1.3 mL of RNAlater™ Solutions (Thermo Fisher Scientific, Waltham, MA, USA) was added to the whole blood, mixed, and then stored in a – 80 °C freezer until analysis. Samples were processed at the same time for RNA extraction, amplification and RT-PR analysis. RNA was extracted using a Ribopure blood kit (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instructions. RNA purity and concentration were determined using the NanoDrop ND-100 spectrophotometer and analysed using ND-1000 version 3.1.2 software. qPCR was performed using TaqMan primer probes (NLRP3, ASC and IL-1β); the evaluation of gene expression was performed by TaqMan® RT–PCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous control for data normalization. The 7900HT fast RT–PCR system (Thermo Fisher Scientific) was used, and relative quantification (RQ) values were calculated using the 2 − ΔΔCt method54. Fold change was calculated using the baseline control cohort average ΔCT as the calibrator for this calculation.
Measurement of serum cytokine levels
Whole blood was incubated in the presence or absence of lipopolysaccharide (LPS) at a final concentration of 10 ng/ml (Sigma Life Science, Dublin, Ireland) at 37 °C for 1 h. The volume of blood used was variable, depending on the volume available per person, at least 200 µL used. Basel levels are defined as the concentration of cytokines without stimulation. Phosphate-buffered saline (PBS) was used in unstimulated samples (defined as “vehicle”) at an equal volume to LPS. After incubation, the serum was separated by centrifugation and stored at − 80 °C for later batch processing. Serum cytokine levels were analysed using a customized multiplex cytokine array by Meso Scale Discovery (Manchester, UK) and analysed on the Sector imager and validated (Meso Scale Discovery). The multiplex assay included mature interleukin-1 beta (IL-1β), interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-I receptor antagonist (IL-Ira), tumour necrosis factor-alpha (TNF-α), tumour necrosis factor -beta (TNF- β), interferon-gamma (IFN-Υ), vascular endothelial growth factor (VEGF), erythropoietin (EPO) and granulocyte–macrophage/colony-stimulating factor (GMCSF). This method employs a 96-well sandwich immunoassay which can quantify up to 12 analytes in 25 µl samples. The plate was then analysed on the SECTOR Imager and validated (Meso Scale Discovery, Rockville, MD, USA; www.mesoscale.com). Sensitivities were < 1 pg/ml for many assays. All assays used the same diluents, diluting linearly from one to fourfold. Non-specific binding between assays was typically < 0.1%. The assay reproducibility was calculated using the intra-variation of the standard curves and was shown to be within an acceptable range. The lower limit of detection for all analytes were as follows: EPO, GM-CSF, IFN-γ, IL-10, IL-18, IL-1RA, IL-1α, IL-1β, IL-2, IL-6, IL-8, TNF-α, TNF-β, VEGF, 0.89, 0.11, 5.17, 0.17, 1.15, 1.93, 0.39, 0.07, 0.71, 0.22, 0.11, 0.55, 0.23, 8.7 pg/ml respectively. Where the sample was below the lower limit of detection the value of the lower limit of detection for that assay was substituted55. All reported results units are in pg/ml.
Statistical methods
Statistical analysis was performed on GraphPad PRISM version 9 using unpaired (unmatched), non-parametric Kruskal–Wallis and Mann Whitney test to compare results between two independent cohorts and both conditions, ‘Veh’ and ‘LPS-treated’. The Kolmogorov–Smirnov test was used to verify normality. Significance was defined as p < 0.05. Results shown are expressed as median and interquartile range (IQR). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. p-value is not present means Not significant (ns) p > 0.05.
Data availability
Raw data was generated at The Children´s Research Laboratory, Discipline of Paediatric Medicine, Trinity College Dublin, the University of Dublin. Derived data supporting the findings of this study are available from the corresponding author (EJM) on request.
References
CDC, C. f. D. C. a. P. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19), <https://emergency.cdc.gov/han/2020/han00432.asp> (2020).
WHO, W. H. O. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19., <https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19> (2020).
ECDC, E. C. f. D. P. a. C. Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children., <https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment> (2020).
Feldstein, L. et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 383, 334–346. https://doi.org/10.1056/NEJMoa2021680 (2020).
Sharma, C. et al. Multisystem inflammatory syndrome in children and Kawasaki disease: A critical comparison. Nat. Rev. Rheumatol. 17, 731–748. https://doi.org/10.1038/s41584-021-00709-9 (2021).
Gruber, C. N. et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell 183, 982-995.e914. https://doi.org/10.1016/j.cell.2020.09.034 (2020).
García-Salido, A. et al. Severe manifestations of SARS-CoV-2 in children and adolescents: From COVID-19 pneumonia to multisystem inflammatory syndrome: A multicentre study in pediatric intensive care units in Spain. Crit. Care 24, 666. https://doi.org/10.1186/s13054-020-03332-4 (2020).
Consiglio, C. R. et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 183, 968-981.e967. https://doi.org/10.1016/j.cell.2020.09.016 (2020).
Hoste, L., Van Paemel, R. & Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 180, 2019–2034. https://doi.org/10.1007/s00431-021-03993-5 (2021).
Giacalone, M., Scheier, E. & Shavit, I. Multisystem inflammatory syndrome in children (MIS-C): A mini-review. Int. J. Emerg. Med. 14, 50. https://doi.org/10.1186/s12245-021-00373-6 (2021).
Nijman, R. G. et al. Pediatric inflammatory multisystem syndrome: Statement by the pediatric section of the european society for emergency medicine and european academy of pediatrics. Front. Pediatr. 8, 490. https://doi.org/10.3389/fped.2020.00490 (2020).
Molloy, E. The Doctor’s Dilemma: Lessons from GB Shaw in a modern pandemic COVID-19. Pediatr. Res. 89, 701–703. https://doi.org/10.1038/s41390-020-0927-1 (2021).
Bari, A., Ch, A., Bano, I. & Saqlain, N. Is leukopenia and lymphopenia a characteristic feature of COVID-19 in children?. Pak. J. Med. Sci. 37, 869–873. https://doi.org/10.12669/pjms.37.3.3848 (2021).
Syrimi, E. et al. The immune landscape of SARS-CoV-2-associated multisystem inflammatory syndrome in children (MIS-C) from acute disease to recovery. iScience 24, 103215. https://doi.org/10.1016/j.isci.2021.103215 (2021).
Feldstein, L. R. et al. Characteristics and outcomes of us children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. Jama 325, 1074–1087. https://doi.org/10.1001/jama.2021.2091 (2021).
Zhao, Y., Yin, L., Patel, J., Tang, L. & Huang, Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: A meta-analysis. J. Med. Virol. 93, 4358–4369. https://doi.org/10.1002/jmv.26951 (2021).
Ravichandran, S. et al. SARS-CoV-2 immune repertoire in MIS-C and pediatric COVID-19. Nat. Immunol. 22, 1452–1464. https://doi.org/10.1038/s41590-021-01051-8 (2021).
Yoshida, Y. et al. Enhanced formation of neutrophil extracellular traps in Kawasaki disease. Pediatr. Res. 87, 998–1004. https://doi.org/10.1038/s41390-019-0710-3 (2020).
Muto, T. et al. White blood cell and neutrophil counts and response to intravenous immunoglobulin in Kawasaki disease. Glob. Pediat. Health 6, 2333794X19884826. https://doi.org/10.1177/2333794X19884826 (2019).
Pouletty, M. et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): A multicentre cohort. Ann. Rheum. Dis. 79, 999–1006. https://doi.org/10.1136/annrheumdis-2020-217960 (2020).
McKenna, E. et al. Neutrophils in COVID-19: Not innocent bystanders. Front. Immunol. 13, 864387. https://doi.org/10.3389/fimmu.2022.864387 (2022).
Seery, V. et al. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. EBioMedicine 67, 103357. https://doi.org/10.1016/j.ebiom.2021.103357 (2021).
García-Salido, A. et al. PIMS-TS immunophenotype: description and comparison with healthy children, Kawasaki disease and severe viral and bacterial infections. Infect. Dis. (Lond) 54, 687–691. https://doi.org/10.1080/23744235.2022.2059561 (2022).
Heidari, B. et al. Expression of CD11b as an adhesion molecule on neutrophils in children with Kawasaki disease. Iran J. Allergy Asthma Immunol. 13, 265–270 (2014).
Kobayashi, T. et al. Increased CD11b expression on polymorphonuclear leucocytes and cytokine profiles in patients with Kawasaki disease. Clin. Exp. Immunol. 148, 112–118. https://doi.org/10.1111/j.1365-2249.2007.03326.x (2007).
Boribong, B. et al. Neutrophil profiles of pediatric COVID-19 and multisystem inflammatory syndrome in children. bioRxiv https://doi.org/10.1101/2021.12.18.473308 (2021).
Porritt, R. A. et al. The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J. Clin. Investig. 131, e151520. https://doi.org/10.1172/jci151520 (2021).
Carter, M. et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 26, 1701–1707. https://doi.org/10.1038/s41591-020-1054-6 (2020).
Ramaswamy, A. et al. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity 54, 1083–1095. https://doi.org/10.1016/j.immuni.2021.04.003 (2021).
Schimke, L. F. et al. Severe COVID-19 shares a common neutrophil activation signature with other acute inflammatory states. Cells 11, 847. https://doi.org/10.3390/cells11050847 (2022).
Karawajczyk, M. et al. High expression of neutrophil and monocyte CD64 with simultaneous lack of upregulation of adhesion receptors CD11b, CD162, CD15, CD65 on neutrophils in severe COVID-19. Ther. Adv. Infect. Dis. 8, 20499361211034064. https://doi.org/10.1177/20499361211034065 (2021).
Kumar, N. P. et al. Systemic inflammation and microbial translocation are characteristic features of SARS-CoV-2-related multisystem inflammatory syndrome in children. Open Forum Infect. Dis. 8, ofab279. https://doi.org/10.1093/ofid/ofab279 (2021).
Teixeira, P. C. et al. Increased LPS levels coexist with systemic inflammation and result in monocyte activation in severe COVID-19 patients. Int. Immunopharmacol. 100, 108125. https://doi.org/10.1016/j.intimp.2021.108125 (2021).
Oliva, A. et al. Low-grade endotoxemia and thrombosis in COVID-19. Clin. Transl. Gastroenterol. 12, e00348. https://doi.org/10.14309/ctg.0000000000000348 (2021).
Takeshita, S. et al. The role of bacterial lipopolysaccharide-bound neutrophils in the pathogenesis of Kawasaki disease. J. Infect. Dis. 179, 508–512. https://doi.org/10.1086/314600 (1999).
Aboudounya, M. M. & Heads, R. J. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediators Inflamm. 2021, 8874339. https://doi.org/10.1155/2021/8874339 (2021).
Khanmohammadi, S. & Rezaei, N. Role of Toll-like receptors in the pathogenesis of COVID-19. J. Med. Virol. 93, 2735–2739. https://doi.org/10.1002/jmv.26826 (2021).
Sohn, K. M. et al. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J. Korean Med. Sci. 35, e343. https://doi.org/10.3346/jkms.2020.35.e343 (2020).
Root-Bernstein, R. Innate receptor activation patterns involving TLR and NLR synergisms in COVID-19, ALI/ARDS and sepsis cytokine storms: A review and model making novel predictions and therapeutic suggestions. Int. J. Mol. Sci. 22, 2108. https://doi.org/10.3390/ijms22042108 (2021).
Lee, P. Y. et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J. Clin. Investig. 130, 5942–5950. https://doi.org/10.1172/jci141113 (2020).
Vella, L. A. et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci. Immunol. 6, eabf7570. https://doi.org/10.1126/sciimmunol.abf7570 (2021).
Beckmann, N. D. et al. Downregulation of exhausted cytotoxic T cells in gene expression networks of multisystem inflammatory syndrome in children. Nat. Commun. 12, 4854. https://doi.org/10.1038/s41467-021-24981-1 (2021).
Moreews, M. et al. Polyclonal expansion of TCR Vbeta 21.3(+) CD4(+) and CD8(+) T cells is a hallmark of multisystem inflammatory syndrome in children. Sci. Immunol. 6, eabh1516. https://doi.org/10.1126/sciimmunol.abh1516 (2021).
Wang, W. T. et al. Inflammasome activation in children with Kawasaki disease and multisystem inflammatory syndrome. Arterioscler. Thromb. Vasc. Biol. 41, 2509–2511. https://doi.org/10.1161/atvbaha.121.316210 (2021).
Peart Akindele, N. et al. Distinct cytokine and chemokine dysregulation in hospitalized children with acute coronavirus disease 2019 and multisystem inflammatory syndrome with similar levels of nasopharyngeal severe acute respiratory syndrome coronavirus 2 shedding. J. Infect. Dis. 224, 606–615. https://doi.org/10.1093/infdis/jiab285 (2021).
DeBiasi, R. L. et al. Multisystem inflammatory syndrome of children: Subphenotypes, risk factors, biomarkers, cytokine profiles, and viral sequencing. J. Pediatr. 237, 125–135. https://doi.org/10.1016/j.jpeds.2021.06.002 (2021).
Diorio, C. et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Investig. 130, 5967–5975. https://doi.org/10.1172/jci140970 (2020).
Caldarale, F. et al. Plasmacytoid dendritic cells depletion and elevation of IFN-γ dependent chemokines CXCL9 and CXCL10 in children with multisystem inflammatory syndrome. Front. Immunol. 12, 654587. https://doi.org/10.3389/fimmu.2021.654587 (2021).
Diorio, C. et al. Proteomic profiling of MIS-C patients indicates heterogeneity relating to interferon gamma dysregulation and vascular endothelial dysfunction. Nat. Commun. 12, 7222. https://doi.org/10.1038/s41467-021-27544-6 (2021).
Eberhard, B. A., Andersson, U., Laxer, R. M., Rose, V. & Silverman, E. D. Evaluation of the cytokine response in Kawasaki disease. Pediatr. Infect. Dis. J. 14, 199–203. https://doi.org/10.1097/00006454-199503000-00006 (1995).
Matsubara, T. et al. Decrease in the concentrations of transforming growth factor-beta 1 in the sera of patients with Kawasaki disease. Scand. J. Rheumatol. 26, 314–317. https://doi.org/10.3109/03009749709105322 (1997).
Sahebnasagh, A. et al. A perspective on erythropoietin as a potential adjuvant therapy for acute lung injury/acute respiratory distress syndrome in patients with COVID-19. Arch. Med. Res. 51, 631–635. https://doi.org/10.1016/j.arcmed.2020.08.002 (2020).
Ehrenreich, H. et al. Erythropoietin as candidate for supportive treatment of severe COVID-19. Mol. Med. 26, 58. https://doi.org/10.1186/s10020-020-00186-y (2020).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Shoari, N. & Dubé, J. S. Toward improved analysis of concentration data: Embracing nondetects. Environ. Toxicol. Chem. 37, 643–656. https://doi.org/10.1002/etc.4046 (2018).
Author information
Authors and Affiliations
Contributions
J.I.C. conducted experiment(s), data analysis and manuscript preparation; L.R. patient recruitment and epidemiological data collection; L.K. conducted experiment(s) and data analysis; J.A. conducted experiment(s) and data analysis for control group; A.M. ethics application and start of the project; D.H. conducted experiment(s) and data analysis for control group; E.R. conducted experiment(s) and data analysis for control group; J.J., B.F., C.O.M., S.G., P.G., T.R.L., K.B., clinical collaborators; E.J.M. was involved in study design & concept, ethics application, supervision, data collection review, analysis and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Isaza-Correa, J., Ryan, L., Kelly, L. et al. Innate immune dysregulation in multisystem inflammatory syndrome in children (MIS-C). Sci Rep 13, 16463 (2023). https://doi.org/10.1038/s41598-023-43390-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43390-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.