Abstract
To present rectal endoscopic findings and toxicity after definitive moderately hypofractionated, intensity-modulated radiotherapy (IMRT) for prostate cancer. We retrospectively reviewed patients who underwent IMRT for prostate cancer and underwent post-radiotherapy endoscopies between 2008 and 2018. Endoscopic findings were reviewed and graded using Vienna Rectoscopy Score (VRS). We have analyzed the association between endoscopic findings and rectal bleeding, and investigated risk factors for rectal bleeding. Total 162 patients met the inclusion criteria of this study. There was a trend of VRS worsening during the initial 3 years after radiotherapy followed by recovery. Rectal bleeding was highest at 1 year after radiotherapy and improved thereafter. The 5-year cumulative incidence of grade ≥ 2 rectal bleeding was 14.8%. In the multivariable Cox regression analysis, cardiovascular disease (hazard ratio [HR] 2.732, P = 0.037), rectal wall V65 (HR 1.158, P = 0.027), and VRS ≥ 3 in first post-radiotherapy endoscopy (HR 2.573, P = 0.031) were significant risk factors for rectal bleeding. After IMRT for prostate cancer, VRS and rectal bleeding worsened over 1–3 years after radiotherapy and recovered. Cardiovascular disease, rectal wall V65, and VRS ≥ 3 in first post-radiotherapy endoscopy were significant risk factors for rectal bleeding.
Similar content being viewed by others
Introduction
Prostate cancer was the second most common cancer and the fifth leading cause of cancer-related deaths in men in 20201. Radiotherapy is a standard treatment option for localized and locally advanced prostate cancer2,3,4. As prostate cancer has low α/β ratio, larger dose per fraction for prostate cancer is potentially associated with increased therapeutic effects5. Multiple randomized trials comparing hypofractionation with conventional fractionation for prostate cancer have proven the non-inferiority of hypofractionation in clinical outcomes6,7,8.
Rectal bleeding is a major late side effect of radiotherapy for prostate cancer9. Most incidences of bleeding are temporary and self-limiting. However, some patients experience repeated episodes of bleeding that require interventions such as sucralfate enemas, endoscopic argon plasma coagulation (APC), blood transfusion, or even hospitalization10. Endoscopic examination provides objective findings of post-radiotherapy changes in the rectum11. Several studies have reported endoscopic changes after radiotherapy11,12,13. However, most studies have focused on the early changes or endoscopic findings at the time of toxic events. Long-term endoscopic changes, especially after hypofractionated radiotherapy, have not been reported.
Therefore, in this study, we evaluated the long-term endoscopic findings after definitive moderately hypofractionated intensity-modulated radiotherapy (IMRT) for prostate cancer to better understand changes in the rectal mucosa after radiotherapy. We also evaluated the correlation between endoscopic findings and rectal bleeding, and investigated the risk factors of rectal bleeding.
Materials and methods
Study design
With approval from the Institutional Review Board of the Samsung Medical Center (SMC IRB 2022-06-079), we retrospectively reviewed the medical records of patients who underwent definitive radiotherapy for localized or locoregional prostate cancer between January 2008 and December 2018 at Samsung Medical Center. Patients who underwent moderately hypofractionated IMRT with 70 Gy in 28 fractions and at least two post-radiotherapy endoscopies were included in this study. Patients who underwent single post-radiotherapy endoscopic evaluation was excluded from current study, as single endoscopic assessment may not adequately reflect rectal mucosal changes over time. Patients who received dose fractionation regimens other than 70 Gy in 28 fractions or those who lacked post-radiotherapy endoscopies were excluded.
Radiotherapy
All patients underwent planning computed tomography (CT) simulations in the supine position with a 2.5 mm slice thickness. Rectal catheter with inflatable balloon was used for prostate immobilization. The balloon was inflated with 60 cc of air for simulation and daily treatment. Planning magnetic resonance imaging (MRI) was performed immediately after planning CT for target contouring assistance with the same setup position and immobilization devices. The CT and MRI images were automatically matched, and the images were checked in all directions and modified, if necessary, by a primary physician.
Target volume delineation and dose prescription principles were as follows. Patients with localized prostate cancer were stratified into one of three risk groups by the D’Amico risk classification14. For patients with low- and intermediate-risk prostate cancer, the prostate gland was contoured as the clinical target volume (CTV)-prostate. For patients with localized high-risk and locoregional prostate cancer, two CTVs were contoured. The prostate gland and the involved seminal vesicle were contoured as the CTV-prostate. The elective pelvic lymph node volume up to the sacral promontory was contoured as CTV-pelvis, as recommended by Lawton et al.15. If a metastatic lymph node was present, it was contoured as the GTV-lymph node (LN). The planning target volume (PTV) expansion from CTVs was a 5 mm expansion from the CTV-prostate and the GTV-LN for the PTV-prostate and the PTV-LN, and a 7 mm expansion from the CTV-pelvis for the PTV-pelvis. With consideration of dose constraints of normal organs, prescribed doses were as follows: (1) PTV-prostate: 70 Gy in 28 fractions, 2.5 Gy per fraction; (2) PTV-LN: 61.6 Gy to 70 Gy in 28 fractions, 2.2 to 2.5 Gy per fraction; (3) PTV-pelvis: 50.4 Gy in 28 fractions, 1.8 Gy per fraction. The rectal wall was contoured above and below 1 cm from the PTV-prostate. The dose constraints applied to the rectal wall were Dmax < 74 Gy, V70 ≤ 7%, V65 ≤ 10%, V60 ≤ 15%, V50 ≤ 20%, and V25 ≤ 50%.
Image-guided radiotherapy was performed with daily pretreatment cone-beam computed tomography to reduce setup uncertainties. Rectal balloon was always inserted at the same depth with same inflated volume every treatment. The balloon was used as a surrogate for the location of target volumes. Fiducial markers or rectal spacers were not used for the patients in the study period.
Assessments
Baseline clinical and treatment characteristics including age, Eastern Cooperative Oncology Group Performance Status, comorbidities, use of antithrombotic medication, initial prostate-specific antigen (PSA) level, stage of prostate cancer, Gleason score, radiotherapy volume, treatment modality, use of androgen deprivation therapy, and dose-volume histogram (DVH) parameters of the rectal wall were collected.
Patients were recommended to visit the follow-up clinic every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. During follow-ups, the patients were requested to undergo rectosigmoidoscopy or colonoscopy annually for 5 years after treatment. Endoscopic findings of the rectal mucosa were reviewed and graded by an experienced gastroenterology endoscopy specialist (J.E.K.) using the Vienna Rectoscopy Score (VRS) suggested by Wachter et al.16. According to the VRS, five different endoscopic components of mucosal damage (mucosal congestion, telangiectasia, ulceration, stricture, and necrosis) are graded from grade 0 to 3. Based on the grades of the individual parameters, VRS is derived as a six-scaled score, from 0 to 5. Examples of the VRS are shown in Fig. 1. Symptomatic rectal bleeding was graded according to the Common Terminology Criteria for Adverse Events, version 5.0: grade 1, mild symptoms with no intervention is indicated; grade 2, moderate symptoms with interventions such as APC is indicated; grade 3, transfusion, invasive intervention, or hospitalization is indicated; grade 4, life-threatening incidences; grade 5, death. Changes in endoscopic findings and severity of rectal bleeding in individual patients were collected and analyzed on the basis of the time from the end of radiotherapy. Endoscopy performed in the first year after radiotherapy was regarded as the first post-radiotherapy endoscopy, and endoscopic findings were used in the investigation of the risk factors for rectal bleeding.
Statistical analyses
Correlation analysis with the Chi-square test and the Fisher’s exact test was performed to determine the association between endoscopic findings and rectal bleeding each year. The cumulative incidence of rectal bleeding was calculated and plotted using the Kaplan–Meier method. Cox regression analysis and binary logistic regression analysis were performed to determine factors associated with grade ≥ 2 rectal bleeding and worst VRS ≥ 3. Factors with P-value < 0.10 in the univariable analysis were considered as potential candidates for multivariable analysis. Out of potential candidates, variables with a variance inflation factor < 10.0 entered the multivariable analysis. The final multivariable model was determined using a backward variable selection method. Statistical significance was set at P-value < 0.05. Statistical analyses were performed using the IBM SPSS Statistics software (version 27.0; IBM, Inc., Armonk, NY, USA).
Ethics approval
The institutional review board of the Samsung Medical Center approved the study. Informed consent was waived by the review board due to the retrospective nature of the study. All procedures performed involving human participants were in accordance with the Declaration of Helsinki as revised in 2013.
Results
Patient and treatment characteristics
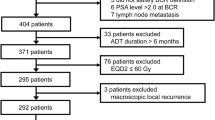
A total of 162 patients met the inclusion criteria of this study. The flowchart of patient selection process is illustrated in Supplementary Fig. 1. The median follow-up time was 4.8 years (range, 3.1−10.9 years). The median age of the patients was 73 years (interquartile range, 67−77 years) and the mean initial PSA was 26.6 ng/mL. A total of 53 patients (32.7%) were under antithrombotic medication at the time of radiotherapy with antiplatelet drugs (48 patients, 29.6%), anticoagulant drugs (3 patients, 1.9%), or both (2 patients, 1.2%). The clinical T stage was T1 in 5 (3.1%), T2 in 64 (39.5%), T3 in 85 (52.5%), and T4 in 8 patients (4.9%). The clinical N stage was N0 in 135 (83.3%) and N1 in 27 patients (16.7%). The risk groups of patients were low-risk in 13 (8.0%), intermediate-risk in 47 (29.0%), high-risk in 75 (46.3%), and locally advanced disease in 27 patients (16.7%). The radiotherapy target volume was prostate gland ± seminal vesicle in 80 patients (49.4%) and whole pelvis in 82 patients (50.6%). Androgen deprivation therapy was administered to 84 patients (51.8%). Baseline patient and treatment characteristics are summarized in Table 1.
Endoscopic findings
Annual post-radiotherapy endoscopy was performed a median of four-times per patient (range, 2−5). The following number of patients underwent endoscopy each year: 149 in the first year (92.0%), 153 in the second year (94.4%), 129 in the third year (79.6%), 87 in the fourth year (53.7%), and 53 in the fifth year (32.7%).
There was a trend of worsening VRS during the initial 3 years after radiotherapy, which recovered afterwards. VRS ≥ 2 was observed in 60.4%, 86.2%, 80.6%, 70.1%, and 71.7% of the patients annually. VRS ≥ 3 was observed in 24.8%, 38.2%, 40.3%, 25.3%, and 26.4% of the patients annually. Out of the five individual parameters of mucosal damage, telangiectasia was the most prominent parameter. Grade ≥ 2 telangiectasia was observed in 58.4%, 86.2%, 80.6%, 67.8%, and 71.7% of the patients annually. Grade 3 telangiectasia was observed in 18.8%, 37.5%, 40.3%, 23.0%, and 26.4% of the patients annually. Strictures or necrosis were not observed. The details of the changes in each parameter are summarized in Fig. 2 and Supplementary Table 1.
Rectal bleeding
Rectal bleeding was observed in 95 patients (58.6%) after radiotherapy. Grade ≥ 2 rectal bleeding was observed in 25 patients (15.4%), and grade 3 rectal bleeding was observed in 6 patients (3.7%). The 5-year cumulative incidences of rectal bleeding were 58.4%, 14.8%, and 2.7% for Grade ≥ 1, Grade ≥ 2, and Grade 3. The proportion of patients with rectal bleeding was highest at 1−2 years after radiotherapy (grade 1 in 45 patients, grade 2 in 14 patients, and grade 3 in 2 patients), and the bleeding decreased afterwards. Although grade 2 patients required APC and grade 3 patients required transfusion or hospitalization, the events were well-managed without specific complications. The cumulative incidence of rectal bleeding and changes in rectal bleeding over time are summarized in Fig. 3.
Correlation between endoscopic findings and rectal bleeding and risk factors for rectal bleeding
Endoscopic findings and rectal bleeding of individual patients were collected and analyzed on annual basis after radiotherapy to determine correlations between these factors. In the correlation analysis, there were statistically significant correlations between factors throughout the follow-up period (from 0−1 year to 3−4 years after radiotherapy, P < 0.001, and 4−5 years after radiotherapy, P = 0.049). The results are summarized in Table 2.
Cox regression analysis was performed to identify risk factors associated with grade ≥ 2 rectal bleeding. In the univariable analysis, cardiovascular disease (hazard ratio [HR] 1.185, 95% confidence interval [CI] 1.111−6.377, P = 0.028) and rectal wall V65 (HR 1.171, 95% CI 1.032−1.329, P = 0.014) were significant risk factors for rectal bleeding. Multivariable analysis revealed that cardiovascular disease (HR 2.732, 95% CI 1.060−7.038, P = 0.037), rectal wall V65 (HR 1.158, 95% CI 1.017−1.318, P = 0.027), and VRS ≥ 3 in the first post-radiotherapy endoscopy (HR 2.573, 95% CI 1.091−6.069, P = 0.031) were significant risk factors for rectal bleeding. There was no clear cut-off value for rectal wall DVH parameters. The results are summarized in Table 3.
Binary logistic regression analysis was performed to find risk factors of worse endoscopic finding, VRS ≥ 3. Multiple DVH parameters of rectal wall were significantly associated with worse endoscopic findings in the univariable analysis: rectal wall V25 (OR 1.036, 95% CI 1.009–1.064, P = 0.009), rectal wall V50 (OR 1.106, 95% CI 1.036–1.182, P = 0.003), rectal wall V60 (OR 1.132, 95% CI 1.044–1.228, P = 0.003), rectal wall V65 (OR 1.154, 95% CI 1.052–1.266, P = 0.002), rectal wall V70 (OR 1.155, 95% CI 1.005–1.328, P = 0.042), respectively. Multivariable analysis showed that rectal wall V25 (OR 1.029, 95% CI 1.001–1.058, P = 0.039), rectal wall V65 (OR 1.134, 95% CI 1.030–1.248, P = 0.011) were significantly associated with worse endoscopic findings. The results of binary logistic regression analysis are summarized in Table 4.
Rectal bleeding and endoscopic findings of patients with initial VRS ≥ 3 over time
Figure 4 shows the cumulative incidence of rectal bleeding and changes of VRS for 37 patients who presented VRS ≥ 3 in the first post-radiotherapy endoscopy (VRS 3 in 33, and VRS 4 in 4 patients). Compared to the study cohort, the group of patients showed significantly higher proportion of patients experiencing rectal bleeding (5-year cumulative incidence, 75.7%, 24.3%, and 5.4% for Grade ≥ 1, Grade ≥ 2, and Grade 3, respectively). Most severe toxic event observed was grade 3 in 2 patients (5.4%) who required blood transfusion due to low hemoglobin level from rectal bleeding. Grade 2 toxic events were observed in 7 patients (18.9%) who underwent APC. Other patients reported self-limited rectal bleeding not requiring active management (19 patients, 51.4%) or did not report any rectal bleeding (9 patients, 24.3%). Also, it is to note that the endoscopic findings improved over time without any significant deterioration.
Discussion
In this study, we reviewed the endoscopic findings, rectal bleeding, and risk factors for rectal bleeding after moderately hypofractionated IMRT for prostate cancer. Endoscopic findings showed that rectal mucosal damage was prominent and worsened until 2−3 years after radiotherapy and recovered afterwards. The proportion of patients with rectal bleeding increased until 1−2 years after radiotherapy and decreased thereafter. We revealed that cardiovascular disease, rectal wall V65, and VRS ≥ 3 in the first post-radiotherapy endoscopy were risk factors of post-radiotherapy rectal bleeding.
The significance and usefulness of endoscopic rectal mucosal changes after radiotherapy have been studied in various clinical settings. Goldner et al. evaluated endoscopic changes after radiotherapy for prostate cancer11. After 70−74 Gy with 2 Gy per fraction, VRS ≥ 2 was observed in 46% and 33% of patients at 12 and 24 months, respectively. There was also a significant correlation between the maximal VRS and rectal side effects. Ohtani et al. reported long-term endoscopic changes and rectal bleeding after brachytherapy for prostate cancer13. The incidence of rectal bleeding was 24%, and there was a statistically significant correlation between VRS and rectal bleeding. Ippolito et al. performed proctoscopy 1 year after definitive or adjuvant radiotherapy for prostate cancer and investigated the role of early mucosal changes in predicting late rectal toxicity12. The 3-year cumulative incidence of grade ≥ 2 rectal toxicity was 24%, with a significant correlation with initial telangiectasia grade and VRS. They concluded that early proctoscopy could predict late rectal bleeding. There are also studies investigating the role of endoscopy after radiotherapy for cervical cancer and rectal cancer17,18.
In this study, VRS ≥ 3 was observed in 100 patients (61.7%) during follow-up, and 14 patients (26.4%) had a VRS 3 until 5 years after radiotherapy.
Compared to the above-mentioned studies, the mucosal damage of patients in our study seems to be relatively severe. There may be several reasons for this difference in mucosal damage. First, there were differences in the patient population. While our study is based on patients who underwent moderately hypofractionated IMRT for prostate cancer with 70 Gy in 28 fractions, previous studies are based on patients treated with conventional fractionated radiotherapy with 3 dimensional conformal radiotherapy (3D-CRT)11, both 3D-CRT and IMRT12, or brachytherapy13. Second, the number of patients with endoscopic evaluation decreased over time, with 149 patients (92.0%) and 152 patients (94.4%) in the first and second year, respectively, and 53 patients (32.7%) in the fifth year after radiotherapy. Also, out of 379 patients who underwent moderately hypofractionated IMRT for prostate cancer, 217 patients refused endoscopic evaluations. As endoscopic evaluation was recommended but not mandatory, there was a tendency of patients without specific complications to refuse further endoscopic evaluation. We speculate that mucosal damage in patients who did not underwent endoscopic evaluation would have improved significantly. Cumulative incidence of rectal bleeding supports the speculation. We have compared cumulative incidence of rectal bleeding between two cohorts: the study cohort (162 patients), and a cohort that includes all patients before the exclusion of those who did not undergo post-radiotherapy endoscopies (379 patients) (Supplementary Fig. 1). The cumulative incidence of rectal bleeding was higher for the study cohort (5-year incidence, Grade ≥ 1, 58.4% vs. 42.2%; Grade ≥ 2, 14.8% vs. 13.7%; Grade 3, 2.7% vs. 2.4%) (Supplementary Fig. 2). This suggests that a significant number of patients without any toxic events were excluded from current study due to the lack of endoscopic findings. This may have affected the high toxic events observed in the study cohort. However, we were unable to present clear evidence concerning this speculation because of lack of data.
Randomized trials and meta-analyses of hypofractionated radiotherapy for prostate cancer have reported outcomes similar to those of conventionally fractionated radiotherapy in terms of tumor control6,19,20,21,22. However, toxicity varies greatly between studies, with the reported incidence of grade ≥ 2 gastrointestinal (GI) toxicity ranging from 4 to 30%22,23,24,25. Direct comparison of toxic events with previously published studies is challenging, as the toxicity grading system, radiotherapy dose prescription, and patient population differ between studies. However, the incidence of rectal bleeding in this study seems to be similar to that reported in other studies (grade ≥ 2, 15.4%; grade 3, 3.7%; no grade 4 toxic events).
Mucosal damage and rectal bleeding after radiotherapy dynamically change over time. In this study, the proportion of patients with high VRS showed an increasing trend over the first 2−3 years after radiotherapy and decreased thereafter (Fig. 2a). The proportion of patients with rectal bleeding increased over the first 1−2 years after radiotherapy and decreased thereafter (Fig. 2b). Abdalla et al. reported that the peak incidence of GI toxicity was observed 2−3 years after radiotherapy for prostate cancer with 60−74 Gy26. Groen et al. reported that grade ≥ 2 GI toxicity increased in the first two years in patients of the FLAME trial27,28. The time of peak incidence differs between studies; however, it is to note that GI toxic events can occur for a prolonged period after the end of treatment.
Several studies have reported that anticoagulation therapy results in increased rectal bleeding after radiotherapy for prostate cancer. Choe et al. reported that patients taking warfarin or clopidogrel had a significantly increased risk of bleeding after radiotherapy for prostate cancer29. Takeda et al. reported that using anticoagulants or antiaggregants resulted in increased late rectal toxicity after radiotherapy for prostate cancer30. The findings were identical in a study by Kim et al., who reported an increased risk of grade ≥ 3 rectal bleeding for patients taking anticoagulants4. Unlike previous studies, as shown in Table 3, the use of antithrombotic medication was not a significant risk factor for rectal bleeding in the current study (HR 1.683, P = 0.196). However, cardiovascular disease was significantly associated with increased rectal bleeding (HR 2.732, P = 0.037). This might result from the differences in the types of antithrombotic medications administered. While most patients receiving antithrombotic medication were taking aspirin for the prevention of cardiovascular disease, patients with cardiovascular disease were taking various antithrombotic drugs in various combinations (from only aspirin to a combination of clopidogrel and new oral anticoagulants). The simple use of antithrombotic medications for the prevention of cardiovascular disease may not have to be considered as a risk factor for rectal bleeding after radiotherapy for prostate cancer; however, this will need further validation.
There are several strategies to reduce rectal toxicity in radiotherapy for prostate cancer. First, using rectal balloon results in prostate immobilization allowing smaller PTV margin and reduces rectal volume receiving high-dose radiation31. Second, fiducial marker can be placed for improving the accuracy of prostate targeting and reducing rectal toxicity32. Third, rectal spacer, which injects absorbable polyethylene glycol hydrogel spacer into the perirectal space, can physically move anterior rectal wall away from prostate, resulting in reduced rectal radiation dose and decreased radiotherapy related toxicities33. Fourth, if gastrointestinal toxic event occurs after radiotherapy, hyperbaric oxygen therapy might have potential to reduce the side effects34,35.
This study reports the long-term endoscopic findings after moderately hypofractionated IMRT for prostate cancer. We believe that this study is the largest study with long-term follow-ups with serial endoscopies. This study provides useful clinical information on actual rectal mucosal changes after radiotherapy for prostate cancer. However, this study had several limitations. First, the results could have been biased owing to the retrospective nature of the study and patients lost during follow-up. Median follow-up time of 4.8 years with shortest duration of 3.1 years seems to be sufficient for assessment of radiation-related late toxicity. We believe that further worsening of rectal toxicity after 3 years is unlikely. However, definitive confirmation is not possible due to the lack of data. Second, endoscopic findings were scored according to the interpretation of the images obtained during endoscopy. As the rectal mucosa is vulnerable to air inflation, which can cause bleeding, it is important to evaluate the mucosal status during the entrance of scope to avoid interference from the endoscopic procedure itself. However, it is difficult to identify if the image was taken during entrance or withdrawal of the scope, which could have led to over- or under-estimation of rectal mucosal findings. Third, comorbidities and medications of individual patients were assessed before treatment initiation, and changes during or after radiotherapy were not assessed. There may have been patients with new anticoagulant or antiplatelet medications, but those aspects were not considered in current study. Fourth, endoscopic evaluation of patients was recommended but not mandatory. The results could have been biased as quite a few patients were excluded from current study due to lack of endoscopic evaluation.
Conclusion
After moderately hypofractionated IMRT for prostate cancer, the VRS worsened during the initial 3 years after radiotherapy and recovered afterwards, with telangiectasia being the most prevalent endoscopic finding. There was a statistically significant correlation between the VRS and clinical rectal bleeding. Cardiovascular disease, rectal wall V65, and VRS ≥ 3 in the first post-radiotherapy endoscopy were significantly associated with rectal bleeding and thus could be considered as risk factors.
Data availability
The data that support the findings of this study are available upon reasonable requests to the corresponding author (W.P.; wonro.park@samsung.com).
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Kanesvaran, R. et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with prostate cancer. ESMO Open 7, 100518. https://doi.org/10.1016/j.esmoop.2022.100518 (2022).
Parker, C. et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 31, 1119–1134. https://doi.org/10.1016/j.annonc.2020.06.011 (2020).
Kim, T. G. et al. Patient-related risk factors for late rectal bleeding after hypofractionated radiotherapy for localized prostate cancer: A single-center retrospective study. Radiat. Oncol. 17, 30. https://doi.org/10.1186/s13014-022-01998-4 (2022).
Vogelius, I. R. & Bentzen, S. M. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: Bad news, good news, or no news?. Int. J. Radiat. Oncol. Biol. Phys. 85, 89–94. https://doi.org/10.1016/j.ijrobp.2012.03.004 (2013).
Widmark, A. et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 394, 385–395. https://doi.org/10.1016/S0140-6736(19)31131-6 (2019).
Hoffman, K. E. et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for Localized prostate cancer. J. Clin. Oncol. 36, 2943–2949. https://doi.org/10.1200/JCO.2018.77.9868 (2018).
Catton, C. N. et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J. Clin. Oncol. 35, 1884–1890. https://doi.org/10.1200/JCO.2016.71.7397 (2017).
Takemoto, S. et al. Treatment and prognosis of patients with late rectal bleeding after intensity-modulated radiation therapy for prostate cancer. Radiat. Oncol. 7, 87. https://doi.org/10.1186/1748-717X-7-87 (2012).
Sebastian, S., O’Connor, H., O’Morain, C. & Buckley, M. Argon plasma coagulation as first-line treatment for chronic radiation proctopathy. J. Gastroenterol. Hepatol. 19, 1169–1173. https://doi.org/10.1111/j.1440-1746.2004.03448.x (2004).
Goldner, G. et al. Proctitis after external-beam radiotherapy for prostate cancer classified by Vienna Rectoscopy Score and correlated with EORTC/RTOG score for late rectal toxicity: results of a prospective multicenter study of 166 patients. Int. J. Radiat. Oncol. Biol. Phys. 67, 78–83. https://doi.org/10.1016/j.ijrobp.2006.08.055 (2007).
Ippolito, E. et al. Early proctoscopy is a surrogate endpoint of late rectal toxicity in prostate cancer treated with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 83, e191-195. https://doi.org/10.1016/j.ijrobp.2011.12.046 (2012).
Ohtani, M. et al. Long-term endoscopic follow-up of patients with chronic radiation proctopathy after brachytherapy for prostate cancer. Diagn. Ther. Endosc. 2016, 1414090. https://doi.org/10.1155/2016/1414090 (2016).
D’Amico, A. V. et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280, 969–974. https://doi.org/10.1001/jama.280.11.969 (1998).
Lawton, C. A. et al. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 74, 383–387. https://doi.org/10.1016/j.ijrobp.2008.08.002 (2009).
Wachter, S. et al. Endoscopic scoring of late rectal mucosal damage after conformal radiotherapy for prostatic carcinoma. Radiother. Oncol. 54, 11–19. https://doi.org/10.1016/s0167-8140(99)00173-5 (2000).
Kim, T. G., Huh, S. J. & Park, W. Endoscopic findings of rectal mucosal damage after pelvic radiotherapy for cervical carcinoma: Correlation of rectal mucosal damage with radiation dose and clinical symptoms. Radiat. Oncol. J. 31, 81–87. https://doi.org/10.3857/roj.2013.31.2.81 (2013).
Lee, J. et al. The role of endoscopic evaluation for radiation proctitis in patients receiving intermediate-dose postoperative radiotherapy for rectal cancer. Jpn. J. Clin. Oncol. 48, 988–994. https://doi.org/10.1093/jjco/hyy126 (2018).
Royce, T. J. et al. Conventional versus hypofractionated radiation therapy for localized prostate cancer: A meta-analysis of randomized noninferiority trials. Eur. Urol. Focus 5, 577–584. https://doi.org/10.1016/j.euf.2017.10.011 (2019).
Datta, N. R., Stutz, E., Rogers, S. & Bodis, S. Conventional versus hypofractionated radiation therapy for localized or locally advanced prostate cancer: A systematic review and meta-analysis along with therapeutic implications. Int. J. Radiat. Oncol. Biol. Phys. 99, 573–589. https://doi.org/10.1016/j.ijrobp.2017.07.021 (2017).
Dearnaley, D. et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 17, 1047–1060. https://doi.org/10.1016/S1470-2045(16)30102-4 (2016).
Lee, W. R. et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J. Clin. Oncol. 34, 2325–2332. https://doi.org/10.1200/JCO.2016.67.0448 (2016).
Di Franco, R. et al. Rectal/urinary toxicity after hypofractionated vs. conventional radiotherapy in high risk prostate cancer: systematic review and meta analysis. Eur. Rev. Med. Pharmacol. Sci. 21, 3563–3575 (2017).
Di Franco, R. et al. Rectal/urinary toxicity after hypofractionated vs. conventional radiotherapy in low/intermediate risk localized prostate cancer: systematic review and meta analysis. Oncotarget 8, 17383–17395. https://doi.org/10.18632/oncotarget.14798 (2017).
Tamihardja, J. et al. Moderately hypofractionated radiotherapy for localized prostate cancer: Updated long-term outcome and toxicity analysis. Strahlenther Onkol. 197, 124–132. https://doi.org/10.1007/s00066-020-01678-w (2021).
Abdalla, I. et al. Evolution of toxicity after conformal radiotherapy for prostate cancer. Prostate Cancer Prostatic Dis. 5, 296–303. https://doi.org/10.1038/sj.pcan.4500608 (2002).
Kerkmeijer, L. G. W. et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: Results from the FLAME randomized phase III trial. J Clin Oncol 39, 787–796. https://doi.org/10.1200/JCO.20.02873 (2021).
Groen, V. H. et al. Anorectal dose-effect relations for late gastrointestinal toxicity following external beam radiotherapy for prostate cancer in the FLAME trial. Radiother. Oncol. 162, 98–104. https://doi.org/10.1016/j.radonc.2021.06.033 (2021).
Choe, K. S., Jani, A. B. & Liauw, S. L. External beam radiotherapy for prostate cancer patients on anticoagulation therapy: How significant is the bleeding toxicity?. Int. J. Radiat. Oncol. Biol. Phys. 76, 755–760. https://doi.org/10.1016/j.ijrobp.2009.02.026 (2010).
Takeda, K. et al. Clinical correlations between treatment with anticoagulants/antiaggregants and late rectal toxicity after radiotherapy for prostate cancer. Anticancer Res. 29, 1831–1834 (2009).
Teh, B. S. et al. The use of rectal balloon during the delivery of intensity modulated radiotherapy (IMRT) for prostate cancer: more than just a prostate gland immobilization device?. Cancer J. 8, 476–483. https://doi.org/10.1097/00130404-200211000-00012 (2002).
O’Neill, A. G., Jain, S., Hounsell, A. R. & O’Sullivan, J. M. Fiducial marker guided prostate radiotherapy: A review. Br. J. Radiol. 89, 20160296. https://doi.org/10.1259/bjr.20160296 (2016).
Armstrong, N. et al. SpaceOAR hydrogel spacer for reducing radiation toxicity during radiotherapy for prostate cancer. A systematic review. Urology 156, e74–e85. https://doi.org/10.1016/j.urology.2021.05.013 (2021).
Kashihara, T. et al. The use of hyperbaric oxygen to treat actinic rectal fistula after SpaceOAR use and radiotherapy for prostate cancer: A case report. BMC Urol. 20, 196. https://doi.org/10.1186/s12894-020-00767-3 (2020).
Yuan, J. H. et al. The effects of hyperbaric oxygen therapy on pelvic radiation induced gastrointestinal complications (rectal bleeding, diarrhea, and pain): A meta-analysis. Front. Oncol. 10, 390. https://doi.org/10.3389/fonc.2020.00390 (2020).
Author information
Authors and Affiliations
Contributions
Conceptualization, W.P.; Data curation, all authors; Formal analysis, B.K.B., J.E.K., and W.P.; Investigation, B.K.B., J.E.K., and W.P.; Methodology, B.K.B., J.E.K., and W.P.; Project administration, W.P.; Manuscript writing—original draft, B.K.B., J.E.K., and W.P.; Manuscript writing – review and editing, all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bae, B.K., Kim, J.E., Pyo, H. et al. Long-term findings of rectal endoscopy and rectal bleeding after moderately hypofractionated, intensity-modulated radiotherapy for prostate cancer. Sci Rep 13, 22099 (2023). https://doi.org/10.1038/s41598-023-43202-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43202-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.