Abstract
The relationship between radiation doses and clinical relapse in patients receiving salvage radiotherapy (SRT) for biochemical recurrence (BCR) after radical prostatectomy (RP) remains unclear. We identified 292 eligible patients treated with SRT between 2005 and 2018 at 15 institutions. Clinical relapse-free survival (cRFS) between the ≥ 66 Gy (n = 226) and < 66 Gy groups (n = 66) were compared using the Log-rank test, followed by univariate and multivariate analyses and a subgroup analysis. After a median follow-up of 73 months, 6-year biochemical relapse-free survival, cRFS, cancer-specific survival, and overall survival rates were 58, 92, 98, and 94%, respectively. Six-year cRFS rates in the ≥ 66 Gy and < 66 Gy groups were 94 and 87%, respectively (p = 0.022). The multivariate analysis revealed that Gleason score ≥ 8, seminal vesicle involvement, PSA at BCR after RP ≥ 0.5 ng/ml, and a dose < 66 Gy correlated with clinical relapse (p = 0.015, 0.012, 0.024, and 0.0018, respectively). The subgroup analysis showed the consistent benefit of a dose ≥ 66 Gy in patients across most subgroups. Doses ≥ 66 Gy were found to significantly, albeit borderline, increase the risk of late grade ≥ 2 GU toxicity compared to doses < 66 Gy (14% vs. 3.2%, p = 0.055). This large multi-institutional retrospective study demonstrated that a higher SRT dose (≥ 66 Gy) resulted in superior cRFS.
Similar content being viewed by others
Introduction
Following radical prostatectomy (RP) for localized prostate cancer, approximately 30% of patients develop biochemical recurrence (BCR) within 10 years1. In the case of BCR after RP, salvage radiotherapy (SRT) to the prostate bed is the only curative treatment. Due to recent advances in RT techniques, including intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT), escalated doses may be delivered with reduced gastrointestinal and genitourinary late toxicities2,3. Several systematic reviews and meta-analyses indicated that a dose escalation in SRT in the range of 60–70 Gy improved biochemical control4,5. Therefore, the American Society for Radiation Oncology (ASTRO)/American Urological Association (AUA) guidelines suggested 64 Gy or slightly higher as the minimum dose to be delivered for biochemical control in SRT6, and 64–72 Gy in a standard fraction is currently recommended in the 2023 National Comprehensive Cancer Network (NCCN) guidelines7. However, the effects of a high dose on late toxicity need to be considered8. Although biopsy-proven gross recurrence may require higher doses according to the NCCN guidelines7, gross tumors are generally not detected on various diagnostic imaging techniques in early SRT with PSA < 0.5 ng/ml9,10. Positron emission tomography (PET) imaging with 68gallium-labeled prostate-specific membrane antigen ligands (68Ga-PSMA) may be promising for the detection of recurrent tumors11; however, 68Ga-PSMA PET is utilized only in some countries and may be difficult to detect minimal tumor burden in early SRT. In the setting of SRT, randomized control trial (RCT) has not yet proven the effects of radiation doses on clinical relapse identified on radiological imaging and/or biopsy. Therefore, in the present large multi-institutional study with a long-term follow-up, we investigated the relationship between radiation doses and clinical relapse-free survival (cRFS) in patients receiving SRT for BCR after RP.
Methods
Study population
We identified 424 patients treated with SRT for BCR after RP between 2005 and 2018 at 15 institutions. All patients had adenocarcinoma of the prostate without evidence of lymph node or distant metastasis at RP. BCR included either prostate-specific antigen (PSA) elevation or persistence after RP: PSA elevation was defined as a PSA increase ≥ 0.10 ng/ml within two or more evaluations12, and PSA persistence as a serum concentration ≥ 0.10 ng/ml one month after RP13. At least CT was performed for the evaluation of local recurrence, lymph node metastasis, and distant metastasis. Magnetic resonance imaging (MRI), bone scintigraphy, and local biopsy were not performed routinely for the evaluation of macroscopic disease. Figure 1 shows a flowchart for patient selection. Patients with missing pathological or clinical information were excluded from the analysis (n = 4). Patients receiving SRT after RP without satisfying the definition of BCR were excluded (n = 3). Patients with PSA at SRT > 2.0 ng/ml (n = 6) or with lymph node metastasis at the final pathology (n = 7) were also excluded due to the increased risk of metastases14. Furthermore, patients receiving SRT combined with long-term (i.e. > 6 months) androgen deprivation therapy (ADT) were excluded (n = 33) because the combination of long-term ADT may influence clinical outcomes15. In addition, patients treated with an equivalent dose in 2-Gy fractions (EQD2) of ≤ 60 Gy were excluded (n = 76) because a total dose of ≤ 60 Gy in conventional fractionation was insufficient for local control16. Patients with macroscopic disease at SRT were also excluded (n = 3). The three patients with macroscopic disease had a tumor only around the prostate bed. These exclusions yielded 292 patients in the study cohort. To assess the relationship between radiation doses and clinical outcomes, the cohort was further divided into the < 66 Gy (n = 66) and ≥ 66 Gy groups (n = 226). This division was based on the median total dose of 66 Gy for the entire patient population (n = 295) as the cutoff and was consistent with findings from previous retrospective series17,18. In the present study, all doses were expressed in EQD2 calculated for prostate cancer (α/β = 1.5 Gy). The present study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. This study was approved by each institutional review board of Nagoya City University Graduate School of Medical Sciences, Kariya Toyota General Hospital, Japanese Red Cross Aichi Medical Center Nagoya Daini Hospital, Japan Community Health care Organization Chukyo Hospital, Suzuka General Hospital, Konan Kosei Hospital, Okazaki City Hospital, Nanbu Tokushukai General Hospital, Nagoya City West Medical Center, Narita Memorial Hospital, Kasugai Municipal Hospital, National Hospital Organization Nagoya Medical Center, Hokuto Hospital, Jisenkai Aizawa Hospital, and Fujieda Heisei Memorial Hospital. Since this was a retrospective observational analysis, the Nagoya City University Ethics Committee waived the need for informed consent as part of the study approval in line with the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. Therefore, research content was disclosed in the form of opt-out on the website.
Treatment procedures
SRT was delivered to the prostate and seminal vesicle bed at a median (range) dose of 66 Gy (61–85). All patients were treated using 6–18-MV photon beams. Conventional fractionation (1.8–2.0 Gy per fraction) was used for 220 (75%) patients, while moderate hypo-fractionation (2.1–3.0 Gy per fraction) was used for 72 (25%) patients. Whole-pelvic radiotherapy (WPRT) was administered to 11 patients (4%) at a median (range) dose of 45 Gy (40–54) at the discretion of radiation oncologists. IMRT and IGRT were used for 191 (65%) and 217 (74%) patients, respectively. Details of our RT methods were previously described8,10. In general, the delineation of targets adhered to the guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group19. The prescription dose and fractionation were determined at the discretion of each radiation oncologist. The use of ADT combined with SRT was at the discretion of each urologist. A luteinizing hormone-releasing hormone analog and/or anti-androgen therapy (i.e., bicalutamide) was used for 16 patients (6%) as a neoadjuvant and/or concurrent short-term ADT. The median (range) duration of ADT was 2.5 (1–6) months.
Clinical outcomes and toxicities
The follow-up time was calculated from the start date of SRT. The primary endpoint was cRFS, defined as the time from the start date of SRT to clinical relapse including local recurrence in the prostate bed, retroperitoneal lymph node metastasis, skeletal metastasis, and visceral metastasis. Metastases were identified on radiological imaging and/or eventual biopsy. Secondary endpoints included biochemical relapse-free survival (bRFS), cancer-specific survival (CSS), and overall survival (OS). Biochemical RFS, CSS, and OS were defined as the time from the start date of SRT to two consecutive PSA values ≥ 0.20 ng/ml, death or complications from prostate cancer, and death from any cause, respectively.
Genitourinary (GU) and gastrointestinal (GI) toxicities following SRT were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Any symptoms related to GU or GI toxicities that occurred or persisted three months after the end of SRT were regarded as late toxicities. Toxicity assessments were conducted at each follow-up visit.
Statistical analysis
Patient and treatment characteristics were compared using Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables between the < 66 Gy and ≥ 66 Gy groups. Univariate and multivariate analyses were conducted with Cox’s proportional hazards models to identify independent risk factors related to clinical relapse. Variables for the multivariate analysis were selected based on their biological importance and alignment with the predictive factors employed in the previous literature14. Survival was estimated with the Kaplan–Meier method, and survival estimates were compared using the Log-rank test between the two dose groups. We conducted a subgroup analysis by prognostic factors identified in the multivariate analysis. To address the potential bias due to differences in patient characteristics between the two groups, we conducted sensitivity analyses comparing survival curves of cRFS by excluding cases with short-term ADT administration or stratifying by the start year of SRT. The start year of SRT was divided at the median value of 2014 (Table 1). Late grade 2 or higher GU and GI toxicities were analyzed by estimating cumulative incidence curves, treating death from any cause as a competing risk. Gray’s test stratified by total doses in EQD2 was performed for late grade 2 or higher GU and GI toxicities. All statistical analyses were conducted using EZR20, which is a graphical user interface for R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria). The threshold for significance was p < 0.05.
Results
Patient characteristics
Table 1 shows patient characteristics. The median dose for all patients was 66.0 Gy (61.0–85.0) in EQD2. Gleason scores and PSA at BCR after RP were significantly higher in the ≥ 66 Gy group. Follow-up times were significantly longer in the < 66 Gy group (p = 0.001, Table 1); however, when stratified by the start year of SRT (2006–2013 and 2014–2018), there were no significant differences in follow-up time between the < 66 Gy and ≥ 66 Gy groups (p = 0.25 and 0.42, respectively, Table 1). Among patients with BCR after SRT (n = 118), 99 (84%) subsequently received ADT. Between the two dose groups, no significant differences were found in the other characteristics (Table 1).
Outcomes and impact of radiation doses
The median follow-up duration was 73 months (range, 5–189) for all patients (n = 292). Among all patients, 25 (8%) died, 6 (2%) of whom died of prostate cancer. In 118 patients (40%) with BCR after SRT, 22 (8%) developed clinical relapse. The median PSA at BCR after SRT was 0.30 (0.01–4.32) ng/ml. Figure 2 shows the survival curves of bRFS, cRFS, CSS, and OS for all patients receiving SRT for BCR after RP. Six-year bRFS, cRFS, CSS, and OS rates were 58% (95% confidence interval [CI], 52–64), 92% (95% CI, 88–95), 98% (95% CI, 95–99), and 94% (95% CI, 90–96), respectively.
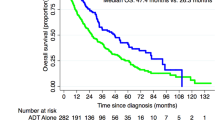
Twenty-two patients (8%) developed clinical relapse: 3 with regional recurrence, 15 with distant metastases, and 4 with both regional recurrence and distant metastases. The sites of distant metastases were bone in 15 patients, the lungs in 3, the liver in 3, and extraregional lymph nodes in 3. No patients developed local recurrence. The median (range) times to clinical relapse from the start date of SRT were 32 months (3–139) for all patients, 36 months (6–73) for the ≥ 66 Gy group, and 22 months (3–139) for the < 66 Gy group. Table 2 summarizes comparisons of biochemical and clinical relapse, prostate cancer death, and overall death between the two dose groups. Doses ≥ 66 Gy significantly improved cRFS (p = 0.022, Fig. 3); the 6-year cRFS rates of the ≥ 66 Gy and < 66 Gy groups were 94% (95% CI, 90–97) and 87% (95% CI, 75–93), respectively (p = 0.022, Table 2). Excluding patients who received short-term ADT (n = 16) yielded similar results regarding the effect of doses ≥ 66 Gy on cRFS (Supplementary Fig. S1a, p = 0.031). In the 2006–2013 period, there was still a significant improvement in cRFS with doses ≥ 66 Gy (Supplementary Fig. S1b, p = 0.040). In the 2014–2018 period, a similar trend was observed (Supplementary Fig. S1c), although there was no statistical significance (p = 0.54).
In the study cohort, 118 patients (40%) developed BCR after SRT. The 6-year bRFS rates of the ≥ 66 Gy and < 66 Gy groups were 56% (95% CI, 49–62) and 66% (95% CI, 52–76), respectively (p = 0.21, Table 2). The 6-year CSS rates of the ≥ 66 Gy and < 66 Gy groups were 100% (95% CI, not estimable) and 91% (95% CI, 79–96), respectively (p < 0.001, Table 2). The 6-year OS rates of the ≥ 66 Gy and < 66 Gy groups were 96% (95% CI, 92–98) and 86% (95% CI, 74–93), respectively (p = 0.006, Table 2).
Prognostic factors for clinical relapse-free survival and a subgroup analysis
Table 3 shows the results of univariate and multivariate analyses for clinical relapse after SRT. Gleason score ≥ 8, SVI, PSA at BCR after RP ≥ 0.5 ng/ml, and EQD2 < 66 Gy correlated with clinical relapse in the univariate and multivariate analyses. Figure 4 shows the number of events and adjusted hazard ratios (HRs) of clinical relapse by prognostic factors in the subgroup analysis. The most prominent benefit with a dose of ≥ 66 Gy was observed in patients with Gleason scores 8–10 (HR, 9.2; 95% CI, 2.6–32; p < 0.001). This benefit was consistent in patients across the majority of subgroups, including PSA at BCR after RP, ECE, and age at SRT. Multivariate analyses of CSS and OS were not performed because of the small number of cancer-specific deaths (n = 6).
Effects of radiation doses by clinical and pathological risk factors. EQD2 equivalent dose in 2-Gy fractions, HR hazard ratio, 95% CI 95% confidence interval, SVI seminal vesicle involvement, PSA prostate-specific antigen, BCR biochemical recurrence, RP radical prostatectomy, ECE extracapsular extension, SRT salvage radiotherapy.
Late toxicities
The symptoms of the late GU and GI toxicities are shown in Table S1. Among all patients (n = 292), late grade 1, 2, 3, 4, and 5 GU toxicities were reported in 43 (15%), 26 (8.9%), 16 (5.5%), 1 (0.3%), and 1 patient (0.3%), respectively. The most frequent symptom of late grade 2 or higher GU toxicity was hematuria in 30 patients (10%) followed by urinary tract obstruction in 8 patients (2.7%). The patient with late grade 4 GU toxicity underwent surgery for bladder tamponade resulting from hematuria. The patient with late grade 5 GU toxicity experienced postrenal acute renal failure due to urinary obstruction. The 6-year cumulative incidence of late grade 2 or higher GU toxicities for all patients was 12% (95% CI, 8.1–16). The 6-year cumulative incidence of late grade 2 or higher GU toxicities was higher in the ≥ 66 Gy group than in the < 66 Gy group with borderline significance (Fig. 5a, 14% [95% CI, 9.7–20] vs. 3.2% [95% CI, 0.6–10], p = 0.055).
Late grade 1, 2, and 3 GI toxicities were reported in 35 (12%), 8 (2.7%), and 8 patients (2.7%), respectively. No grade 4 or 5 GI toxicities were reported. The most frequent symptom of late grade 2 or higher GI toxicity was rectal hemorrhage in 15 patients (5.1%). The 6-year cumulative incidence of late grade 2 or higher GU toxicities for all patients was 5.4% (95% CI, 3.1–8.4). There was no significant difference in the 6-year cumulative incidence of late grade 2 or higher GI toxicities between the ≥ 66 Gy and < 66 Gy groups (Fig. 5b, 6.0% [95% CI, 3.3–9.6] vs. 3.3% [95% CI, 0.6–10], p = 0.37).
Discussion
We investigated the relationship between radiation doses and cRFS in patients who received SRT for BCR after RP. The present results demonstrated significantly better cRFS with a SRT dose of ≥ 66 Gy (p = 0.022, Fig. 3), which supports the dose recommendations in the ASTRO/AUA guidelines (64 Gy or slightly higher) 6 and 2023 NCCN guidelines (64–72 Gy) 7 even in terms of clinical relapse. To the best of our knowledge, this is the first study to show the benefit of a higher SRT dose, which reduced clinical relapse. Furthermore, the subgroup analysis confirmed a consistent benefit with a dose of ≥ 66 Gy in patients across most subgroups.
Several observational studies14,21,22 and a meta-analysis5 on SRT reported the advantage of dose escalations for biochemical control. However, the SAKK 09/10 trial23 and a Chinese single-center trial 24 did not verify the benefit of doses ≥ 70 Gy for bRFS or cRFS. In contrast, the present study showed significantly better cRFS in the ≥ 66 Gy group (p = 0.022, Fig. 3). This may be partly attributed to our study population having more poor prognostic factors, i.e., Gleason scores 8–10 (35%) and a T stage ≥ pT3b (15%), than other studies17,21,23,25. Since higher Gleason scores correlated with BCR and distant metastasis after SRT21,26, previous studies with more favorable prognostic factors may have included patients who did not require dose-escalated SRT. The present subgroup analysis showed the marked benefit of doses ≥ 66 Gy in patients with Gleason scores 8–10, but not with Gleason score ≤ 7 (Fig. 4).
The difference in prescribed doses may have influenced the present results. We selected 66 Gy as a dose cut-off based on previous retrospective series17,18, which reported that SRT doses ≥ 66 Gy improved biochemical control. A meta-analysis by King et al.5 showed a sigmoidal dose–response curve with a tumor control dose 50 of approximately 66 Gy. Some of the patients in our cohort received low doses (61–64 Gy) because the recommended doses were 60–66 Gy for SRT according to the guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group published in 200819. This may have contributed to the difference in cRFS between the two dose groups (p = 0.020, Fig. 3) in contrast to the findings of the SAKK 09/10 trial (70 Gy vs. 64 Gy)23 and Chinese trial (72 Gy vs. 66 Gy)24. A dose range of 60–64 Gy is considered to be insufficient for local control16 according to the SWOG study27, which showed that the 10-year risk of local failure was 9% even after adjuvant radiation therapy. A possible interpretation of these findings is that at least 64 Gy is needed to eradicate microscopic disease in the prostate bed, and also that further benefits may not be expected at doses ≥ 70 Gy. However, a recent matched-pair analysis25 suggested that doses ≥ 70 Gy were particularly beneficial in high-risk patients. Therefore, selection bias regarding some pathological features may have largely affected clinical outcomes. While patient characteristics exhibited imbalances in terms of short-term ADT administration, excluding patients who received short-term ADT yielded the similar result regarding the improvement in cRFS with doses ≥ 66 Gy (Supplementary Fig. S1a). The limited influence of short-term ADT in our study could be ascribed to the high rate of secondary ADT use after BCR following SRT (84%) and the relatively low rate of WPRT (4%, as shown in Table 1). In addition, the median follow-up time between the two groups differed by 30 months (Table 1, p = 0.001). To address the potential bias in detecting metastatic disease, the data were split into the two periods (2006–2013 and 2014–2018) with similar follow-up durations (Table 1, p = 0.25 and 0.42). In each period, we observed a consistent trend of cRFS improvement with dose escalation (Supplementary Figs. S1b and S1c). The increased prevalence of IMRT, which allows for high-conformity irradiation8 may have contributed to the higher incidence of patients receiving doses ≥ 66 Gy between 2014 and 2018. Furthermore, the difference in the total dose could have been influenced by the increasing recommended doses outlined in guidelines6.
In contrast to previous studies17,18, higher doses of ≥ 66 Gy did not significantly improve bRFS (p = 0.21, Table 2). Heterogeneity in pathological and clinical characteristics (Table 1) may have influenced our results. In the present study, the ≥ 66 Gy group had worse prognostic factors: Gleason scores 8–10 were detected in 39 and 21% of patients in the ≥ 66 Gy and < 66 Gy groups, respectively (p = 0.013, Table 1); PSA at BCR after RP were 0.42 and 0.32 ng/ml in the ≥ 66 Gy and < 66 Gy groups, respectively (p = 0.012, Table 1). Since a higher Gleason score and PSA at BCR are associated with a higher incidence of BCR4,9,21,28, this imbalance in prognostic factors may have offset the difference in bRFS between the two dose groups. These prognostic factors are also associated with distant metastasis21,26 and death by any cause28. Therefore, conversely, in the present study, improved cRFS with higher doses of ≥ 66 Gy (p = 0.022, Fig. 3) may be strengthened because patients in the ≥ 66 Gy group had less favorable prognostic factors, due to which the between-group difference may have been underestimated (i.e., bias towards null).
We observed clinical relapse in 22 patients (7%). Sites of recurrence were as follows: 3 with regional recurrence, 15 with distant metastases, and 4 with both regional recurrence and distant metastases, but not local recurrence. This result was partly consistent with previous findings showing that the most frequent pattern of recurrence in an 8-year follow-up was distant metastasis (21%), followed by regional (6%) and local recurrence (2.2%)29. The assumption that SRT achieves local control and prevents or delays metastases is supported by the favorable 6-year disease-free survival observed even in patients with high-risk factors such as GS8–10 and short PSA doubling time14. However, no local recurrence was observed in either dose group. This may be attributed to difficulties in detecting local recurrence compared to regional recurrence and bone metastasis. There is no consensus on monitoring local recurrence after SRT7. Even biopsy has limited predictive value for at least two years post-RT due to delayed tumor regression30. The low PSA level (median, 0.30 ng/ml) and high utilization rate of ADT (84%) at BCR after SRT may also have affected the capability of detecting local recurrence. Furthermore, the retrospective nature of the present study may have led to the infrequent use of MRI and local biopsy.
WPRT and ADT may exert effects even on subclinical distant metastasis, and recent RCTs showed improvements in clinical outcomes by the addition of WPRT31 and concurrent short-term ADT32 to SRT. However, in the present study, WPRT and concurrent short-term ADT were rarely used (≤ 6%) because evidence remained unestablished when we initiated the present study. Nevertheless, we observed a significant 7% improvement in 6-year cRFS in the ≥ 66 Gy group (Table 2, p = 0.022), and the pattern of recurrence exclusively involved regional recurrence and distant metastasis. Future studies need to consider emerging evidence from several RCTs31,32 that will facilitate the application of ADT and WPRT, and the effects of dose-escalated SRT to local lesions in well-selected patients will become clear.
In the present study, the 6-year cumulative incidence of late grade 2 or higher GU toxicities was 12%, which aligned with the findings in previous literature9,23,33,34. Nevertheless, the observed trend shown in Fig. 5a suggests that doses ≥ 66 Gy substantially increased the risk of grade 2 or higher GU toxicity. Given the lack of clear benefits in biochemical control with doses ≥ 70 Gy from the two RCTs23,24 and the previous finding that total dose ≥ 68 Gy was identified as an independent risk factor for late GU toxicity8, the optimal total dose may have been in the range of approximately 66–68 Gy in our study population. Further studies are warranted to explore the trade-off between tumor control and adverse effects.
The present study had several limitations. Due to its retrospective nature, a selection bias was inevitable. For example, decisions regarding prescribed doses were at the discretion of radiation oncologists, and this may have introduced undetectable confounders. However, patient characteristics in each group were almost homogenous. In the present study, sensitivity analyses were conducted to mitigate potential bias arising from differences in short-term ADT use and follow-up duration between the two groups. Moreover, imaging techniques depended on each institution’s practice; MRI and local biopsy were not routinely performed during the follow-up period. This may have reduced the sensitivity of detecting local and regional recurrence. We also did not perform 68Ga-PSMA PET, which has a higher diagnostic capability to detect small-volume metastases35. In addition, we did not perform a multivariate analysis of CSS or OS because of the small number of events. Further studies with a longer follow-up period are warranted to assess clinical outcomes.
In conclusion, this large multi-institutional retrospective study demonstrated that a higher dose (≥ 66 Gy) resulted in superior 6-year cRFS in patients receiving SRT for BCR after RP. Future prospective studies need to investigate the impact of dose-escalated SRT on cRFS in well-selected patients.
Data availability
The datasets generated and/or analyzed during the present study are not publicly available due to ethical reasons, but are available from the corresponding author upon reasonable request.
References
Stephenson, A. J. et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. JNCI J. Natl. Cancer Inst. 98, 715–717. https://doi.org/10.1093/jnci/djj190 (2006).
Michalski, J. M. et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: The NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 4, e180039. https://doi.org/10.1001/jamaoncol.2018.0039 (2018).
Moore, K. L. et al. Quantifying unnecessary normal tissue complication risks due to suboptimal planning: A secondary study of RTOG 0126. Int. J. Radiat. Oncol. Biol. Phys. 92, 228–235. https://doi.org/10.1016/j.ijrobp.2015.01.046 (2015).
Ohri, N., Dicker, A. P., Trabulsi, E. J. & Showalter, T. N. Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur. J. Cancer 48, 837–844. https://doi.org/10.1016/j.ejca.2011.08.013 (2012).
King, C. R. The dose–response of salvage radiotherapy following radical prostatectomy: A systematic review and meta-analysis. Radiother. Oncol. 121, 199–203. https://doi.org/10.1016/j.radonc.2016.10.026 (2016).
Thompson, I. M. et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J. Urol. 190, 441–449. https://doi.org/10.1016/j.juro.2013.05.032 (2013).
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer, version 1.2023. Sep 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed Sep 23, 2022).
Tomita, N. et al. Impact of advanced radiotherapy techniques and dose intensification on toxicity of salvage radiotherapy after radical prostatectomy. Sci. Rep. 10, 1–8. https://doi.org/10.1038/s41598-019-57056-9 (2020).
Tomita, N. et al. Early salvage radiotherapy for patients with PSA relapse after radical prostatectomy. J. Cancer Res. Clin. Oncol. 135, 1561–1567. https://doi.org/10.1007/s00432-009-0603-7 (2009).
Tomita, N. et al. Early salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy: Its impact and optimal candidate. Asia. Pac. J. Clin. Oncol. 16, 273–279. https://doi.org/10.1111/ajco.13341 (2020).
Fendler, W. P. et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: A prospective single-arm clinical trial. JAMA Oncol. 5, 856–863. https://doi.org/10.1001/jamaoncol.2019.0096 (2019).
Mohler, J. L. et al. Prostate cancer, version 1.2016. J. Natl. Compr. Canc. Netw. 14, 19–30. https://doi.org/10.6004/jnccn.2016.0004 (2016).
Mottet, N. et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 79, 243–262. https://doi.org/10.1016/j.eururo.2020.09.042 (2021).
Stephenson, A. J. et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J. Clin. Oncol. 25, 2035–2041. https://doi.org/10.1200/JCO.2006.08.9607 (2007).
Shipley, W. U. et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N. Engl. J. Med. 376, 417–428. https://doi.org/10.1056/NEJMoa1607529 (2017).
Swanson, G. P. et al. Predominant treatment failure in postprostatectomy patients is local: Analysis of patterns of treatment failure in SWOG 8794. J. Clin. Oncol. 25, 2225–2229. https://doi.org/10.1200/JCO.2006.09.6495 (2007).
Pisansky, T. M. et al. Salvage radiation therapy dose response for biochemical failure of prostate cancer after prostatectomy—A multi-institutional observational study. Int. J. Radiat. Oncol. Biol. Phys. 96, 1046–1053. https://doi.org/10.1016/j.ijrobp.2016.08.043 (2016).
Bernard, J. R. et al. Salvage radiotherapy for rising prostate-specific antigen levels after radical prostatectomy for prostate cancer: Dose-response analysis. Int. J. Radiat. Oncol. Biol. Phys. 76, 735–740. https://doi.org/10.1016/j.ijrobp.2009.02.049 (2010).
Sidhom, M. A. et al. Post-prostatectomy radiation therapy: Consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother. Oncol. 88, 10–19. https://doi.org/10.1016/j.radonc.2008.05.006 (2008).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458. https://doi.org/10.1038/bmt.2012.244 (2013).
Tendulkar, R. D. et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J. Clin. Oncol. 34, 3648–3654. https://doi.org/10.1200/JCO.2016.67.9647 (2016).
Goenka, A. et al. Long-term outcomes after high-dose postprostatectomy salvage radiation treatment. Int. J. Radiat. Oncol. Biol. Phys. 84, 112–118. https://doi.org/10.1016/j.ijrobp.2011.10.077 (2012).
Ghadjar, P. et al. Dose-intensified versus conventional-dose salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy: The SAKK 09/10 randomized phase 3 trial. Eur. Urol. 80, 306–315. https://doi.org/10.1016/j.eururo.2021.05.033 (2021).
Qi, X. et al. Toxicity and biochemical outcomes of dose-intensified postoperative radiation therapy for prostate cancer: results of a randomized phase III trial. Int. J. Radiat. Oncol. Biol. Phys. 106, 282–290. https://doi.org/10.1016/j.ijrobp.2019.09.047 (2020).
Böhmer, D. et al. Impact of dose escalation on the efficacy of salvage radiotherapy for recurrent prostate cancer—A risk-adjusted, matched-pair analysis. Cancers Basel. 14, 1320. https://doi.org/10.3390/cancers14051320 (2022).
Fossati, N. et al. Impact of early salvage radiation therapy in patients with persistently elevated or rising prostate-specific antigen after radical prostatectomy. Eur. Urol. 73, 436–444. https://doi.org/10.1016/j.eururo.2017.07.026 (2018).
Thompson, I. M. et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA 296, 2329–2335. https://doi.org/10.1001/jama.296.19.2329 (2006).
Stish, B. J. et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J. Clin. Oncol. 34, 3864–3871. https://doi.org/10.1200/JCO.2016.68.3425 (2016).
Jackson, W. C. et al. Anatomical patterns of recurrence following biochemical relapse after post-prostatectomy salvage radiation therapy: a multi-institutional study. BJU Int. 120, 351–357. https://doi.org/10.1111/bju.13792 (2017).
Crook, J. et al. Postradiotherapy prostate biopsies: What do they really mean? Results for 498 patients. Int. J. Radiat. Oncol. Biol. Phys. 48, 355–367. https://doi.org/10.1016/S0360-3016(00)00637-4 (2000).
Pollack, A. et al. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial. Lancet 399, 1886–1901. https://doi.org/10.1016/S0140-6736(21)01790-6 (2022).
Carrie, C. et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 17, 747–756. https://doi.org/10.1016/S1470-2045(16)00111-X (2016).
Feng, M. et al. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 68, 1417–1423. https://doi.org/10.1016/j.ijrobp.2007.01.049 (2007).
Nath, S. K. et al. Toxicity analysis of postoperative image-guided intensity-modulated radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 78, 435–441. https://doi.org/10.1016/j.ijrobp.2009.08.023 (2010).
Marlon, P. et al. Sensitivity, specificity, and predictors of positive 68Ga–prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur. Urol. 70, 926–937. https://doi.org/10.1016/j.eururo.2016.06.021 (2016).
Acknowledgements
This study was supported by a Grant-in-Aid for Research in Nagoya City University Number 2213003.
Author information
Authors and Affiliations
Contributions
S.T. and N.T. conceived the study concept and study design. S.T., N.T., M.N., K.U., M.I., S.A., M.I., Y.T., S.O., Y.M., K.N., Y.O., A.M., A.M., and S.T. performed compilation and synthesis of the data. S.T. and N.T. carried out statistical analyses. All authors participated in interpretation of the results and writing of the report, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takano, S., Tomita, N., Niwa, M. et al. Impact of radiation doses on clinical relapse of biochemically recurrent prostate cancer after prostatectomy. Sci Rep 14, 113 (2024). https://doi.org/10.1038/s41598-023-50434-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50434-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.