Abstract
In this survey, the symptomatic leaves of Clivia miniata were collected from a greenhouse in Karaj city of Iran. The isolation and morphological investigation showed Scytalidium-like fungus associated with leaf blight symptom. The phylogenetic analysis of the internal transcribed spacer along with partial sequences of rDNA large subunit and translation elongation factor 1-α (tef-1α) genomic regions confirmed the identification of the recovered isolate as Neoscytalidium dimidiatum. The pycnidial morph of the fungus didn’t observe both in vitro and in vivo. The pathogenicity test on C. miniata and C. nobilis was also conducted to fulfill the Koch’s postulates. To our Knowledges, this is the first report of N. dimidiatum causing leaf blight disease on C. miniata and C. nobilis worldwide, as well as these host plants are new for N. dimidiatum in the world.
Similar content being viewed by others
Introduction
The genus Neoscytalidium belongs to the Botryosphaeriaceae family (Ascomycota), produces two types of anamorphs which are known as arthric with Scytalidium-like conidia and pycnidial that produces Fusicoccum-like conidia1. The arthric and pycnidial states were named Torula dimidiata and Hendersonula toruloidea respectively1. Later, Scytalidium hyalinum was introduced, which has the arthric mycelium2. Hendersonula toruloidea was revised and transferred into a new genus, Nattrassia, with N. mangiferae as the type species. The Scytalidium-like synanamorph was named as Scytalidium dimidiatum3. During a phylogenetic revision of the Botryosphaeriaceae family, polyphyletic nature of the genus Scytalidium was revealed, and Neoscytalidium was introduced as a new genus and S. dimidiatum was considered as a synonym for N. dimidiatum4. Neoscytalidium novaehollandiae was introduced as new species based on the production of muriform and Dichomera-like conidia, differing from N. dimidiatum5. Scytalidium hyalinum has been identified as a melanin-deficient mutant of S. dimidiatum, and introduced as N. dimidiatum var. hyalinum3,6. The N. hyalinum was chosen as the type species of Neoscytalidium, instead of N. dimidiatum2. Considering the fact that “dimidiatum” (1882) is an older epithet than “hyalinum” (1977), it was proposed that the correct type species is N. dimidiatum. Neoscytalidium orchidacearum was introduced as a new species only on orchids in Thailand, up to now. It has been mentioned that the fungus isolated as N. dimidiatum from rhinosinusitis in Iran, may be another new species3,7. Neoscytalidium oculus was reported as a new species based on the absence of a coelomycetous synanamorph from keratitis8. Until 2020, it was believed that Neoscytalidium has four species including N. dimidiatum, N. novaehollandiae, N. orchidacearum, and N. oculus9. The similarity of the ex-type sequence of N. dimidiatum, N. novaehollandiae, and N. orchidacearum was confirmed based on the ITS, tef1 and tub2 genomic regions. Neoscytalidium novaehollandiae and N. orchidacearum were considered as synonyms for N. dimidiatum, and it was mentioned that the N. dimidiatum is the only species in the genus Neoscytalidium10. This suggestion was confirmed by the phylogeny of the ITS, LSU, chs-1 and tub2 genomic regions11. Recently, N. hylocereum was reported as a new species causing canker on Hylocereus polyrhizus in Thailand12.
Neoscytalidium species can be plant pathogens, endophytes, saprobes, and latent pathogens. It is also considered as a mycosis agent in human and animals2,6,9,13. This genus distributed all over the world except Arctic and Antarctic areas2. Although, they are almost observed on woody plant hosts, but they can be found on leafy plants, soils, and mammals6,8,13,14. Neoscytalidium dimidiatum is an opportunistic pathogen and can cause veriety of diseases in plants1,2,6,9,13,15,16,17,18,19,20,21,22,23,24 as well as in human3,6,7,8,11,13,14,15,25.
In Iran, Neoscytalidium dimidiatum has been isolated from pistachio trees as one of the causal agents of young shoots dieback in Kerman province26. It has also been reported as a causal agent of Citrus branch wilt, death and decline disease in Khouzestan province27. The N. mangiferae was reported as the causal agent of dieback and trunk cankers on Ficus religiosa and dieback on Psidium guajava in Jiroft City, Kerman province28. It has also been isolated from Acer, Platanus, Magnolia, Eryobotria, Eryobotria, Morus, Ulmus, Cupressus, Robinia, Eucalyptus, Citrus, Juglans and Malus as a causal agent of decline disease in Shiraz City, Fars province29. Neoscytalidium hyalinum has been isolated from wood lesion and decline symptoms of willow (Salix spp.) and poplar (Populus spp.) in Shiraz City, Fars province30. It has been reported as a associated fungus with trunk disease on Calligonum amoenum in Kerman province31. Also, this species was isolated from dieback and canker symptoms of the date palm in Fars, Kerman, Khouzestan, Hormozgan, Isfahan, and Sistan and Balochestan provinces32. Neoscytalidium dimidiatum has also been reported as a pathogen associated with Persian oak decline in Ilam province33. Neoscytalidium hyalinum has been reported causing fruit rot on Cucumis melo in Fars province34. This fungus has also been isolated from neem tree (Azadirachta indica) decline symptom in Hormozgan province35. Neoscytalidium dimidiatum has been reported as a associated fungus with walnut decline in Kerman, Kermanshah, Hamedan, Kurdistan, and Isfahan provinces36. This species has also been reported as a associated fungus of sooty canker and dieback on Ficus benghalensis in Kish Island, Hormozgan province37. Also the fungus has recently been isolated as a associated fungus with necrotic wood tissues of pomegranate in Kerman and Fars provinces38.
The genus Clivia (Amaryllidaceae) is an important herbaceous plant that are grown worldwide for cut-flower trade39,40. Clivia miniata (Lindl.) Verschaff., is the most well-known species, known as Natal lily, bush lily, Kaffir lily, Boslelie, or Umayime in different regions39. Also, the medicinal, pharmacological, and phytochemical features of this species have been studied39,41.
Fungal diseases of the Clivia spp. have been investigated many times in the world. Stagnospora curtisii has been reported as a associated fungus of leaf and stalk spot of Clivia spp. in New Zealand42. Colletotrichum gloeosporiodes has been reported causing anthracnose disease on C. miniata in South Africa43. Colletotrichum trichellum has also been reported causing anthracnose on C. miniata in Korea44. Macrophoma agapanthii as a causal agent of leaf dieback, and Sclerotium rolfsii as a causal agent of collar rot on Clivia spp. have been reported in South Aferica45. Colletotrichum boninense has been reported as a causal agent of anthracnose on C. miniata in Japan, and later the name of the species has been changed to C. karsti46,47. Fusicoccum luteum has been identified causing leaf spot and plant collapse in C. miniata in Northland48. Colletotrichum cliviae has been reported as a causal agent of anthracnose on C. miniata in China, and later the name of the species has been changed to C. cliviicola49,50. Alternaria tenuissima, Blennoria sp., Phoma sp., and Strelitziana cliviae have been reported on Clivia as plant pathogens40. Fusarium solani and F. proliferatum have been identified as the causal agents of root rot on C. miniata in China51. Fusarium proliferatum has been reported causing sheath rot on C. miniata in China52. Fusarium oxysporum has also been reported as the causal agent of basal stem rot on C. miniata in China53. Fusarium solani has been reported causing sheath rot on C. miniata in China41. Botrytis cinerea (China), C. himantophylli (Canada), Gloeosporium crini (Korea), Gloeosporium sp. (China), Myrothecium leucotrichum (Florida), Pestalotia glandicola (Japan), and Physalospora cliviae (Southern Africa) have also been reported on Clivia54. Colletotricum trichellum has been reported causing anthracnose on C. miniata in Tehran province, in Iran55.
According to the importance of Clivia species conservation from extinction39, identification of the biotic infectious agents, especially fungi, is very important. Despite the economic importance of Clivia spp. in Iran, there is only one study regarding the diseases of this plant. So, the main aim of the present study was the isolation and morphological and molecular characterization of the causal agent of the leaf blight disease on C. miniata and fulfilling the Koch’s postulates to confirm the pathogenicity of the recovered fungal agent.
Materials and methods
Sampling and isolation
The C. miniata leaves with leaf blight symptom were collected from a greenhouse of the Department of Horticulture, College of Agriculture and Natural resources, University of Tehran, Karaj (N35° 48′ 20.4″ E 050° 59′ 53.9″) according to the guidelines of the University of Tehran. The leaf samples were surface sterilized by 70% ethanol. The boundaries of the diseased and healthy parts of the leaf samples were cut into 1 \(\times\) 1 cm small pieces, submerged in 70% ethanol, and then in 2% sodium hypochlorite, each step for 2 min. The leaf pieces were washed three times with distilled water, and then were transferred onto the sterile paper towel to dry in room temperature. The specimens were placed onto the 1.7% water agar culture medium and incubated at 26 ℃ in dark condition for seven days. When the mycelia grow from the leaf pieces, they were purified using the hyphal tip method, and the hyphal tips were transferred onto the 2% potato dextrose agar culture medium and incubated at the same above mentioned conditions.
Morphological identification
As the fungal colony grows, its diameter was measured until it filled the nine cm in diameter Petri dish. The surface and reverse colour of the five-days and 15-day old colony was recorded according to the Rayner’s colour chart56. The microscopic slide mounts were prepared from 15-day old colony using the lactophenol, and in the microscopic slides, the mycelium and spore features were examined under the olympus BH2 light microscope. In order to fruit body formation in the recovered fungal isolate, the isolate was grown on 2% pine needle agar (PNA) culture medium (2% water agar with double sterilized pine needle in autoclave) and the inoculated Petri dishes were kept under the 12 h near UV light/12 h dark condition at 25 ℃, and the colony was inspected every day until the 30 days. Morphological identification of the recovered isolate was done based on the description provided by Crous et al.4.
Molecular identification
DNA extraction
The modified Zhong and Steffenson’s57 protocol was used to extract the genomic DNA from the recovered fungal isolate. In brief, 10–20 g of mycelium was harvested from the four-day old colony on PDA culture medium, and the lysis buffer was added and the mycelia were ground very well. The mixture was transferred to a sterile microtube and vortexed. The microtube was placed on the 65 ℃ hot-plate for 15 min and inverted every five minutes. Then, it was placed on the ice and kept at − 20 ℃ for 10 min. In order to remove proteins, chloroform:isoamyl alcohol (24:1) was added and kept in the same above mentioned condition. The mixture was centrifuged at 10,000 rpm for 10 min. The supernatant was transferred to a new microtube, chloroform was added and the mixture was centrifuged again. The supernatant was transferred to a new microtube and equal volume of isopropanol was added. Three phases were appeared in the mixture, where the middle phase containing the nucleic acid. The mixture was inverted, centrifuged at 12,000 rpm for 10 min, and the supernatant was discarded. The 70% ethanol was added and the mixture was centrifuged at 10,000 rpm for three minutes. After removing the supernatant, the microtube was placed upside-down on the 65 ℃ hot-plate for 30 min. Finally, 30 µl deionized sterile water was added and spined shortly. The extracted DNA was kept at 4 ℃ overnight, and then stored at -20 ℃ for future use. The success of the DNA extraction was investigated using the electrophoresis in 1% agarose gel and also by NanoDropping.
Polymerase chain reaction and sequencing
The internal transcribed spacer (ITS), partial sequence of large subunit (LSU), and partial sequence of translation elongation factor 1-α (tef1-α) genomic regions were amplified using the ITS1 (5̍-TCCGTAGGTGAACCTGCGG-3̍) and ITS4 (5̍-TCCTCCGCTTATTGATATGC-3′), LROR (5′-ACCCGCTGAACTTAAGC-3′) and LR5 (5′-ATCCTGAGGGAAACTTC-3′), EF1-728F (5′-CATCGAGAAGTTCGAGAAGG-3′) and EF1-986R (5′-TACTTGAAGGAACCCTTACC-3′) primer pairs, respectively58,59,60. The PCR mixture (25 µl total volume) consisted of 12.5 µl PCR Mastermix, 8.5 µl deionized water, 0.5 µl for ITS and 1 µl for LSU and tef1-α of primers (10 pmol), 3 µl (for ITS) and 2 µl of 30 ng/µl extracted DNA (for LSU and tef1-α). The PCR condition was as follow for ITS, LSU, and tef1-α, respectively: initial denaturation (95 ℃ for 90s, 95 ℃ for 2min, 95 ℃ for 5min), 35 cycles of denaturation (94 ℃ for 30s, 95 ℃ for 30s, 94 ℃ for 50s), annealing (52 ℃ for 30s, 51 ℃ for 45s, 56 ℃ for 45s), and extension (72 ℃ for 30s, 72 ℃ for 1min, 72 ℃ for 1min), and with the final extension (72 ℃ for 6min, 72 ℃ for 8min, 72 ℃ for 10min)61. The accuracy of the PCR products was evaluated by electrophoresis in 1% agarose gel and the PCR products of ITS and both of LSU and tef1-α genomic regions were sent for sequencing to Bio-Magic-Gene International Company and Takapouzist (Iran), respectively.
Phylogeny analysis
The obtained sequences were deposited in the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/) and also these sequences were subjected to BLAST search in NCBI database using the Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/). The closest related sequences of the species and genera to these queries were obtained from GenBank, and all sequences were edited by Chromas software version 2.6.6. The sequences of three genomic regions were concatenated in each isolate. The datasets were aligned with MUSCLE program of the MEGA version 11 software using the default settings. The best model for generating maximum likelihood phylogenetic tree was determined by MEGA software using all sites of gaps or missing data treatment as the default setting. The maximum likelihood and maximum parsimony phylogenetic trees were constructed for ITS-TEF, ITS-LSU and ITS-LSU-TEF concatenated sequences. For generating each tree, the bootstrap method with 1000 replications was used. The all sites of gaps or missing data were also considered in the tree reconstruction. Finally, the phylogenetic trees were built in MEGA version 11 software and their topologies were compared.
Pathogenicity test
The Koch’s postulates were conducted to confirm the pathogenicity of the recovered isolate. For each Clivia species, one pot having one plant was used. For pathogenicity test, 20cm in diameter and 18cm in height plastic pots were used. Each pot had one three-year old growing and healthy plant that was propagated offset. Three replicates were used in the treatment as well as in the control. The soil composition of the pots was a mixture of garden soil, sand, and rotted leaf soil that each of them made a third of the mixture. The surface of the four healthy leaves of each plant in each pot was disinfected with 70% ethanol13. In each leaf, four points were wounded using a sterile 6 mm in diameter cork borer. The mycelial plugs (6 mm in diameter) were taken from the margin of the seven-day old colony and placed upside-down on the created wounds and wrapped with cellophane. The mycelium free PDA plugs were used for the inoculation of the wounds in control plants. Each pot was covered by black plastic bag for three days to provide dark condition and preserve the moisture, and after three days the plastic bag was removed. The pots were irrigated every two days. The tests were monitored daily, until the symptom of the disease appeared. The experiment was performed twice on C. miniata and C. nobilis. Furthermore, the experiment was conducted in two different temperature ranging 26–28 ℃ and 30–32 ℃, to investigate the effect of this factor on the pathogenicity of the recovered fungus.
Results
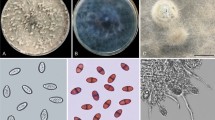
The obtained axenic isolate filled the nine cm in diameter Petri dish after five days. The average growth rate of the isolate was 1.77 cm/day. The surface colour of the five-day old colony was white becoming pale mouse grey at the center and it was white on the reverse. The colony colour was olivaceous grey and buff for the surface and the reverse of the 15-day old colony, respectively. The isolate produced powdery to the touch aerial mycelia and Scytalidium-like arthroconidia. The conidia were various in shape including globose to subglobose, ellipsoidal, cylindrical, oblong, obtuse, truncate, and articulated from the mycelia as singular or in chain. They were hyaline to pale brown, thick walled, 0–2 septate, and 4–28 (9.85) \(\times\) 2.5–5 (7.04) µm in size (n = 50). The hyphae rarely had swellings (Fig. 1). The arthroconidia were aggregated on the pine needle on PNA culture medium, but the differentiation of the hyphae and formation of the pycnidia did not occur. These morphological characteristics of the recovered isolate were similar to the genus Neoscytalidium according to the Crous et al.4 description.
Morphological features of the Neoscytalidium dimidiatum, isolate GKH-2. (a and b) Surface and reverse of five-day old colony; (c and d) Surface and reverse of the 15-day old colony; (e–g) Aseptate to 2-septate arthroconidia; (h) Swelling in hypha; and (i) Arthric chain. Scale bars: e and f = 6µm; g = 8 µm; h and i = 4 µm.
The PCR amplified 554, 916, and 297 bp fragments from ITS, LSU, and tef1-α genomic regions, respectively. These sequences were deposited in the GenBank (NCBI) with accession numbers of MZ047291, OQ306534, and OQ383346, respectively. The results of the BLAST searches of the recovered isolate (GKH-2) sequences with the related N. dimidiatum sequences were shown in the Table S1. The selected sequences for phylogenetic analysis were presented in Table 1. The maximum likelihood phylogenetic tree was generated for ITS-TEF (Fig. 2), ITS-LSU (Fig. 3) and ITS-LSU-TEF (Fig. 4) using the Tamura 3-parameter and rate among sites has invariant sites (T92 + I), Kimura 2-parameter with distribute Gamma category 2 (K2 + G), and Tamura 3-parameter with distribute Gamma category 2 (T92 + G), respectively. These parameters were the best model that was suggested by MEGA software. To generate ITS-TEF and ITS-LSU-TEF phylogenetic trees, the sequence of tef1-α genomic region for N. orchidacearum and N. oculus was not available in GenBank as well as the LSU genomic region of N. hylocereum for generating ITS-LSU and ITS-LSU-TEF phylogenetic trees, so these taxa were omitted from the further analyses. The maximum parsimony tree was also built for the investigated sequences (Fig. S1, S2 and S3) and the tree topology of the two phylogenetic trees for each concatenated sequence were compared. As expected, they were similar, indicating the accuracy of the phylogenetic study. In the phylogenetic trees, the isolate GKH-2 of the present study was placed in a clade along with the Neoscytalidium isolates from Turkey with 83% bootstrap support in ITS-LSU phylogenetic tree, with 84% bootstrap support in ITS-LSU-TEF phylogenetic tree and with 56% in ITS-TEF phylogenetic tree, and then it was identified as N. dimidiatum. This isolate was deposited in the microbial culture collection of Agricultural Biotechnology Research Institute of Iran Culture Collection, Karaj, Iran, with the accession number of ABRIICC 10,347.
Maximum likelihood phylogenetic tree of the concatenated ITS and tef1-α sequences, generated by MEGA ver. 11 software. The suggested T92 + I model by MEGA software was used. Phyllosticta phoenicis was used as an outgroup taxon. The recovered fungal isolate in the present study was shown in boldface. The bootstrap values more than 50% have been presented on the branches.
Maximum likelihood phylogenetic tree of the concatenated ITS and LSU sequences, generated by MEGA ver. 11 software. The suggested K2 + G model by MEGA software was used. Phyllosticta phoenicis was used as an outgroup taxon. The recovered fungal isolate in the present study was shown in boldface. The bootstrap values more than 50% have been presented on the branches.
Maximum likelihood phylogenetic tree of the concatenated ITS, LSU and tef1-α sequences, generated by MEGA ver. 11 software. The suggested T92 + G model by MEGA software was used. Phyllosticta phoenicis was used as an outgroup taxon. The recovered fungal isolate in the present study was shown in boldface. The bootstrap values more than 50% have been presented on the branches.
In the pathogenicity test, symptom of the disease appeared five days after the plant inoculation in all inoculated sites in C. miniata leaves in 30–32 ℃, and in the same temperature condition, the symptom in C. nobilis leaves appeared 23 days after the plant inoculation in some of the inoculated sites. At the temperature condition of 26–28 ℃, the symptom of the disease was observed 16 days after the inoculation only in one inoculated site in C. miniata, whereas the symptoms were appeared on C. nobilis 31 days after the inoculation in one inoculated site. The control plants remained symptomless until the end of the experiment. The blight symptom initiated from the wounded and inoculated sites as a brown-rotted lesions, and the areas around the lesions were chlorotic (Fig. 5). As the disease developed, the brown region at the center of the spot became necrotic. Eventually, the disease led the leaf to die. The inoculated fungus was reisolated from these newly produced symptoms and then it was identified as N. dimidiatum, fulfilling the Koch’s postulates.
Discussion
The N. dimidiatum (GKH-2) was isolated from the leaf blight symptom of C. miniata. It was identified based on the morphological and molecular characterization. The phylogenetic analysis based on the three genomic regions showed that the isolate GKH-2 belongs to the clade that the N. dimidiatum isolates from Turkey were located, and these isolates separated from the clade that accommodates the other Neoscytalidium isolates. These two clades were separated with 99% (in the maximum likelihood phylogenetic tree) and 100% (in the maximum parsimony phylogenetic tree) bootstrap value. The separation of the Neoscytalidium isolates into two clades, which places isolates from Turkey in one clade independently from other Neoscytalidium isolates was in concordance with Zhang et al.10 and Güney et al.17,62. It is likely that the genus Neoscytalidium might be polyphyletic. According to the morphological investigation, isolate GKH-2 was very similar to the N. dimidiatum described by Crous et al.4, but it produced swollen hyphae rarely and two-septate arthroconidia was also common. The pycnidium formation in the isolate GKH-2 was not observed. Previously, the N. oculus isolate IOM325287 was introduced as a new Neoscytalidium species based on the phylogenetic analysis as well as lack of pycnidium formation8. Regarding to several studies, lack of pycnidium formation is dependent on various factors, such as temperature1,9,62,63,64,65,66, and considering this character as a key feature in the description of the species would be inaccurate. Recently, in the phylogenetic study of the Neoscytalidium species, it is observed that all species of Neoscytalidium are placed in the same clade, which this clade was named as N. dimidiatum, except for the isolates from Turkey that located in another clade, which this clade was named as Neoscytalidium sp. It has been concluded that the N. dimidiatum is the only species in the genus Neoscytalidium, so the other species were transferred to this species10. For the identification and introduction of Neoscytalidium species, all aspects should be considered and polyphasic studies are necessary. Attention to the morphological characteristics and ecological niches such as host along with the phylogenetic studies, can lead to better understanding of the species concept in the genus Neoscytalidium. According to the previous studies, N. novaehollandiae is distinguished from N. dimidiatum by producing two types of conidia, as well as by phylogenetic features5. The N. orchidacearum produces hyaline conidia, and it was found only on orchid plants in Thailand so far. Considering these features along with phylogenetic characteristics, this species can be separated from N. dimidiatum and N. novaehollandiae3,25. As the Zhang et al.10 have mentioned, this opinion that the genus Neoscytalidium is monotypic needs further studies, especially in the aspect of genetic population.
Regarding to N. orchidacearum and N. hylocereum, it seems that host plant has an important role in the evolution and speciation of the genus Neoscytalidium. Recently, two isolates of N. dimidiatum (isolates Keningau and Keningau02) have been reported causing stem canker on Selenicereus megalanthus in Malaysia67. These isolates were separated from N. dimidiatum and N. novaehollandiae based on the ITS, tef1-α and tub genomic regions in the phylogenetic tree as well as from the N. hylocereum isolates of the Wonglom et al.12 study. It seems that the isolates Keningau and Keningau02 might be the different species from N. dimidiatum and it is suggested that they have to be examined in detail.
Although N. dimidiatum almost infects the woody plants, but there are some reports that this species causes disease on leafy plants. The N. dimidiatum has been reported as the leaf blight causal agent on white spider lily (Hymenocallis littoralis) in Malaysia, Cattleya × hybrid in Taiwan, Sansevieria trifasciata in Brazil, Origanum onites and Melissa officinalis in Turkey18,19,22,68,69. Based on our observation of the surveyed greenhouse, about 16 percent of the growing Clivia plants had the leaf blight symptom, similar to the sampled symptom, showing the importance of the emerging leaf blight disease on Clivia plants caused by N. dimidiatum. We did not investigate the special favorable conditions for the infection of the Clivia plants by the pathogen, except two temperature ranges 26–28 ℃ and 30–32 ℃. But, like any other plant disease, the leaf blight disease occurrence on Clivia might be affected by any kind of stresses such as temperature and water stresses. The effect of temperature on pathogenicity of the recovered isolate was investigated in this study. The temperature ranging from 26 to 28 ℃ could not stimulate the pathogenicity of N. dimidiatum isolate GKH-2. The temperature ranging from 30 to 32 ℃ was optimal to isolate GKH-2 to cause disease on Clivia leaves. This obtained result is in concordance to the fact that N. dimidiatum is a thermophilic fungus and tends to cause diseases in the tropical and subtropical regions14,62. The optimal temperature is an environmental factor that required to induce the pathogenicity and development of the disease by Neoscytalidium species. It has been demonstrated that the temperature is the effective factor for disease onset70. It has also been mentioned that the temperature is a critical factor for successful infection and disease severity caused by pythiaceous fungi71. It has demonstrated that the highest fig fruit colonization by N. dimidiatum occur at 30 ℃ and the mycelium of N. dimidiatum can not develop at 10 and 15 °C72.
According to the results of the pathogenicity test, the isolate GKH-2 was pathogenic on both C. miniata and C. nobilis. Regarding to the time after inoculation for symptom development on C. miniata and the severity of the symptoms, and in comparison with C. nobilis, it seems that the isolate GKH-2 was more pathogenic on C. miniata. Neoscytalidium dimidiatum is known as an opportunistic fungus that penetrates through the wounds and causes diseases under the stress conditions6,62. However, It has been mentioned that this fungus can also cause diseases in healthy hosts13,62. To our best of knowledge, this is the first report of N. dimidiatum causing leaf blight disease on C. miniata and C. nobilis, worldwide.
Data availability
Most of the datasets generated during and/or analyzed during the current study are available in the manuscript. The newly generated sequences are deposited in the GenBank (MZ047291, OQ306534 and OQ383346).
References
Nouri, M. T., Lawrence, D. P., Yaghmour, M. A., Michailides, T. J. & Trouillas, F. P. Neoscytalidium dimidiatum causing canker, shoot blight and fruit rot of almond in California. Plant Dis. 102, 1638–1647 (2018).
Phillips, A. et al. The Botryosphaeriaceae: genera and species known from culture. Stud. Mycol. 76, 51–167 (2013).
Huang, S.-K. et al. Morphology and phylogeny of Neoscytalidium orchidacearum sp. Nov. (Botryosphaeriaceae). Mycobiology 44, 79–84 (2016).
Crous, P. W. et al. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 55, 235–253 (2006).
Pavlic, D. et al. Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 100, 851–866 (2008).
Mohd, M. H., Salleh, B. & Zakaria, L. Identification and molecular characterizations of Neoscytalidium dimidiatum causing stem canker of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. J. Phytopathol. 161, 841–849 (2013).
Bakhshizadeh, M., Hashemian, H. R., Najafzadeh, M. J., Dolatabadi, S. & Zarrinfar, H. First report of rhinosinusitis caused by Neoscytalidium dimidiatum in Iran. J. Med. Microbiol. 63, 1017–1019 (2014).
Calvillo-Medina, R. P. et al. Identification and biofilm development by a new fungal keratitis aetiologic agent. Mycoses 62, 62–72 (2019).
Alizadeh, M., Safaie, N., Shams-Bakhsh, M. & Mehrabadi, M. Neoscytalidium novaehollandiae causes dieback on Pinus eldarica and its potential for infection of urban forest trees. Sci. Rep. 12, 9337 (2022).
Zhang, W. et al. Evaluating species in Botryosphaeriales. Pers. Mol. Phyl. Evol. Fungi 46, 63–115 (2021).
Crous, P. W. et al. New and interesting fungi. 4. Fungal Syst. Evol. 7, 255 (2021).
Wonglom, P., Pornsuriya, C. & Sunpapao, A. A new species of Neoscytalidium hylocereum sp. Nov. causing canker on red-fleshed dragon fruit (Hylocereus polyrhizus) in Southern Thailand. J. Fungi 9, 197 (2023).
Türkölmez, Ş, Derviş, S., Çiftçi, O., Serçe, Ç. U. & Dikilitas, M. New disease caused by Neoscytalidium dimidiatum devastates tomatoes (Solanum lycopersicum) in Turkey. Crop Prot. 118, 21–30 (2019).
Polizzi, G. et al. in II International Symposium on Citrus Biotechnology 892, 237–243.
Kee, Y. J., Suhaimi, N. N., Zakaria, L. & Mohd, M. H. Characterisation of Neoscytalidium dimidiatum causing leaf blight on Sansevieria trifasciata in Malaysia. Aust. Plant Dis. 12, 1–4 (2017).
Derviş, S., Özer, G. & Türkölmez, Ş. First report of Neoscytalidium dimidiatum causing tuber rot of potato in Turkey. J. Plant Pathol. 102, 1295–1296 (2020).
Güney, İG., Bozoğlu, T., Özer, G., Türkölmez, Ş & Derviş, S. First report of Neoscytalidium dimidiatum associated with dieback and canker of common fig (Ficus carica L.) in Turkey. J. Plant Dis. Prot. 129, 701–705 (2022).
Nadiah, N., Mohamed Nor, N., Latiffah, Z. & Masratul Hawa, M. First report of leaf blight on white spider lily caused by Neoscytalidium dimidiatum in Malaysia. New Dis. Rep. 35, 16–16 (2017).
Monteles, R. P. et al. Neoscytalidium dimidiatum causes leaf blight on Sansevieria trifasciata in Brazil. Aust. Plant Dis. Notes 15, 1–4 (2020).
Dy, K. S., Wonglom, P., Pornsuriya, C. & Sunpapao, A. Morphological, molecular identification and pathogenicity of Neoscytalidium dimidiatum causing stem canker of Hylocereus polyrhizus in southern Thailand. Plants 11, 504 (2022).
Ören, E., Palacıoğlu, G., Koca, G., Ozan, G. N. & Bayraktar, H. First report of Neoscytalidium dimidiatum causing branch dieback and canker on apple in Turkey. J. Plant Pathol. 104, 429–429 (2022).
Alkan, M., Özer, G., Koşar, İ, Güney, İG. & Derviş, S. First report of leaf blight of Turkish oregano (Origanum onites) caused by Neoscytalidium dimidiatum in Turkey. J. Plant Pathol. 104, 471–471 (2022).
Oksal, E., Yiğit, T. & Özer, G. First report of Neoscytalidium dimidiatum causing shoot blight, dieback and canker of apricot in Turkey. J. Plant Pathol. 102, 579–580 (2020).
Yi, R. H., Ling Lin, Q., Mo, J. J., Wu, F. F. & Chen, J. Fruit internal brown rot caused by Neoscytalidium dimidiatum on pitahaya in Guangdong province, China. Aust. Plant Dis. Notes 10, 1–4 (2015).
Suwannarach, N., Kumla, J. & Lumyong, S. Leaf spot on cattleya orchid caused by Neoscytalidium orchidacearum in Thailand. Can. J. Plant Path. 40, 109–114 (2018).
Aminaee, M. & Ershad, D. Die–back of young shoots of pistachio trees in Kerman province. in Proc. of 11th Plant Protection Congress, Rasht, (1987).
Heydarian, A. & Alizadeh, A. Citrus die-back and decline caused by Nattrassia mangiferae and its other hosts in Khouzestan province. in Proc. of 12th Iran. Plant Protection Congress, Karaj, P. 230 (1995).
Mirzaee, M., Mohammadi, M. & Rahimian, H. Nattrassia mangiferae, the cause of die-back and trunk cankers of Ficus religiosa and branch wilt of Psidium guajava in Iran. J. Phytopathol. 150, 244–247 (2002).
Jamali, S. & Banihashemi, Z. The pathological and physiological study of Nattrassia mangiferae the cause of shade trees decline in Shiraz city. Iran. J. Plant Pathol. 46, 105–109 (2010).
Hashemi, H. & Mohammadi, H. Identification and characterization of fungi associated with internal wood lesions and decline disease of willow and poplar trees in Iran. For. Pathol. 46, 341–352 (2016).
Nazmadini, S., Mohammadi, H. & Kazemzadeh Chakusary, M. First report of Phaeoacremonium venezuelense and Neoscytalidium hyalinum from Calligonum amoenum in Iran. in Proc. of 23th Iranian Plant Protection Congress, Gorgan (2018).
Rahimi, Z., Banihashemi, Z. & Mirtalebi, M. Two new species of Neoscytalidium the causal agents of Nattrassia canker from Iran. in Proc. of 23th Iranian Plant Protection Congress, Gorgan, p. 16 (2018).
Alidadi, A., Kowsari, M., Javan-Nikkhah, M., Jouzani, G. S. & Rastaghi, M. E. New pathogenic and endophytic fungal species associated with Persian oak in Iran. Eur. J. Plant Pathol. 155, 1017–1032 (2019).
Mirtalebi, M., Sabahi, F. & Banihashemi, Z. Fruit rot caused by Neoscytalidium hyalinum on melon in Iran. Aust. Plant Dis. Notes 14, 1–4 (2019).
Ghasemi-Sardareh, R. & Mohammadi, H. Characterization and pathogenicity of fungal trunk pathogens associated with declining of neem (Azadirachta indica A. Juss) trees in Iran. J. Plant Pathol. 102, 1159–1171 (2020).
Bagherabadi, S., Zafari, D. & Maharachchikumbura, S. S. Neoscytalidium dimidiatum as one of the fungal agents associated with walnut decline in Iran. J. Nuts 13, 31–39 (2022).
Yeganeh, S. & Mohammadi, H. Sooty canker, a destructive disease of banyan (Ficus benghalensis L.) trees in landscapes of Kish Island (Iran). Urban For. Urban Green. 72, 127573 (2022).
Kadkhoda-Hematabadi, S., Mohammadi, H. & Sohrabi, M. Morphological and molecular identification of plant pathogenic fungi associated with necrotic wood tissues of pomegranate trees in Iran. J. Plant Pathol. https://doi.org/10.1007/s42161-023-01308-1 (2023).
Musara, C., Aladejana, E. B. & Aladejana, A. E. Clivia miniata (Lindl.) Bosse, (Amaryllidaceae): Botany, medicinal uses, phytochemistry and pharmacological properties. J. Appl. Pharm. Sci. 11, 012–018 (2021).
Becker, C. A. Identification and Characterisation of Some Phytopathogens Infecting South African Indigenous Ornamental Plants (2014).
Sun, Y. et al. First report of Fusarium solani causing leaf sheath rot of Bush Lily in China. Plant Dis. 106, 1992 (2022).
Hill, C. New plant disease records in New Zealand, 1974–1982. N. Z. J. Bot. 20, 355–359 (1982).
Whitlock, V. Vol. 70 800–800 (Amer Phytopathological Soc 3340 Pilot Knob Road, St Paul, MN 55121, 1986).
Kim, W. G., Hong, S. K. & Cho, W. D. Occurrence of anthracnose on English Ivy caused by Colletotrichum trichellum in Korea. Mycobiology 29, 107–109 (2001).
Stroh, H. Pests and disease affecting Clivia in South Africa. Clivia 4, 69–80 (2002).
Moriwaki, J., Sato, T. & Tsukiboshi, T. Morphological and molecular characterization of Colletotrichum boninense sp. Nov. from Japan. Mycoscience 44, 0047–0053 (2003).
Damm, U. et al. The Colletotrichum boninense species complex. Stud. Mycol. 73, 1–36 (2012).
Braithwaite, M. et al. A survey of subtropical nursery plants for fungal diseases in Northland. N. Z. Plant Prot. 59, 132–136 (2006).
Yang, Y. et al. Colletotrichum anthracnose of Amaryllidaceae. Fungal Divers. 39, 123–146 (2009).
Damm, U., Sato, T., Alizadeh, A., Groenewald, J. Z. & Crous, P. W. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud. Mycol. 92, 1–46 (2019).
Pan, S. W. R. W. F. & Jinliang, H. L. First report of Fusarium solani and Fusarium proliferatum causing root rot of Clivia miniata in China. In Proceedings of the Academic Annual Meeting of the Chinese Society of Phytopathology (2019).
Li, Y., Yan, Z., Wang, Y. & Zhou, Z. First report of Fusarium proliferatum causing leaf sheath rot on Clivia miniata in Henan province, China. Plant Dis. 104, 1552 (2020).
Liu, L. et al. First report of Fusarium oxysporum causing basal stem rot of Clivia miniata in China. Plant Dis. 104, 1561 (2020).
Farr, D. F. & Rossman, A. Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA, https://nt.ars-grin.gov/fungaldatabases/ (2023).
Mirabolfathy, M. The incidence of anthracnose of Clivia miniata in Tehran. Iran. J. Plant Pathol 25, 1–4 (1989).
Rayner, R. W. A mycological colour chart. Mycol. Colour Chart (1970).
Zhong, S. & Steffenson, B. J. Virulence and molecular diversity in Cochliobolus sativus. Phytopathology 91, 469–476 (2001).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 18, 315–322 (1990).
Vilgalys, R. & Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246 (1990).
Carbone, I. & Kohn, L. M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91, 553–556 (1999).
Fan, X.-L., Bezerra, J. D., Tian, C. M. & Crous, P. W. Families and genera of diaporthalean fungi associated with canker and dieback of tree hosts. Pers. Mol. Phyl. Evolut. Fungi 40, 119–134 (2018).
Güney, İG., Özer, G., Türkölmez, Ş & Derviş, S. Canker and leaf scorch on olive (Olea europaea L.) caused by Neoscytalidium dimidiatum in Turkey. Crop Protect. 157, 105985 (2022).
Sadowsky, A., Solel, Z. & Sztejnberg, A. Effect of heat-stress predisposition on the development of Scytalidium wilt of, Star Ruby’grapefruit, caused by Scytalidium lignicola. Eur. J. Plant Pathol. 117, 123–127 (2007).
Gusella, G., Morgan, D. P. & Michailides, T. J. Further investigation on limb dieback of fig (Ficus carica) caused by Neoscytalidium dimidiatum in California. Plant Dis. 105, 324–330 (2021).
Sabernasab, M., Jamali, S., Marefat, A. & Abbasi, S. Morphological and molecular characterization of Neoscytalidium novaehollandiae, the cause of Quercus brantii dieback in Iran. Phytopathol. Mediterr. 58, 347–358 (2019).
Hong, C.-F., Gazis, R., Crane, J. H. & Zhang, S. Prevalence and epidemics of Neoscytalidium stem and fruit canker on pitahaya (Hylocereus spp.) in South Florida. Plant Dis. 104, 1433–1438 (2020).
Khoo, Y. W., Tan, H. T., Khaw, Y. S., Li, S.-F. & Chong, K. P. First report of Neoscytalidium dimidiatum causing stem canker on Selenicereus megalanthus in Malaysia. Plant Dis. 107, 222 (2023).
Chang, C.-W., Chen, C.-Y., Wang, C.-L. & Chung, W.-H. First report of leaf blight on Cattleya× hybrid caused by Neoscytalidium dimidiatum in Taiwan. J. Plant Pathol. 102, 921–921 (2020).
Özer, G., Günen, T. U., Koşar, İ, Güney, İG. & Derviş, S. First report of Neoscytalidium dimidiatum causing blight of Melissa officinalis in Turkey. J. Plant Dis. Prot. 129, 197–199 (2022).
Sweetingham, M., Thomas, G., Yang, H. & Shea, G. Anthracnose–the pathogen, epidemiology and the management package. Highlights Lupin Res. Dev. West. Aust. 1998, 8–9 (1998).
Raftoyannis, Y. & Dick, M. W. Effects of inoculum density, plant age and temperature on disease severity caused by pythiaceous fungi on several plants. Phytoparasitica 30, 67–76 (2002).
Gusella, G., Fiore, G., Vitale, A., Felts, D. G. & Michailides, T. J. New findings on the effects of different factors involved in fig limb dieback caused by Neoscytalidium dimidiatum in California. Eur. J. Plant Pathol. https://doi.org/10.1007/s10658-023-02685-0 (2023).
Acknowledgements
Present research has been done based on a support of University of Tehran. So, the authors pleased to appreciate the University of Tehran.
Author information
Authors and Affiliations
Contributions
Z.Z. performed the research and wrote the primary manuscript and Kh.-B.F. designed and supervised the research and revised the primary manuscript. In addition, Kh.-B.F. is corresponding author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaeimian, Z., Fotouhifar, KB. First report of Neoscytalidium dimidiatum as the causal agent of leaf blight on Clivia miniata. Sci Rep 13, 16110 (2023). https://doi.org/10.1038/s41598-023-43144-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43144-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.