Abstract
How far are species distributed on the abyssal plains? Spanning from 3000 to 6000 m below sea level, abyssal plains cover three-quarters of the ocean floor and are the largest but also least explored habitat on Earth. The question of vertical and horizontal distribution is central to understanding biogeographic and population genetic processes within species inhabiting the deep-sea benthos. Amphipod crustaceans are an important and dominant taxon in this ecosystem. As they are brooders, their dispersal capacities are more limited compared to species with free-swimming larvae, and with the exception of a few scavenging species deep-sea amphipods are restricted to a single ocean. Based on an integrative taxonomic approach (morphology, COI, 16S and 18S) we demonstrate the occurrence of a predatory amphipod species, Rhachotropis abyssalis, in three oceans: the Antarctic Ross Sea, the Northwest Pacific and the North Atlantic; regions more than 20,000 km apart. Although such extensive geographic distributions may represent a rare exception for brooding predators, these findings might also be no exception at all, but a reflection of the rare sampling and rare taxonomic investigation of invertebrate predators in the deep-sea. Our findings highlight our abysmal state of knowledge regarding biodiversity and biogeography on abyssal plains.
Similar content being viewed by others
Introduction
Abyssal plains, ranging from 3000 to 6000 m depth, make up the largest fraction of the ocean floor, with estimates to over 75%1. Despite being the largest interconnected benthic habitat, our understanding of its biodiversity and the distribution of its species is still very limited. Large-scale biodiversity assessments are still challenging for abyssal depth (e.g. 2) due to the difficult sampling process and the patchy distribution of many species (e.g.3). Because the abyss was considered as a large, homogeneous habitat it was initially assumed to harbour a rather species-poor benthic fauna with many species being geographically widespread4,5,6,7,8. This is in part attributed to the first deep-sea expeditions, which appeared to sample the same or highly similar species in various oceans9,10,11. Numerous later studies have shown that the abyssal fauna has a rich species diversity and the cosmopolitan distribution of many species is contentious (e.g.12,13,14,15,16,17). The extent of geographic range may influence species ability to recover after disturbance but at the same time wrong estimation of such range may lead to overestimation of the resilience potential of certain taxon. That is why it is crucial to correctly infer the biogeographic ranges of deep-sea species. The benthic invertebrate fauna of abyssal plains is often dominated by small megafaunal taxa with sizes of a few centimeters, like elpidiid holothurians and macrofaunal species like peracarid crustaceans or polychaetes12,13,14. These taxa can be differentiated by either having planktonic larvae, which provide a strong and wide dispersal capacity, and breeding taxa, which lack a free-swimming larval stage. Taxa with planktonic larvae can be passively dispersed over thousands of kilometers and numerous studies have identified and genetically verified examples of species with multi-oceanic distributions (e.g.12,16,17,18). Brooding taxa like Peracarida or some Polychaeta lack such dedicated dispersal stages and their dispersal and distribution is largely dependent on the mobility of the adults19. This limits the geographic distributions of brooding species, accelerates endemism and the potential for cryptic speciation20. Genetic analyses of brooding species with putative cosmopolitan distributions usually revealed assemblages of cryptic species, each with narrower geographic distributions within a single ocean21,22,23. A prominent case to highlight this is the scavenging amphipod Eurythenes gryllus (Lichtenstein in Mandt, 1822), which is one of the most intensively studied deep-sea amphipods and had been long assumed to occur globally, but molecular studies showed that it might consist of at least 15 species-level lineages24,25,26,27.
Amphipoda are one of the most abundant and taxonomically diverse groups in deep-sea benthic environments28 and are like all peracarids brooders. Multiocean distributions of deep-sea amphipods have only been confirmed genetically for scavengers29. Scavengers are particularly mobile and actively search food falls. Therefore, it is not surprising that transoceanic or cosmopolitan distributions were supported for some abyssal scavenging amphipods by genetic data19,29,30,31,32,33, e.g. Hirondellea dubia Dahl, 1959, was found in several trenches30 but also here the majority of species appear to be geographically restricted (e.g.,33).
Amphipoda of the family Eusiridae are fast moving predators with a worldwide distribution34. Most eusirid species are restricted to certain combinations of bottom water temperatures and bottom depths. For example, the investigation of the biogeographic distribution of Eusiridae species around Iceland found a marked separation along the Greenland-Iceland-Faroe (GIF) Ridge with 28 out of 36 species occurring only within a single water mass35. Here the ridge and the different water masses restricted the distribution of species. Within Eusiridae, the genus Rhachotropis contains 64 species (World Amphipoda Database36). Rhachotropis has the widest geographic (all oceans) and bathymetric (0–9460 m) distribution of all amphipod genera37,38. Rhachotropis species are found in all oceans and major basins of the world: Arctic, Atlantic Ocean, Mediterranean Sea, Caribbean Sea, Indian Ocean, Pacific Ocean and the Southern Ocean37. They have been collected in all water depths, from the shelf (e.g.35,39,40,41) to abyssal and hadal sampling sites42,43, in trenches44, as well as around hydrothermal vents45. The various Rhachotropis species show different ecological and bathymetric preferences. For example, Rhachotropis aculeata (Lepechin, 1780) has a wide temperature tolerance (−1 °C to + 6 °C), and a relatively narrow vertical distribution, 100–600 m. Whereas Rhachotropis saskia Lörz & Jażdżewska, 2018 has a molecularly confirmed vertical distribution extending three kilometers (4987–8196 m)38. Rhachotropis abyssalis Lörz, 2010 was collected on a seamount in the Ross Sea at 3380 m depth and described as species new to science in 201043. None of the seven Antarctic species was previously found in waters outside of the Southern Ocean. On a recent expedition to the North Atlantic specimens of Rhachotropis were collected that strongly resembled the Antarctic species R. abyssalis.
In our study we test the putative multi-oceanic distribution of the predatory amphipod Rhachotropis abyssalis Lörz, 2010 of the family Eusiridae via an integrative taxonomic approach. Determining whether a single geographically widely distributed species is present or multiple species with restricted geographic distributions depends highly on the researcher’s interpretation of the available data and the underlying species concept46,47,48. In this study, we started out by employing a classical taxonomic approach identifying species by diagnostic morphological characters (sensu the Phylogenetic Species Concept by Wheeler and Platnick49) to identify Rhachotropis abyssalis. This was then complemented with genetic data to test for the presence of cryptic species-level diversity. In particular for molecular genetic data, the choice of the species concept can have strong implications46,47,48. Although reproductive isolation (sensu the Biological Species Concept50) would have been the desired criterion, this criterion can not be employed here as the populations occur in extreme allopatry (thousands of kilometers apart). If species and/or populations occur in sympatry, reproductive isolation can be inferred genetically from mito-nuclear concordance (see48). We therefore follow a more general Evolutionary Species Concept for the integrative approach that emphasizes that a species “maintains its identity from other such entities through time and over space and that has its own independent evolutionary fate and historical tendencies”51. Here extensive genetic differentiation irrespective of the geographic distribution may suffice to differentiate species (see also52).
The hypothesis to be tested via integrative taxonomy: One species of amphipod, a brooding predator, occurs in multi-oceans in abyssal depth.
Results
Morphology
Amphipoda specimens sampled during IceDIVA2 by RV Sonne in the Labrador Sea and North Atlantic strongly resembled specimens collected in the Ross Sea described as Rhachotropis abyssalis (Fig. 1). Morphological investigations of the defining characters used to separate species of studied genus, such as the rostrum being longer than the head, antenna as long as body, eyes absent, first coxa produced, smooth pereonites, pereopod 7 longer than body, all pleonites bearing dorsal process, telson cleft, long narrow showed no main differences of the specimens of R. abyssalis from the different oceans. The species specific morphological characters clearly defined all specimens as Rhachotropis abyssalis. Minute morphological differences between the Ross Sea and the North Atlantic specimens (Fig. 2) remain in the range of intraspecific variations. The different appearance of Ross Sea versus Atlantic specimens is due to the fixation process (the position when the animals were preserved); proportions of the maxilliped article 2 to 3 are the same (Fig. 2a,b). The coxa 1 reaches the end of the head and is weakly pointy (Fig. 2e,f); the head of the Atlantic specimens seems slightly longer because the animal is bent more to its dorsal side. The proportions of article 2 and 3 of antenna 1 are the same in Ross Sea and Atlantic specimens (Fig. 2e,f). While the palm, propodus and dactylus of gnathopod 1, show no differences amongst the specimens, the carpus is slightly more extended in the Ross than in the Atlantic specimens (Fig. 2g,h). Uropod 1 and uropod 2 showed the same proportions of peduncle to rami lengths in the Ross Sea as in the Atlantic specimens (Fig. 2c,d). No further morphological differences were discovered in the Pacific specimens when comparing them to the Atlantic material and the type specimens from the Ross Sea.
Sequencing output
In total 19 new sequences of three genes were obtained. They were uploaded to GenBank under accession numbers: COI: OQ622273-OQ622280, 16S: OQ622391-OQ622399, 18S: OQ622283-OQ622285. Relevant voucher information, taxonomic classifications, and sequences of all studied genes are deposited in the dataset “DS-RHABYSS” in the Barcode of Life Data System (BOLD) (www.boldsystems.org)47. A summary of all data (including collection data and GenBank accession numbers) is available in the Table 1. The attempts to obtain sequences of the 16S and 18S gene fragment from the type material of Rhachotropis abyssalis from the Ross Sea failed.
In COI, the North Atlantic and the Ross Sea populations of R. abyssalis each feature a single haplotype, whereas the Northwest Pacific (NW Pacific) population features four haplotypes separated by one or two mutations from each other (Fig. 3). Similarly, in 16S a single North Atlantic haplotype and two NW Pacific haplotypes (separated by one mutation) are recovered. The 18S sequences differ by three mutations around position 200 of the alignment where one transversion and two-nucleotide deletion is observed for the NW Pacific individual. COI genetic p-distances between NW Pacific and Ross Sea haplotypes are 1.56% (nine mutations) and 2.42–2.60% (14 or 15 mutations) between these and the North Atlantic haplotypes. In 16S, the p-distance was 1.79–2.05% (7 or 8 mutations) between NW Pacific and North Atlantic haplotypes. In the Bayesian analyses (Fig. 4, Supplementary Fig. S1) all R. abyssalis sequences cluster closely together and are genetically clearly differentiated from all other Rhachotropis species.
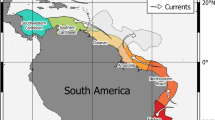
Geographic and molecular distance of the abyssal amphipod Rhachotropis abyssalis (A) Distribution of Rhachotropis abyssalis. Top left—world, top right—Northwest Pacific, bottom right—Antarctic, bottom left—North Atlantic. Pink diamond indicates the record from Ocean Biogeographic Information System (OBIS) for which neither voucher nor the sequence was available for the authors. (B) Median Joining networks of COI, 16S and 18S haplotypes of R. abyssalis with indication of their geographic origin. Code after area presents station code. The approximate geographic distances between the studied regions are given on the side of arrows.
Bayesian tree of COI sequences of all amphipods identified as Rhachotropis as well as identified to higher taxonomic levels but ascribed to BINs belonging to studied genus available in BOLD and GenBank. NWP – Northwest Pacific, NA – North Atlantic, RS – Ross Sea, Antarctic. The tree branches collapsed following the BINs ascription. Number after the name indicates the BIN, followed by the number of sequences and haplotypes for each branch. Right panel shows the results of species delimitation. BIN: Barcode Index Numbers by BOLD, ASAP: Assemble Species by Automatic Partitioning (the species partitions were identical for the K2P and p-distances), sGMYC: general mixed Yule Coalescent with single threshold, mGMYC: general mixed Yule Coalescent with multiple thresholds. Rhachotropis abyssalis shown on a yellowish background.
Species delineation
In Assemble Species by Automatic Partitioning (ASAP) as well as both Generalized Mixed Yule Coalescent (GMYC) analyses, the three R. abyssalis populations were grouped into a single species in the best-scoring partition (Fig. 4), only ASAP partitions employing COI thresholds < 1.5% (while having low ASAP-scores) separated the three populations into different species. By contrast, BOLD ascribed the sequences of the three populations to three different BINs: BOLD:AAC9695 (Ross Sea), BOLD:ADF6532 (NW Pacific), BOLD:AFA1318 (North Atlantic) (Fig. 4).
Discussion
Our detailed morphological and molecular genetic investigations confirm the cosmopolitan distribution of Rhachotropis abyssalis in the Ross Sea, the Northwest Pacific and North Atlantic.
The observed morphological variation and genetic distances between the three R. abyssalis populations fall within the intraspecific morphological variability of Rhachotropis species38,39 and the genetic variability typically observed within crustaceans including marine amphipods (e.g.,37,53,54). Notably, interspecific distances for closely related amphipod and isopod (peracarid crustaceans with similar life-history traits) species usually exceed 3% by far, in most cases even 5 or >10% [e.g.,37,54,55,56,57]. Only BINs suggested that these represent three distinct species and over-splitting of species by BINs has been reported for amphipods38,58,59. Treating these three geographically extremely divergent populations as a single species is, therefore, supported by classical morphology-based taxonomic approaches as well by molecular genetic approaches employing an Evolutionary Species Concept51.
The occurrence in the Ross Sea, the Northwest Pacific and North Atlantic is an extensive geographic distribution with at least 8400, 14,000 and 20,000 km separating the three studied populations. To our knowledge, this is the first record of a benthic predator with a brooding life-style that exhibits such a geographically widespread, multi-oceanic distribution in the abyss. How R. abyssalis achieved this extensive distribution remains an open question. Rhachotropis species are known to be good swimmers37,53. However, as predators they are likely to have bursts of fast swimming rather than swimming continuously over long distances. Amphipods of the genus Rhachotropis have slender bodies and long antennae, their long and skinny legs imply that they stalk over soft sediment, which contrasts with lysianassoid scavengers whose compact bodies, short legs and short antennae are better suited for swimming. Therefore, it is highly unlikely that the geographic distribution observed herein is the result of single long-distance dispersal events across thousands of kilometers. Continuous range expansion is more plausible and suggests that R. abyssalis occurred and probably still occurs in other regions of the vast abyssal plains. Not only geographic distance itself, also geologic structures like ridges or trenches have been shown to be effective dispersal barriers for other brooding amphipods and isopods22,60,61,62. Rhachotropis abyssalis must have either crossed or sidestepped such dispersal barriers to occur multi-oceanic. Taylor and Roterman63 have pointed out that hardly any population genetic studies have been carried out for benthic deep-sea invertebrates, greatly limiting our understanding of population genetic and phylogeographic processes in this vast ecosystem. Detailed population genetic analyses could not be performed for R. abyssalis due to the limited number of available populations and individuals. It is a common problem for many deep-sea benthic taxa resulting among others from their rarity and patchiness of distribution64.
It should be borne in mind that our results are based on three geographically disjunct populations, the actual distribution of the species may be much more continuous. Rhachotropis abyssalis probably has been overlooked in other regions as the overall sampling activities in the abyss are poor and predators like R. abyssalis probably have rather low population densities and are not attracted by baited traps like scavengers.
An additional Ocean Biodiversity Information System (OBIS) based record of R. abyssalis from north of Norway and the depth of 770 m (Fig. 3) has to be treated with caution. We did not have access to the voucher specimen, OBIS does not provide the name of its identifier and any genetic data are available so we were unable to verify it. Caution is particularly required because the cited record would greatly extend the presently confirmed vertical distribution of R. abyssalis (currently known from ~2300 vertical meters to ~4900 vertical meters).
It is interesting to note that the NW Pacific population is the genetically most diverse and divergent population; especially in contrast to the North Atlantic population, which is genetically uniform (despite 1200 km between sampling stations) and genetically closer to the Ross Sea population (despite being separated by 20,000 km, the largest geographic distance in our study). This may imply later and more recent colonisation of the North Atlantic and an origin of R. abyssalis in the Pacific, though this is highly speculative with the limited data available. We assume that the populations of R. abyssalis are not only geographically but also genetically more interconnected and continuous than currently evident. It is expected that future research expeditions may uncover additional populations of the species in other regions not studied here.
Many known deep-sea amphipods are scavengers, though this is biased by the sampling methods. Collection of these Amphipoda in abyssal and hadal depths is comparatively easier and more cost-effective as they are preferentially attracted and caught in large numbers reaching sometimes thousands of individuals per trap16,28,29,30,31. As a consequence, large portion of more thorough studies of deep-sea Amphipoda focused mainly on scavengers (e.g.,27,65,66,67,68,69). Another classic gear deployed in abyssal plains was a boxcorer, which is often missing fast swimming epibenthic taxa like predatory amphipods70. Deep-sea predatory amphipods, such as Rhachotropis, are mainly caught via epibenthic sledges34,38,57,71,72. Deploying dragged gear with small mesh-sizes in the deep sea is far more time consuming—and therefore more expensive—than using still gear such as boxcores or traps, but yields often more than 90% of species new to science (e.g. 70,72). Abyssal plains are generally undersampled, but especially for mobile predatory invertebrates our knowledge regarding the diversity and biogeographic connections is abysmal.
The IUCN (the International Union for Conservation of Nature) World Conservation Congress called for the protection of at least 30% of each marine habitat globally and at least 30% of all the ocean for worldwide effective marine biodiversity conservation by 203071,73,74,75. One of the localities from which we newly reported R. abyssalis is the North Atlantic Current and Evlanov Sea Basin (NACES) Marine Protected Area (MPA)—a 600,000 km2 area. Our finding of R. abyssalis highlights how poorly explored the marine deep-sea benthos still is, even such a widely distributed species as R. abyssalis was unknown for the whole Pacific and Atlantic Oceans including the NACES marine protected area.
Materials and methods
Amphipod recovery and morphological identification
The study is based on the amphipods collected during three expeditions: TANGAROA to the Antarctic (Ross Sea)76, KuramBio I conducted in the Kuril-Kamchatka Trench area (Northwest Pacific [NW Pacific])77 and IceDIVA2 in the Labrador Sea and North Atlantic78. Samples were taken by a camera-epibenthic sledge (C-EBS) at abyssal depths79,80 and an epibenthic-sledge (EBS)81. Both gears are equipped with supra- and epibenthic samplers possessing two plankton nets (500 µm) on top of each other leading to two cod ends (300 µm). All samples were fixed in precooled (− 20 °C) undenatured 96% ethanol and treated as described in 82. Large amphipod specimens were immediately sorted on deck, fixed in − 20° precooled 98% ethanol and later transferred to 96% ethanol. Specimens were photographed immediately after sampling. Samples collected in the NW Pacific were photographed after preservation in 96% ethanol, therefore no coloured pigments remained. Amphipods were measured from the tip of the rostrum to the end of the telson. Specimens and/or extractions are stored at the NIWA collection Wellington (New Zealand), University of Lodz (Poland), the Hamburg Zoological Museum (Germany) and Natural History Museum Vienna (Austria) (see Supplementary Material S1).
DNA barcoding
Molecular analysis was based on the type material of R. abyssalis from the Ross Sea (two individuals), nine individuals obtained during IceDIVA2 from North Atlantic and six individuals collected during KuramBio I from the NW Pacific. Sequence data of the Rhachotropis abyssalis was either already published or produced de novo. Three genes, two mitochondrial (cytochrome c oxidase subunit I [COI] and 16S rRNA) and one nuclear (18S rRNA) were studied, the two sequences of the Ross Sea material and seven sequences of the NW Pacific material had been published previously37,71.
The DNA was extracted from one pleopod of fixed museum material. The pleopod was transferred into 1 M Tris-HCL (pH 7.5) for 10 minutes to wash ethanol from the tissue. The pleopod was then transferred into a new vial of 45 µl Tris-HCL. 5 µl proteinase K was added to the vial and left for 24 h on a shaker at 56 °C and 300 rpm. The DNA was extracted from the vial with magnetic beads (Steinbrenner, MagSi DNA Beads). The standard beads cleanup protocol was used with a 1.8x ratio to gather most of the DNA, but excluded the smallest fragments. The DNA was then eluted in 20 µl ultrapure water and the concentration measured on a Qubit 3.0 Fluorometer. Each sample gained approximately 100 ng of DNA.
The COI was amplified for the newly obtained material with the LCO1490-JJ/HCO2198-JJ [5′-CHACWAAYCATAAAGATATYGG/5′-AWACTTCVGGRTGVCCAAARAATCA] primer pair83 and the reaction conditions described in Hou et al.84. The 16S rRNA gene was amplified using 16SFt_amp/16SRt_amp2 [5′GCRGTATIYTRACYGTGCTAAGG/5′-CTGGCTTAAACCGRTYTGAACTC]59 and the reaction conditions as presented by Lörz et al.38. The amplification of 16S was done for four individuals from the North Atlantic and four individuals collected in the NW Pacific. The nuclear 18S rRNA gene of two individuals from North Atlantic, and one individual from NW Pacific was amplified with the 18SF/18SR [5′-CCTAYCTGGTTGATCCTGCCAGT/5′-TAATGATCCTTCCGCAGGTT] primer pair described together with the established PCR protocol by Englisch et al.85. In some cases additional forward (18S4F, 5′-CCAAGGAAGRCAGCAGGCACG) and reverse (18S2R, 5′-GAGTCCCGTGTTGAGTCAATTAAGC) primers were used86.
Sequences were obtained by Macrogen Europe, the Netherlands on the Applied Biosystems 3730xl capillary sequencer. Sequencing was bidirectional (COI and 16S of North Atlantic individuals) or done only in forward direction (all 18S and 16S of NW Pacific individuals). All chromatograms were visually inspected, primer sequences were trimmed and sequencing errors corrected in Geneious 10.1.2. The sequences were initially blasted using default parameters on NCBI BLAST and in case of the COI gene translated into amino acid sequences to confirm that no stop codons were present.
Data assembly and analysis
The newly obtained sequences were supplemented by the published data and produced three separate datasets, each representing one gene. Separate alignments for each gene were performed with MAFFT 787,88 using the G-INS-i algorithm.
All COI and 16S sequences for Rhachotropis available in GenBank89 and BOLD90 were downloaded and aligned (in BOLD also all unidentified sequences assigned to Rhachotropis BINs91 were included) to assess the differentiation of R. abyssalis form all other Rhachotropis species. The final COI alignment was 578 bp in length and consisted of 194 sequences representing 28 Operational Taxonomic Units (OTUs) while the 16S alignment was 391 bp long (43 sequences, 19 OTUs). For both datasets the sequences of the same individual of Cleonardo neuvillei, species representing another genus of the family Eusiridae, were used (COI - MZ197284.1, 16S - MZ197459). To visualize the genetic differentiation from all other Rhachotropis species, a Bayesian Inference phylogenetic analyses92 was performed for each gene separately (please note, it is not our intention to resolve the internal phylogeny within Rhachotropis, this would require a more extensive set of genetic markers) using MrBayes 3.2.793. Bayesian analyses were run for 10*106 generations, with nruns=4 and chains=6, sampled every 1000th generation, discarding the first 25% as burn-in. Neighbor-Joining tree utilizing the p-distance and pairwise deletion option was calculated for each gene in MEGA X94. All graphics were adjusted for presentation in the software Adobe®Illustrator®CS6.
Pairwise genetic distances (uncorrected p-distances) were computed with MEGA X94. The delimitation of species was done using several distance- and tree-based methods and the COI dataset. Distance-based methods included Barcode Index Number [BIN] System as part of BOLD91 and Assemble Species by Automatic Partitioning (ASAP;95). BINs represent operational taxonomic units (OTUs) and are generated in BOLD after sequences are submitted via distance-based algorithms (single linkage clustering followed by Markov clustering). ASAP uses pairwise genetic distances to assemble individuals into groups and proposes species partitioning ranked according to a scoring system95. ASAP was conducted on the COI alignment using simple p-distance and K2P distances, respectively. We employed the general mixed Yule Coalescent (GMYC) as a tree-based approach for species delimitation96. The required ultrametric tree for GMYC was obtained by running a phylogenetic analysis in BEAST v2.4.692, employing the GTR model of evolution and a Yule coalescent prior and running the analysis for 25*106 generations. Each COI haplotype was included only once. Convergence was assessed with Tracer v1.797 and the first 10% of retained trees discarded as burn-in. GMYC was run in RStudio98 with the “single” and “multiple” options.
To best visualise molecular divergence in relation to the geographic distribution within and between populations, Median Joining Networks were generated in PopART 1.799 for COI and 16S. The geographic distance between the three populations was estimated with consideration of deep-sea bottom currents proposed by Stow et al.100.
Data availability
The datasets generated and/or analysed during the current study are available in the Genbank repository, COI accession numbers: OQ622273—OQ622280; 16S accession numbers OQ622391-OQ622399; 18S accession numbers: OQ622283-OQ622285.
References
Ramirez-Llodra, E. et al. Deep diverse and definitely different unique attributes of the world’s largest ecosystem. Biogeosciences 7, 2851–2899. https://doi.org/10.5194/bg-7-2851-2010 (2010).
Howell, K. L. et al. A decade to study deep-sea life. Nat. Ecol. Evol. 5, 265–267. https://doi.org/10.1038/s41559-020-01352-5 (2021).
Brandão, S. N. & Yasuhara, M. Challenging deep-sea cosmopolitanism: taxonomic re-evaluation and biogeography of ‘Cythere dasyderma Brady, 1880’ (Ostracoda). J. Micropalaeontol. 32, 109–122. https://doi.org/10.1144/jmpaleo2012-009 (2013).
Etter, R. J. et al. Phylogeography of a pan-Atlantic abyssal protobranch bivalve: implications for evolution in the Deep Atlantic. Mol. Ecol. 20, 829–843. https://doi.org/10.1111/j.1365-294X.2010.04978.x (2011).
Brandt, A. Zur Besiedlungsgeschichte des antarktischen Schelfes am Beispiel der Isopoda (Crustacea, Malacostraca) = Colonization of the antarctic shelf by the isopoda (Crustacea, Malacostraca), Berichte zur Polarforschung (Reports on Polar Research) 98, https://doi.org/10.2312/BzP_0098_1991 (1991).
Wägele, J. W. Polymorphism and distribution of Ceratoserolis trilobitoides (Eights, 1833) (Crustacea, Isopoda) in the Weddell Sea and synonymy with C. cornuta (Studer, 1879). Polar Biol. 6, 127–137. https://doi.org/10.1007/BF00274875 (1986).
Vinogradova, N. G. Zoogeography of the Abyssal and Hadal Zones in Adv. Mar. Biol. 32, https://doi.org/10.1016/S0065-2881(08)60019-X (Academic Press, Cambridge, MA, USA, 1997), pp. 325–387.
Bruun, A. F. “Chapter 22: Deep sea and abyssal depths“ in Treatise on Marine Ecology and Paleoecology, https://doi.org/10.1130/MEM67V1-p641 (Geological Society of America, 1957), pp. 641–673.
Malyutina, M. V., Frutos, I. & Brandt, A. Diversity and distribution of the deep-sea Atlantic Acanthocope (Crustacea, Isopoda, Munnopsidae), with description of two new species, Deep. Sea Res. Part II Top. Stud. Oceanogr. 148, 130–150. https://doi.org/10.1016/j.dsr2.2017.11.003 (2018).
Etter, R. J., Rex, M. A., Chase, M. R. & Quattro, J. M. Population differentiation decreases with depth in deep-sea bivalves. Evolution 59, 1479–1491. https://doi.org/10.1111/j.0014-3820.2005.tb01797.x (2005).
Barnard, J. Gammaridean Amphipoda from Depths of 400 to 6000 Meters. Galathea Rep. 5, 23–128 (1961).
Flores, P. C. R., Seid, C. A., Rouse, G. W. & Giribet, G. Cosmopolitan abyssal lineages? A systematic study of East Pacific deep-sea squat lobsters (Decapoda: Galatheoidea: Munidopsidae). Invertebr. Syst. 37(1), 14–60. https://doi.org/10.1071/IS22030 (2023).
Sigwart, J. D. et al. Heterogeneity on the abyssal plains: A case study in the Bering Sea. Front. Mar. Sci. https://doi.org/10.3389/fmars.2022.1037482 (2023).
Hutchings, P. & Kupriyanova, E. Cosmopolitan polychaetes–fact or fiction? Personal and historical perspectives. Invertebr. Syst. 32, 1–9. https://doi.org/10.1071/IS17035 (2018).
Horton, T. et al. Molecular phylogenetics of deep-sea amphipods (Eurythenes) reveal a new undescribed species at the porcupine abyssal plain, North East Atlantic Ocean. Prog. Ocean. 183, 102292. https://doi.org/10.1016/j.pocean.2020.102292 (2020).
Guggolz, T., Meißner, K., Schwentner, M. & Brandt, A. Diversity and distribution of Laonice species (Annelida: Spionidae) in the tropical North Atlantic and Puerto Rico Trench. Sci. Rep. 9, 9260. https://doi.org/10.1038/s41598-019-45807-7 (2019).
Lindley, J. Continuous plankton records: The geographical distribution and seasonal cycles of decapod crustacean larvae and pelagic post-larvae in the north-eastern Atlantic Ocean and the North Sea. J. Mar. Biol. Assoc. 67(1), 145–167. https://doi.org/10.1017/S0025315400026424 (1987).
McManus, M. A. & Woodson, C. B. Plankton distribution and ocean dispersal. J. Exp. Biol. 215(6), 1008–1016. https://doi.org/10.1242/jeb.059014 (2012).
Thurston, M. Scavenging abyssal amphipods from the North-East Atlantic ocean. Mar. Biol. 51, 55–68 (1979).
Pearse, J. S., Bosch, I. Brooding in the Antarctic: Östergren had it nearly right“ in Echinoderms through time, David, B., Guille, A., Feral, J.-P., Roux, M. Eds. (B. Balkema, Rotterdam, 1994) pp. 111–120.
Brandt, A., Blazewicz-Paszkowycz, M., Bamber, R. N., Mühlenhardt-Siegel, U. & Havermans, C. Are there widespread peracarid species in the deep sea (Crustacea: Malacostraca)? Pol. Polar Res. 33, 139–162. https://doi.org/10.2478/v10183-012-0012-5 (2012).
Weston, J. N. et al. Barriers to gene flow in the deepest ocean ecosystems: Evidence from global population genomics of a cosmopolitan amphipod. Sci. Adv. 8(43), 6672. https://doi.org/10.1126/sciadv.abo6672 (2022).
Brix, S. et al. Molecular species delimitation and its implications for species descriptions using desmosomatid and nannoniscid isopods from the VEMA fracture zone as example taxa. Deep. Sea Res. Part II 148, 180–207. https://doi.org/10.1016/j.dsr2.2018.02.004 (2018).
Havermans, C. et al. Genetic and Morphological Divergences in the Cosmopolitan Deep-Sea Amphipod Eurythenes gryllus Reveal a Diverse Abyss and a Bipolar Species. PLoS ONE 8, e74218. https://doi.org/10.1371/journal.pone.0074218 (2013).
Havermans, C. Have we so far only seen the tip of the iceberg? Exploring species diversity and distribution of the giant amphipod Eurythenes. Biodiversity 17, 12–25. https://doi.org/10.1080/14888386.2016.1172257 (2016).
d’Udekem d’Acoz, C. & Havermans, C. Contribution to the systematics of the genus Eurythenes S.I. Smith in Scudder, 1882 (Crustacea: Amphipoda: Lysianassoidea: Eurytheneidae). Zootaxa 3971, 1–80. https://doi.org/10.11646/zootaxa.3971.1.1 (2015).
Ritchie, H., Jamieson, A. J. & Piertney, S. B. Phylogenetic relationships among hadal amphipods of the Superfamily Lysianassoidea: Implications for taxonomy and biogeography. Deep Sea Res. Part I 105, 119–131. https://doi.org/10.1016/j.dsr.2015.08.014 (2015).
Frutos, I., Brandt, A., Sorbe, J. C. Deep-sea suprabenthic communities: The forgotten biodiversity in Marine Animal Forests, Rossi, S., Bramanti, L., Gori, A., Orejas, C. Eds., https://doi.org/10.1007/978-3-319-21012-4_21 (Springer International Publishing, Cham, 2017), pp. 475–503.
Weston, J. N. J. & Jamieson, A. J. The Multi-Ocean Distribution of the Hadal Amphipod, Hirondellea dubia Dahl, 1959 (Crustacea, Amphipoda). Front. Mar. Sci. https://doi.org/10.3389/fmars.2022.824640 (2022).
Blankenship, L. E., Yayanos, A. A., Cadien, D. B. & Levin, L. A. Vertical zonation patterns of scavenging amphipods from the Hadal zone of the Tonga and Kermadec Trenches. Deep. Sea Res. Part I 53, 48–61. https://doi.org/10.1016/j.dsr.2005.09.006 (2006).
Horton, T. et al. Are abyssal scavenging amphipod assemblages linked to climate cycles?. Prog. Ocean. 184, 102318. https://doi.org/10.1016/j.pocean.2020.102318 (2020).
Duffy, G. A., Gutteridge, Z. R. S., Thurston, M. H. & Horton, T. A comparative analysis of canyon and non-canyon populations of the deep-sea scavenging amphipod Paralicella caperesca. J. Mar. Biol. Assoc. 1, 1–13. https://doi.org/10.1017/S0025315415002064 (2015).
Kniesz, K., Jażdżewska, A. M., Martínez Arbizu, P. & Kihara, T.-C. DNA barcoding of scavenging amphipod communities at active and inactive hydrothermal vents in the Indian Ocean. Front. Mar. Sci. https://doi.org/10.3389/fmars.2021.752360 (2022).
Lörz, A.-N. Deep-Sea Rhachotropis (Crustacea: Amphipoda: Eusiridae) from New Zealand and the Ross Sea with key to the Pacific Indian Ocean and Antarctic species. Zootaxa https://doi.org/10.5281/zenodo.195450 (2010).
Weisshappel, J. B. Distribution and diversity of the hyperbenthic amphipod family Eusiridae in the different seas around the Greenland-Iceland-Faeroe-Ridge. Sarsia 85, 227–236. https://doi.org/10.1080/00364827.2000.10414575 (2000).
Horton, T. et al. World Amphipoda Database. Rhachotropis S.I. Smith,. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=101528 on 2023-07-13 (2023).
Lörz, A.-N., Linse, K., Smith, P. J. & Steinke, D. First molecular evidence for underestimated biodiversity of Rhachotropis (Crustacea, Amphipoda), with description of a new species. PLoS ONE 7(3), e32365. https://doi.org/10.1371/journal.pone.0032365 (2012).
Lörz, A.-N., Jażdżewska, A. M. & Brandt, A. A new predator connecting the abyssal with the hadal in the Kuril-Kamchatka Trench, NW Pacific. PeerJ 6, e4887. https://doi.org/10.7717/peerj.4887 (2018).
d’Udekem d’Acoz, C., Vader, W., Legeżińska, J. On a diminutive Rhachotropis species from the North Sea, with a key to European Rhachotropis (Crustacea, Amphipoda, Eusiridae), Bollettino del Museo Civico di Storia Naturale di Verona 31, 31–49 (2007).
Lowry, J. K. & Springthorpe, R. T. New calliopiid and eusirid amphipods from eastern Australian waters (Crustacea: Amphipoda: Calliopiidae: Eusiridae). Proc. Biol. Soc. Wash. 118, 38–47 (2005).
Lörz, A.-N. An enigmatic Rhachotropis (Crustacea: Amphipoda: Eusiridae) from New Zealand. Zootaxa 4006(2), 383–391 (2015).
Thurston, M. H. Abyssal benthic Amphipoda (Crustacea) from the East Iceland basin. Bull. Br. Museum (Nat. History) 38(1), 43–67 (1980).
Lörz, A.-N. Deep-Sea Rhachotropis (Crustacea: Amphipoda: Eusiridae) from New Zealand and the Ross Sea with key to the Pacific Indian Ocean and Antarctic species. Zootaxa 254, 2482. https://doi.org/10.5281/zenodo.195450 (2010).
Dahl, E. Amphipoda from depths exceeding 6000 m, Galathea reports, 211–240 (1959).
Bellan-Santini, D. Rhachotropis species (Crustacea: Amphipoda: Eusiridae) of hydrothermal vents and surroundings on the Mid-Atlantic Ridge, Azores Triple Junction zone. J. Nat. Hist. 40(23–24), 1407–1424. https://doi.org/10.1080/00222930600956809 (2006).
Tan, D. S. H., Ali, F., Kutty, S. H. & Meier, R. The need for specifying species concepts: how many species of silvered langurs (Trachypithecus cristatus group) should be recognized?. Mol. Phylogenet. Evol. 49, 688–689 (2008).
Padial, J. M. & de la Riva, I. A response to recent proposals for integrative taxonomy. Biol. J. Linn. Soc. 101, 747–756 (2010).
Schwentner, M. et al. Cyclestheria hislopi (Crustacea: Branchiopoda): A group of morphologically cryptic species with origins in the Cretaceous. Mol. Phylogenet. Evol. 66, 80–810. https://doi.org/10.1016/j.ympev.2012.11.005 (2013).
Wheeler, Q. D. & Platnick, N. I. The phylogenetic species concept (sensu Wheeler and Platnick). In Species concepts and phylogenetic theory (eds Wheeler, Q. D. & Meier, R.) 55–69 (Columbia University Press, New York, 2000).
Mayr, E. Systematics and the Origin of Species from the Viewpoint of a Zoologist (Columbia University Press, 1942).
Wiley, E. O. & Mayden, R. L. The evolutionary species concept. In Species concepts and phylogenetic theory (eds Wheeler, Q. D. & Meier, R.) 70–89 (Columbia University Press, New York, 2000).
Schwentner, M., Timms, B. V. & Richter, S. Evolutionary systematics of the Australian Eocyzicus fauna (Crustacea: Branchiopoda: Spinicaudata) reveals hidden diversity and phylogeographic structure. J. Zoolog. Syst. Evol. Res. 52, 15–31. https://doi.org/10.1111/jzs.12038 (2014).
Cartes, J. E. & Sorbe, J. C. Deep-water amphipods from the Catalan Sea slope (western Mediterranean): Bathymetric distribution, assemblage composition and biological characteristics. J. Nat. Hist. 33, 1133–1158. https://doi.org/10.1080/002229399299978 (1999).
Costa, F. O., DeWaard, J. R., Boutillier, J., Ratnasingham, S. & Hebert, P. D. N. Biological identifications through DNA barcodes: The case of the Crustacea. Can. J. Fish. Aquat. Sci. 64, 272–295. https://doi.org/10.1139/f07-008 (2007).
Schwentner, M. & Lörz, A.-N. Population genetics of cold-water coral associated Pleustidae (Crustacea, Amphipoda) reveals cryptic diversity and recent expansion off Iceland. Mar. Ecol. 42, e12625. https://doi.org/10.1111/maec.12625 (2021).
Riehl, T., Lins, L. & Brandt, A. The effects of depth, distance, and the Mid-Atlantic Ridge on genetic differentiation of abyssal and hadal isopods (Macrostylidae). Deep Sea Res. Part 148, 74–90 (2018).
Brix, S. et al. Molecular species delimitation and its implications for species descriptions using desmosomatid and nannoniscid isopods from the VEMA fracture zone as example taxa. Deep Sea Res. Part II 148, 180–207 (2018).
Tempestini, A., Rysgaard, S. & Dufresne, F. Species identification and connectivity of marine amphipods in Canada’s three oceans. PLoS ONE 13, e0197174. https://doi.org/10.1371/journal.pone.0197174 (2018).
Lörz, A.-N., Tandberg, A. H. S., Willassen, E. & Driskell, A. Rhachotropis (Eusiroidea, Amphipoda) from the North East Atlantic. ZooKeys 731, 75–101. https://doi.org/10.3897/zookeys.731.19814 (2018).
Bober, S., Brix, S., Riehl, T., Schwentner, M. & Brandt, A. Does the mid-atlantic ridge affect the distribution of abyssal benthic crustaceans across the Atlantic Ocean?. Deep. Sea Res. Part II(148), 91–104. https://doi.org/10.1016/j.dsr2.2018.02.007 (2018).
Bober, S., Riehl, T., Henne, S. & Brandt, A. New Macrostylidae (Isopoda) from the Northwest Pacific Basin described by means of integrative taxonomy with reference to geographical barriers in the abyss. Zool. J. Linn. Soc. 182(3), 549–603 (2018).
Bober, J., Brandt, A., Frutos, I. & Schwentner, M. Diversity and distribution of Ischnomesidae (Crustacea: Isopoda: Asellota) along the Kuril-Kamchatka Trench–a gentic perspective. Prog. Oceanogr. 178, 102174. https://doi.org/10.1016/j.pocean.2019.102174 (2019).
Taylor, M. L. & Roterman, C. N. Invertebrate population genetics across Earth’s largest habitat: The deep-sea floor. Mol. Ecol. 26(19), 4872–4896 (2017).
Kaiser, S., Barnes, D. K. & Brandt, A. Slope and deep-sea abundance across scales: Southern Ocean isopods show how complex the deep sea can be. Deep Sea Res. Part II(54), 1776–1789. https://doi.org/10.1016/j.dsr2.2007.07.006 (2007).
Shulenberger, E. & Hessler, R. R. Scavenging abyssal benthic amphipods trapped under oligotrophic central North Pacific Gyre waters. Mar. Biol. 28, 185–187. https://doi.org/10.1007/BF00387296 (1974).
Shulenberger, E. & Barnard, J. L. Amphipods from an abyssal trapset in the North Pacific Gyre. Crustaceana 31, 241–258. https://doi.org/10.1163/156854076X00035 (1976).
Barnard, J. L. & Ingram, C. L. Lysianassoid Amphipoda (Crustacea) from deep-sea thermal vents. Smithson. Contrib. Zool. 449, 1–80. https://doi.org/10.5479/si.00810282.499 (1990).
De Broyer, C., Nyssen, F. & Dauby, P. The crustacean scavenger guild in Antarctic shelf, bathyal and abyssal communities. Deep Sea Res. II 51(14–16), 1733–1752 (2004).
Horton, T., Thurston, M. H. & Duffy, G. A. Community composition of scavenging amphipods at bathyal depths on the Mid-Atlantic Ridge. Deep Sea Res. Part II 98(Part B), 352–359. https://doi.org/10.1016/j.dsr2.2013.01.032 (2013).
Frutos, I., Kaiser, S., Pułaski, Ł, Studzian, M. & Błażewicz, M. Challenges and advances in the taxonomy of deep-sea peracarida: From traditional to modern methods. Front. Mar. Sci. 9, 154. https://doi.org/10.3389/fmars.2022.799191 (2022).
Jażdżewska, A. M. & Mamos, T. High species richness of Northwest Pacific deep-sea amphipods revealed through DNA barcoding. Prog. Oceanogr. 178, 102184. https://doi.org/10.1016/j.pocean.2019.102184 (2019).
Kaiser, S. et al. Community structure of abyssal macrobenthos of the South and equatorial Atlantic Ocean–identifying patterns and environmental controls. Deep Sea Res. Part I 197, 104066. https://doi.org/10.1016/j.dsr.2023.104066 (2023).
Zhao, Q. et al. Where marine protected areas would best represent 30% of ocean biodiversity. Biol. Conserv. 244, 108536. https://doi.org/10.1016/j.biocon.2020.108536 (2020).
CBD 15/4, “Kunming-Montreal Global Biodiversity Framework.” (Tech. Rep. CBD/COP/15/L25, 2022).
IUCN, “Increasing marine protected area coverage for effective marine biodiversity conservation” (Tech. Rep.WCC 2016Res050EN, World Conservation Congress, Hawai‘i, United States of America, 2016).
Ocean Survey 20/20 International Polar Year and Census of Antarctic Marine Life Ross Sea voyage (TAN0802) biodiversity data. Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research, Wellington, New Zealand, 8748 records, Online http://nzobisipt.elasticbeanstalk.com/resource.do?r=mbis_caml released on Dec 12, 2013. https://doi.org/10.15468/jmsxe8 accessed via GBIF.org on 2023-07-18.
Brandt, A. & Malyutina, M. V. The German-Russian deep-sea expedition KuramBio (Kurile Kamchatka biodiversity studies) on board of the RV Sonne in 2012 following the footsteps of the legendary expeditions with RV Vityaz. Deep. Sea Res. Part II 111, 1–9. https://doi.org/10.1016/j.dsr2.2014.11.001 (2015).
Brix, S., Taylor, J., Kieneke, A., Linse, K., Martínez Arbizu, P. Icelandic marine animals: genetics and ecology meets diversity along latitudinal gradients in the deep sea of the Atlantic Ocean 2, cruise no. SO286, 04.11.2021 - 09.12.2021, Emden (Germany) - Las Palmas (Spain), DOI: https://doi.org/10.48433/cr_so286 (2022).
Brandt, A. et al. Abyssal macrofauna of the Kuril-Kamchatka trench area (northwest pacific) collected by means of a camera-epibenthic sledge. Deep Sea Res. II(111), 175–187 (2015).
Brandt, A. & Malyutina, M. V. The German-Russian deep-sea expedition KuramBio (Kurile Kamchatka biodiversity studies) on board of the RV Sonne in 2012 following the footsteps of the legendary expeditions with RV Vityaz. Deep Sea Res. II(111), 1–9 (2015).
Brenke, N. An epibenthic sledge for operations on marine soft bottom and bedrock. Mar. Technol. Soc. J. 39, 10–21 (2005).
Riehl, T. et al. Field and laboratory methods for DNA studies on deep-sea isopod crustaceans. Pol. Polar Res. 35(2), 203–224 (2014).
Astrin, J. J. & Stüben, P. Phylogeny in cryptic weevils: molecules, morphology and new genera of western Palaearctic Cryptorhynchinae (Coleoptera: Curculionidae). Invertebr. Syst. https://doi.org/10.1071/IS07057 (2008).
Hou, Z., Fu, J. & Li, S. A molecular phylogeny of the genus Gammarus (Crustacea: Amphipoda) based on mitochondrial and nuclear gene sequences. Mol. Phylogenet. Evol. 45, 596–611. https://doi.org/10.1016/j.ympev.2007.06.006 (2007).
Englisch, U., Coleman, C. O. & Wägele, J. W. First observations on the phylogeny of the families Gammaridae, Crangonyctidae, Melitidae, Niphargidae, Megaluropidae and Oedicerotidae (Amphipoda, Crustacea), using small subunit rDNA gene sequences. J. Nat. Hist. 37, 2461–2486. https://doi.org/10.1080/00222930210144352 (2003).
Pitz, K. M. & Sierwald, P. Phylogeny of the millipede order Spirobolida (Arthropoda: Diplopoda: Helminthomorpha). Cladistics 26, 497–525. https://doi.org/10.1111/j.1096-0031.2009.00303.x (2010).
Katoh, K., Misawa, K., Kuma, K.-I. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066. https://doi.org/10.1093/nar/gkf436 (2002).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. https://doi.org/10.1093/molbev/mst010 (2013).
Sayers, E. W. et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 50(D1), D20–D26. https://doi.org/10.1093/nar/gkab1112,PMID:34850941,PMCID:PMC8728269(2022) (2022).
Ratnasingham, S. & Hebert, P.D.N. BOLD: The barcode of life data system (http://www.barcodinglife.org). Mol. Ecol. Notes 7, 355–364, https://doi.org/10.1111/j.1471-8286.2007.01678.x (2007).
Ratnasingham, S. & Hebert, P. D. N. A DNA-Based Registry for All Animal Species: The Barcode Index Number (BIN) System. PLoS ONE 8, e66213. https://doi.org/10.1371/journal.pone.0066213 (2013).
Bouckaert, R. et al. BEAST 2: A software platform for bayesian evolutionary analysis. PLoS Comput. Biol. 10, e1003537. https://doi.org/10.1371/journal.pcbi.1003537 (2014).
Ronquist, F. et al. MRBAYES 32: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 61, 539–542 (2012).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Puillandre, N., Brouillet, S. & Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 21, 609–620. https://doi.org/10.1111/1755-0998.13281 (2021).
Pons, J., Barraclough, T. G., Gomez-Zurita, J., Cardoso, A. & Vogler, A. P. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst. Biol. 55, 595–609. https://doi.org/10.1080/10635150600852011 (2006).
Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. posterior summarization in bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904. https://doi.org/10.1093/sysbio/syy032 (2018).
R Core Team, R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2022).
Bandelt, H. J., Forster, P. & Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16, 37–48. https://doi.org/10.1093/oxfordjournals.molbev.a026036 (1999).
Stow, D., Smillie, Z., Esentia, I. P. Deep-Sea Bottom Currents: Their Nature and Distribution.” in Encyclopedia of Ocean Sciences: Earth Systems and Environmental Sciences 4, J. K. Cochran, H. Bokuniewicz, P. Yager, Eds. https://doi.org/10.1016/B978-0-12-409548-9.10878-4 (Academic Press. 2018).
Acknowledgements
The material was sampled during expeditions led by: Dr. Malcolm Clark (RV Tangaroa, Ross Sea), Prof. Dr. Angelika Brandt (RV Sonne, Kuril-Kamchatka) and Dr. Saskia Brix (RV Sonne, North Atlantic). Karen Jeskulke and Nicole Gatzemeier (DZMB, Hamburg, Germany) managed the sample sorting, databasing and took the on board photographs during the Atlantic expedition. We acknowledge the curation of the material by Sadie Mills, National Institute of Water & Atmospheric Research, Wellington, New Zealand and Kathrin Philipps-Bussau, Leibniz-Institut zur Analyse des Biodiversitätswandels, Hamburg, Germany.
Funding
Open Access funding enabled and organized by University Hamburg Projekt DEAL. German Research Foundation grant IceAGE Amphipoda LO2543/1–1 (ANL). The Federal Ministry of Education and Research Germany (BMBF) grant 03G0286NA to S. Brix, IceDivA2 expedition providing North Atlantic specimens. The BMBF grant 03G0223A to A. Brandt, KuramBio expedition providing Pacific specimens. This is publication 43 of the Kurambio project. Polish National Science Center grant No. 2022/45/B/NZ8/02667 (AMJ). Sampling permission was provided via Russia port authority, permission 49 from 5.4.2016. Specimens provided by the NIWA Invertebrate collection were collected on the TAN0802 voyage funded by the New Zealand Government under the International Polar Year Census of Antarctic Marine Life Project, and the TAN0402 biodiversity survey of the western Ross Sea and Balleny Islands (BIOROSS) undertaken by NIWA and financed by the former New Zealand Ministry of Fisheries.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.N.L., M.S., A.M.J. Data curation: A.M.J., M.S. Formal Analysis: A.M.J., A.N.L. Funding acquisition: A.N.L. Methodology: ANL, MS, AMJ, SB. Visualization: ANL, AMJ. Writing – original draft: ANL, MS, AMJ. Writing – review & editing: ANL, MS, AMJ, SB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lörz, AN., Schwentner, M., Bober, S. et al. Multi-ocean distribution of a brooding predator in the abyssal benthos. Sci Rep 13, 15867 (2023). https://doi.org/10.1038/s41598-023-42942-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42942-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.