Abstract
An in-depth study of the Early Pleistocene European remains of Hippopotamus has allowed the first detailed description of the incidence and types of dental alterations related to palaeopathologies and potentially linked to climatic and environmental factors. The results of a long-term qualitative and quantitative assessment highlight the importance of nutrient deficiencies on the development of dental enamel hypoplasia in Hippopotamus. Glacial cyclicity and the resulting changes in humidity and plant community structure conditioned the local environments critical for the survival of this taxon. Two main intervals of putative constrained nutritionally restrictions were detected at ca. 1.8 Ma and ca. 0.86 Ma (i.e., MIS63 and MIS21, respectively). Statistical comparisons show an increase in the frequency of dental hypoplasia between these two chronological periods, thus reinforcing the idea of increased seasonality in the circum-Mediterranean environments during the Early Pleistocene.
Similar content being viewed by others
Introduction
Paleoclimatic background
Throughout the Pliocene and Pleistocene, long-term trends of climatic cooling and increased glacial cycle amplitude are suggestive of significant changes in the dynamics of the Earth climate system1. In addition, a sustained trend towards increased aridification and seasonality in Europe throughout the Pleistocene is generally accepted2, which forced a progressive transition from tropical-subtropical ecosystems to present-day temperate ones3,4. In response to the aforementioned changes, the European large mammal assemblages biodiversity recorded an increase in the frequency of species adapted to more open environments, which was coincident with the first hominin dispersal out of Africa5,6,7. Moreover, a progressive increase in the amplitude of climate oscillations is documented since 1.4 Ma onwards, including a shift from 41-kyr to ca. 100-kyr orbital rhythm, an increase in the long-term average ice volume, and the establishment of a strong asymmetry in glacial ice volume cycles (i.e., ‘The Early-Middle Pleistocene Transition’ [EMPT] sensu Head and Gibbard2).
Background on Dental Enamel hypoplasia and Hippopotamus paleoecology
Dental Enamel Hypoplasia (DEH) is a disruption in enamel secretion by ameloblasts during amelogenesis, which is linked to physiological stresses8. There are three broad categories of DEH according to FDI (Federation Dentaire International9) and, according to Hillson10 and Hillson and Bond11, DEH displays a wide array of expressions, comprising of vertical or horizontal grooves, pits, or broad bands of missing or incomplete enamel. In large mammals, such disturbances are often associated with periods of environmental and/or nutrient stresses, resulting in a metabolic stress that causes this pathological state8,12. Many studies of the relationships between DEH and the environmental conditions in which the individuals lived have been published13,14,15. The position, width, and depth of DEH can provide information on the age of occurrence of anomaly, the period of stress event, and the severity of the stressors16,17.
Among the large mammals that inhabited Europe during the Pleistocene, the large-sized Hippopotamus spp. had several environmental requirements (such as the presence of permanent bodies of water or the abundance of grassland or aquatic macrophytes) making them relevant for climatic studies18. Their climatic range has been considered narrow, conditioning the use of their presence as a clear indicator of warm and humid climates19. However, preferential diet of individuals of Early Pleistocene European populations of (ca.3200 kg) Hippopotamus antiquus is still debated. Specifically, the discussion continues between a grazing behaviour similar to the dietary preferences of extant Hippopotamus amphibius20 and a diet centred on aquatic macrophytes, as suggested by biogeochemical data from the late Early Pleistocene site of Venta Micena21.
Despite the specific ecological requirements related to water availability and the characteristic continuous growth of their anterior dentition, DEH in hippopotamuses has been scarcely reported20,22 and no comprehensive study has been published so far, especially in relation to changes in the biotic or abiotic factors of the environment. In any case, no comprehensive study of DEH in hippopotamuses or any long-term projection of this phenomenology against changes in the biotic or abiotic environment has been carried out so far.
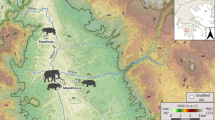
Following the observation of a large representation of DEH in the anterior dentition of the European hippopotamuses (H. antiquus) found in several Early Pleistocene layers at different sites in Europe (Fig. 1), this paper evaluates the question of whether this pathological state may be associated with changes in climatic condition that may resulted in nutritional stress.
Geographical and chronological location of the samples included in this study. Chronological representation also includes epochs/ages, the marine isotopic curve and the most important climatic events in Europe during the Early Pleistocene. Upper Valdarno comprises specimens from: Upper Valdarno general area (more details unknown), Castelfranco, Cava Bacchi, Figline, Renacci, Vacchereccia, Malpasso and Il Colombaiolo. Above the known chronological ranges of each locality (lines or dots), sites with documented hypoplasia are marked in orange; in blue, sites where it was not detected. Marine Isotopic Stages information from Lisiecki and Raymo1. Climatic Events information from Kahlke et al.42.
Results
Chronological and geographical distribution of Enamel Hypoplasia
The analysis of the sample of incisors and canines showed 105 pathologies related to the production of dental enamel among the 310 specimens analysed (30 among 74 Minimum Number of Individuals; Table S1). Twelve of the 23 Early Pleistocene sites studied around Southern and Central Europe document specimens with enamel hypoplasia, although with different incidence (Figs. 1, 2 and S1; Table S1). Of these sites, only the samples of Upper Valdarno and Vallparadís Section have enough specimens to be significative (see “Material and methods”).
Skeletal elements included in the analysis and the different degrees of severity of Linear Enamel Hypoplasia (LEH) observed in each element. Skull specimen: AC3864. 1: upper incisors, 2: upper canines, 3: lower incisors, 4: lower canines. From minimum to maximum severity of LEH: periodic appearance of lines with change of enamel colouring; onset of enamel loss from the enamel-dentine contact limits; appearance of periodic bands with absence of enamel with more than half of the development of the enamel layer. Scale bar equals 20 mm.
Samples of specimens showing Dental Enamel Hypoplasia are observed essentially in two periods: in the ca. 1.9–1.7 Ma interval at the Upper Valdarno sites and in the ca. 1.0–0.86 Ma interval at the Vallparadís Section. Additionally, five specimens displaying hypoplasia are found in Barranco León (BL01-K50-6; Fig. 3i), Fuente Nueva 3 (FN3-T10-6B, FN303-O93-4; Fig. 3m,n), Cava Santarelli (Madonna della Strada; Fig. 3o) and Saticula (Fig. 3w) in the 1.4–1.2 Ma interval (Table S1; Fig. 1). It is important to consider the possibility of obtaining random (or at least uninterpretable) results in sites where the sample of specimens is < 10. This situation especially affects some of the possible observations in the 1.6–1.0 Ma timeframe (Table S1; Fig. 1).
Examples of anterior dentition of Hippopotamus antiquus with Dental Enamel Hypoplasia (DEH) observed in the sample evaluated. (a): upper incisor with LEH (IGF 790), (b): upper canine with LEH (IGF 774), (c): lower incisor with LEH (IGF 804) and (d): lower canine with LEH (IGF 788) from Upper Valdarno; (e): upper incisor with LEH (IPS127144), (f): upper incisor with LEH (IPS61622), (g): upper canine with malformation (IPS98656), (h): upper canine with a traumatic episode and malocclusion (IPS48787), (i): lower incisor with LEH (IPS127126), (j): lower incisor with LEH (IPS879) and (k): lower canine with PEH (IPS127044) from post-Jaramillo Units of Vallparadis Section; (l): upper incisor from Barranco León with LEH (BL01-K52-1); (m): upper incisor with PIEH (MNCN19365) and (n): lower canine with LEH (Hu-1-105) from Huescar-I43; (o): lower canine with LEH from Cava Santarelli-Madonna della Strada44; (p): upper incisor with LEH (FN3-T10-6B) and (q): lower canine with LEH (FN303-O93-4) from Fuente Nueva-3; (r)1,2 (g.2410), (r)3 (g.2407), (r)4 (g.2408), (r)5 (320 B29): upper incisors with LEH from Chambezon; (s): upper canine with LEH and t2,3: upper incisor with LEH preserved in the skull from Collecurti exposed in the Museo Paleontologico Archeologico Serravalle di Chienti; (u): lower canine with LEH (IPS14519) from Esparraguera; (v): lower canine with LEH from Untermassfeld20; (w): upper canine with LEH from Saticula45. LEH: Linear Enamel Hypoplasia, PEH: Pit Enamel Hypoplasia, PIEH: Plane Enamel Hypoplasia. Scale bar equals 20 mm.
Types and incidence of pathologies
Different pathologies such as traumatic episodes (Fig. 3h), malformations (Fig. 3g) or malocclusion (Fig. 3h) coincide with Linear Enamel Hypoplasia (LEH) (Fig. 2), Pit Enamel Hypoplasia (PEH) (Fig. 3k) and Plane Enamel Hypoplasia (PlEH) (Fig. 3m). Clear banding patterns with some enamel waviness, lacking pigmentation and episodes of enamel loss are observed mainly in canines and upper incisors with LEH and PlEH (Fig. 3a–e, i, j, l–v).
In the case of the Upper Valdarno, 52.8% of the observed specimens had enamel pathologies (Fig. 4a1). These pathologies were mainly found in the lower canines (Fig. 4a2; Table S1). On the other hand, 39.5% of the specimens showed enamel pathologies in the post-Jaramillo layers of Vallparadís Section (Fig. 4b1; Table S1). In this sample, pathologies in the upper canines stand out compared to those observed in the lower ones (Fig. 4b2).
Schematic representation of the main data included in the Dental Enamel Hypoplasia (DEH) analysis of the two most representative samples. (a): Upper Valdarno (ca. 1.8 Ma), (b): post-Jaramillo Units of Vallparadis Section (ca. 0.86 Ma). a1, b1: percentage of anterior teeth with DEH. a2, b2: teeth-type distribution of the DEH. a3, b3: number of upper incisors with DEH; a4, b4: number of upper canines with DEH; a5, b5: number of lower incisors with DEH; a6, b6: number of lower canines with DEH. a7, 8: reference upper canine with Linear Enamel Hypoplasia (LEH) from Upper Valdarno (NMB Va.2518), b7, 8: reference upper canine with LEH from Vallparadís Section (IPS127141). a9, b9: boxplot of the frequency measurements of isolated episodes of LEH in upper canines, indicating significative differences between samples (Kruskal–Wallis test) with the letters a and b. The area outlined in grey represents the estimated range of annual growth in extant hippos (Hippopotamus amphibius). CGUU: Cal Guardiola Upper Unit, VEMU: Vallparadís Estacio Middle Unit. Scale bar equals 20 mm.
The evaluation of the frequency of episodes of LEH in the upper canines (Table S2) resulted in a sample of 7 specimens with 34 measurable oscillations in the Upper Valdarno sample, and 15 specimens with 102 oscillations in the sample from the post-Jaramillo layers of the Vallparadís Section. Oscillations with a mean amplitude of 16.7 mm (Fig. 4a7–9) were found in the Upper Valdarno. In contrast, a mean of 14.8 mm (Fig. 4b7–9) was found in the post-Jaramillo Vallparadís Section. Comparison of the means using the non-parametric Kruskal–Wallis test provided a p-value of 0.04463, showing the presence of significant differences between the two samples, which are indicated as subscripts under the error bars in the boxplots shown in Fig. 3a12, b15. Also striking are the extreme cases, with a minimum of 8.4–9.6 mm and a maximum of 26.2–28.5 mm.
Discussion
Enamel hypoplasia in Early Pleistocene Hippopotamus
Few cases of DEH associated with trauma have been reported, as most pathological specimens show a clear periodicity in the DEH episodes, which allows discarding one-off events in them (see Fig. 3h and Kierdorf and Kahlke20). Congenital DEH, which is the pathology least reported in previous studies, shows characteristic typologies that differentiate it from most of the cases observed in the sample studied here8. The wide geographical and chronological extension of the sample analysed here depicting these pathologies allows to discard an intoxication aetiology as the cause of the observed DEH22,23. Given the characteristic repetitive periodicity of hypoplasia episodes assessed in the pathological anterior dentition, the most likely cause of most pathologies observed in the sample studied here appears to be the repetitive and cyclical lack of nutrients. This interpretation is in line with that inferred in other studies, including those involving DEH in extant hippopotamuses20,22 and other ungulates14,15,24.
Hypoplasia frequency in Early Pleistocene Hippopotamus
Lower canine growth was studied especially in the extant common hippopotamus, with direct observations of a ratio of 13–14 mm/year in an old (48 years old) female and 28–30 mm/year in a young (8 years old) one25. On the other hand, estimates of growth ratios by Uno et al.26 provide values of 33.5–74.7 mm/year for the lower canines and 19.4 ± 3.1 mm/year for the upper ones. Harris et al.27 considered 30 mm/year as a standard measure of growth for the lower canines, while Souron et al.28 highlighted that these values can reach 39.1 mm/year for the lower canines and 30 mm/year for the upper ones. In our case, the estimated frequencies of LEH events in the mean measurements obtained for the upper canines were 16.7 mm and 14.8 mm (Fig. 4a9, b9). The sample analysed comes from individuals of the extinct species H. antiquus, which according to estimates made by Palmqvist et al.21 could reach a size twice as large as that observed by Nowak and Walker29 for the living common hippo (ca. 3200 kg vs. ca. 1500 kg). With the available data, it is difficult to ensure a specific seasonality to the observed episodes of hypoplasia, but everything points to a periodicity of approximately 1–2 episodes per year. Thus, it would be reasonable to consider that these episodes mark the season(s) with most severe weather, which would limit the access of hippopotamuses to their food resources, or with recurrent aridity periods that prevented the access to lacustrine systems with a water table of enough depth.
Paleoenvironmental inferences
If we focus on the paleoenvironmental context of the samples, the Upper Valdarno specimens (ca. 1.9–1.7 Ma) record apparently drastic episodes for the physiology of individuals of European hippopotamus populations. In fact, in the coeval localities of this basin, seasonal drought events have been hypothesised, especially in the site of Poggio Rosso3. These aridity episodes fit with the proposed alternations of cold/warm phases of the European climate, which would in turn results in changes in the distribution of open/closed environments2,30. Ultimately, disrupting the access to the vegetation on which the hippopotamuses fed.
Between 1.7 and 1.4 Ma the climate seems to have been associated with more favourable conditions, with the expansion of grasslands31. This environment is close to ideal conditions for the maintenance of hippopotamus populations32 and might reflect in the absence of DEH in the samples of Venta Micena and Monte Argentario. Nevertheless, we remain cautious in this interpretation, as only two specimens from each site were evaluated. In fact, linear dental hypoplasia was found in specimens of horse Equus altidens from Venta Micena, which suggests unfavourable conditions at least for this grazing and fully terrestrial species33. Furthermore, previous biogeochemical studies based on the relative abundance of carbon- and nitrogen-stable isotopes retrieved from bone collagen in the late Early Pleistocene site of Venta Micena (Baza Basin, SE Spain) have shown unexpectedly high δ15N values in the remains of H. antiquus compared to other ungulates recorded at the site. The values were even higher than those measured in the hypercarnivores of the palaeocommunity (e.g., sabertooth cats, hyenas, and wild dogs). These high values of H. antiquus in Venta Micena were interpreted as reflecting a diet based mainly on aquatic plants, including the macrophytes and algae that grew in the oligosaline waters of the lacustrine environments of the Baza Basin21,34. The feeding behaviour of extant common hippopotamuses is preferentially, and almost exclusively, based on grazing on terrestrial vegetation, and browsing habits have only been reported when grasses are not available35. If the interpretation of the Venta Micena isotopic data was confirmed, the feeding habits of the hippopotamuses found at this site would be particularly different from those of the extant populations and might be linked to environmental conditions that limited the proliferation of grazing areas.
In the period 1.4–1.1 Ma, a progressive increase in the amplitude of climate oscillations is recorded being associated with the beginning of the EMPT2, which resulted in an appreciable diffusion of Mediterranean vegetation with the intercalation of arid phases and greater seasonality30. The sample from this chronological range includes only five pathological specimens (10.2% of the total number of analysed specimens from this chronological range; e.g., Barranco León D; Fig. 1; Table S1). None of the 23 specimens from the Lower Unit of Cal Guardiola (MIS35) show any type of DEH. The palaeoecological reconstructions of Cal Guardiola in these chronologies point to a mixed environment, with the presence of wooded and open areas, leading to the inference of a climate with some permanent humidity36 (Fig. 1).
The absence of DEH in the samples dated to ca, 1.0 Ma (MIS31) in the Lower Unit of Vallparadís Estació is noteworthy, although the sample from these localities is very limited (3 specimens). In other samples of the same chronology, such as Untermassfeld or Collecurti, several specimens with pathologies were reported but the prevalence and severity of DEH in them is not excessively high20. Palaeoenvironmental reconstructions of these localities, specifically Vallparadís Estació layer EVT12, point to the near absence of DEH in the hippopotamus remains recorded, which coincides with a period of aridity and expansion of grasslands at least in the Mediterranean Europe36,37.
During the chronological span comprised between MIS24 to MIS22 (ca. 900 ka), a long and severe glacial phase is recorded2,38. The samples dated to ca. 0.90–0.86 Ma include a prevalence of sites with a high incidence of DEH, such as the post-Jaramillo units of Vallparadís Section. In the later site, an increase in humidity and tree cover has been detected, which could limit the expansion of grasslands37. It should also be noted that these are the first records of H. antiquus after MIS24-22 marking a change in biodiversity of terrestrial communities, so it is possible that plant biodiversity also continued to be affected.
Finally, it is important to make special mention of the significant differences reported in the frequency of the episodes of upper canine hypoplasia between the samples from the Upper Valdarno (ca. 1.9–1.7 Ma) and those from the post-Jaramillo Vallparadís Section (ca. 0.86 Ma) (see Fig. 4a9, b9 and Sect. “Materials and methods”). This phenomenon highlights the presence of a marked climatic temporality and an increase in its frequency that may be in line with postulated changes in seasonality during the late Early Pleistocene around the Mediterranean Basin39.
Material and methods
Materials
Specimens of the anterior dentition of Early Pleistocene hippopotamuses (H. antiquus) from different European sites have been analysed (Table S1; Figs. 1 and S1). These dental elements have been selected for analysis for the continuous growth of the anterior dentition throughout the life of the individuals, which allows the assessment of changes in the type of enamel secretion by ameloblasts at different stages of their life cycle, with a time resolution of few days25. Moreover, preliminary observations of cases of dental hypoplasia in the postcanine dentition have not reported any remarkable cases of DEH.
We have selected those samples of European localities dated to the late Early Pleistocene (i.e., Late Villafranchian and Epivillafranchian), with an established presence of hippopotamuses and accessibility of data to the authors (either primary access or through the literature). For a detailed list of the localities studied and all characteristics related to each sample, see Figs. 1, S1 and Table S2.
310 tooth specimens of the anterior dentition were evaluated. The Upper Valdarno sites40 and the post-Jaramillo levels of the Vallparadís Section 41 were treated as two unique samples in this study, since in both cases the accumulations that make up the samples are very close geographically and minimal differences in the chronology of deposition are accepted.
Methods
The criteria for the detection of enamel pathologies have been standardised following the typification set out by Goodman and Rose8, paying special attention to LEH treated in detailed works such as Dobney and Ervynck14. In the specific case of LEH, Fig. 2 shows the different degrees of severity observed in each type of tooth element included in the analysis sample.
Data collection was carried out by direct observation of most samples studied and of those specimens figured in the literature (see Table S1). All cases of dental enamel pathologies found in the anterior dentition were documented in detail, noting the anatomical determination of the element and the typology of the pathologies, taking photographs and scanning the specimens at different 3D resolutions with the most accurate scanner available at each institution (Creality CR—SCAN 01, Precision: 0.1 mm, Resolution: 0.5 mm; Artec Eva, Precision: 0.1 mm, Resolution: 0.2 mm; Artec Space Spider, Precision: 0.05 mm, Resolution: 0.1 mm; Artec Micro, Precision: 0.01 mm, Resolution: 0.029 mm). All data were included in databases for individual observation, detailed analyses, and replicability of the evaluation (Tables S1, S2 and S3).
Sites where the sample was small, or in which the representation of pathologies was null or minimal, were evaluated only qualitatively. The prevalence of hypoplasia in samples from the Upper Valdarno sites and the post-Jaramillo layer of the Vallparadís Section was compared using descriptive statistics and qualitative characteristics (Figs. 3 and 4).
The upper canines were selected for the quantitative comparison of the frequency of LEH episodes in those cases where a sequential pattern was detected, because their growth rates are approximately known in the living H. amphibius25,26,27. Frequency measurements were standardized as the measurement of the distance from the middle of one normal enamel attachment phase to the next, measuring from the most proximal part of the canine to the most distal, in 3D models using Meshlab software (Fig. S2, Table S2). For the comparison of the frequency of the LEH episodes between the samples from the Upper Valdarno and the post-Jaramillo Vallparadís Section, box plots of the individual measurements of each episode were made (Fig. 4). A statistical comparison of the differences between the frequencies of the two samples was carried out. Firstly, the normality of the samples was checked using the Shaphiro-Wilk test, observing that both the whole data (W = 0.9455, p (normal) = 3.512*10–5) and the samples of the Upper Valdarno (W = 0.9328, p (normal) = 0.03769) and the post-Jaramillo Vallparadís Section (W = 0.9492, p (normal) = 0.00064) showed departures from normality. Given the absence of normality, the Kruskal–Wallis non-parametric test for comparison of means was performed.
Additionally, direct observations of the sample of Hippopotamus amphibius specimens available at the Natural History Museum (London), the Muséum National d'Histoire Naturelle (Paris), the Museo Nacional de Ciencias Naturales (Madrid) and the Museo Anatómico de la Universidad de Valladolid (Valladolid) were made to check the possibility of including a current comparative representation of the incidence and degree of Enamel Hypoplasia. Very few cases of enamel hypoplasia (Fig. S3) were observed in this sample, mostly related to malocclusion (Fig. S3g, i, j), congenital pathologies (Fig. S3m, n) or infections of the entire dental series (Fig. S3p, q, r). Two of the individuals with these pathologies came from zoos (Fig. S3i–f, h), while the other seven were wild or of unknown origin (Fig. S3g, i–r). In none of the cases was the degree of linear enamel hypoplasia as severe as in the Hippopotamus antiquus sample, so it was not possible to compare the two samples or to assess quantitatively differences in the frequency of hypoplasia episodes.
Data availability
All data generated or analyzed during the study are included in this article and in its Supplementary Information files.
References
Lisiecki, L. E. & Raymo, M. E. Plio-Pleistocene climate evolution: Trends and transitions in glacial cycle dynamics. Quat. Sci. Rev. 26(1–2), 56–69 (2007).
Head, M. J. & Gibbard, P. L. Early-Middle Pleistocene transitions: Linking terrestrial and marine realms. Quat. Int. 389, 7–46 (2015).
Bertini, A., Magi, M., Mazza, P. P. & Fauquette, S. Impact of short-term climatic events on latest Pliocene land settings and communities in Central Italy (Upper Valdarno basin). Quat. Int. 225(1), 92–105 (2010).
Magri, D., Di Rita, F., Aranbarri, J., Fletcher, W. & González-Sampériz, P. Quaternary disappearance of tree taxa from Southern Europe: Timing and trends. Quat. Sci. Rev. 163, 23–55 (2017).
Arribas, A. & Palmqvist, P. On the ecological connection between sabre-tooths and hominids: Faunal dispersal events in the lower Pleistocene and a review of the evidence for the first human arrival in Europe. J. Archaeol. Sci. 26, 571–585 (1999).
Leroy, S. A., Arpe, K. & Mikolajewicz, U. Vegetation context and climatic limits of the Early Pleistocene hominin dispersal in Europe. Quat. Sci. Rev. 30(11–12), 1448–1463 (2011).
Sorbelli, L. et al. Earliest bison dispersal in Western Palearctic: Insights from the Eobison record from Pietrafitta (Early Pleistocene, central Italy). Quat. Sci. Rev. 301, 107923 (2023).
Goodman, A. H. & Rose, J. C. Assessment of systemic physiological perturbations from dental enamel hypoplasias and associated histological structures. Am. J. Phys. Anthropol. 33(S11), 59–110 (1990).
Commission on Oral Health, Research and Epidemiology. An epidemiological index of developmental defects of dental enamel (DDE Index). Int. Dent. J. 32(2), 159–167 (1982).
Hillson, S. Dental Anthropology (Cambridge University Press, 1996).
Hillson, S. & Bond, S. Relationship of enamel hypoplasia to the pattern of tooth crown growth: A discussion. Am. J. Phys. Anthropol. 104(1), 89–103 (1997).
Simon, S. C. Linear enamel hypoplasia as a proxy for environmental stress in fossil apes. Saint Mary’s University, Halifax, Nova Scotia. PhD thesis (2022).
Bacon, A. M. et al. Linear enamel hypoplasia in large-bodied mammals of Pleistocene northern Vietnam, with a special focus on Pongo. Quat. Int. 563, 38–50 (2020).
Dobney, K. & Ervynck, A. Interpreting developmental stress in archaeological pigs: The chronology of linear enamel hypoplasia. J. Archaeol. Sci. 27(7), 597–607 (2000).
Nawaz, M. K. & Khan, M. A. Enamel hypoplasia analysis of middle miocene mammals from Chabbar Syedan, Punjab, Pakistan. Pak. J. Zool. 54(6), 2651 (2022).
Ameen, M., Khan, A. M., Ahmad, R. M., Iqbal, A. & Akhtar, M. Appraisal of dental enamel hypoplasia in the Middle Miocene deinotheriidae: Implications of the Siwalik paleoenvironment of Pakistan. Rev. Bras. Paleontol. 24(4), 357–368 (2021).
Moggi-Cecchi, J., Pacciani, E. & Pinto-Cisternas, J. Enamel hypoplasia and age at weaning in 19th-century Florence. Italy. Am. J. Phys. Anthropol. 93(3), 299–306 (1994).
Mazza, P. P. A. & Bertini, A. Were Pleistocene hippopotamuses exposed to climate-driven body size changes?. Boreas 42(1), 194–209 (2013).
Adams, N. F., Candy, I. & Schreve, D. C. An early Pleistocene hippopotamus from Westbury Cave, Somerset, England: Support for a previously unrecognized temperate interval in the British Quaternary record. J. Quat. Sci. 37(1), 28–41 (2022).
Kierdorf, U., & Kahlke, R. D. (2022). Pathological findings on remains of hippopotamids from the Early Pleistocene site of Untermassfeld in The Pleistocene of Untermassfeld near Meiningen (Thüringen, Germany) (ed. Kahlke, R. D.) 1251–1272 (Monographien des RGZM Band 40, 4, 2020).
Palmqvist, P., Rodríguez-Gómez, G., Figueirido, B., García-Aguilar, J. M. & Pérez-Claros, J. A. On the ecological scenario of the first hominin dispersal out of Africa. Anthropologie 126(1), 102998 (2022).
Franz-Odendaal, T., Chinsamy, A. & Lee-Thorp, J. High prevalence of enamel hypoplasia in an early Pliocene giraffid (Sivatherium hendeyi) from South Africa. J. Vertebr. Paleontol. 24(1), 235–244 (2004).
Boisserie, J. R. & Merceron, G. Correlating the success of Hippopotaminae with the C4 grass expansion in Africa: Relationship and diet of early Pliocene hippopotamids from Langebaanweg, South Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 308(3–4), 350–361 (2011).
Niven, L. B., Egeland, C. P. & Todd, L. C. An inter-site comparison of enamel hypoplasia in bison: Implications for paleoecology and modeling Late Plains Archaic subsistence. J. Archaeol. Sci. 31(12), 1783–1794 (2004).
Passey, B. H. et al. Inverse methods for estimating primary input signals from time-averaged isotope profiles. Geochim. Cosmochim. Acta 69(16), 4101–4116 (2005).
Uno, K. T. et al. Bomb-curve radiocarbon measurement of recent biologic tissues and applications to wildlife forensics and stable isotope (paleo) ecology. Proc. Natl. Acad. Sci. U.S.A. 110(29), 11736–11741 (2013).
Harris, J. M., Cerling, T. E., Leakey, M. G. & Passey, B. H. Stable isotope ecology of fossil hippopotamids from the Lake Turkana Basin of East Africa. J. Zool. 275(3), 323–331 (2008).
Souron, A., Balasse, M. & Boisserie, J. R. Intra-tooth isotopic profiles of canines from extant Hippopotamus amphibius and late Pliocene hippopotamids (Shungura Formation, Ethiopia): Insights into the seasonality of diet and climate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 342, 97–110 (2012).
Nowak, R. M. & Walker, E. P. Walker’s Mammals of the World Vol. 1 (JHU Press, 1999).
Magri, D., Di Rita, F. & Palombo, M. R. An Early Pleistocene interglacial record from an intermontane basin of central Italy (Scoppito, L’Aquila). Quat. Int. 225(1), 106–113 (2010).
Saarinen, J. et al. Pliocene to Middle Pleistocene climate history in the Guadix-Baza Basin, and the environmental conditions of early Homo dispersal in Europe. Quat. Sci. Rev. 268, 107132 (2021).
Eltringham, S. K. The Hippos: Natural History and Conservation. Poyser Natural History Series, 256 (1999).
Espigares, M. P., Palmqvist, P., Ros-Montoya, S., Garrido, A. M. & Martínez-Navarro, B. Presencia de osteopatologÍas en el yacimiento de Venta Micena (Orce, Granada, España). Anales de la Real Academia de Ciencias Veterinarias de Andalucía Oriental 27, 134–148 (2014).
García-Aguilar, J. M. et al. A reassessment of the evidence for hydrothermal activity in the Neogene-Quaternary lacustrine environments of the Baza basin (Betic Cordillera, SE Spain) and its paleoecological implications. Quat. Sci. Rev. 112, 226–235 (2015).
Cerling, T. E. et al. Stable isotope ecology of the common hippopotamus. J. Zool. 276, 204–212 (2013).
Sorbelli, L. et al. A review on Bison schoetensacki and its closest relatives through the early-Middle Pleistocene transition: Insights from the Vallparadís Section (NE Iberian Peninsula) and other European localities. Quat. Sci. Rev. 261, 106933 (2021).
Strani, F. et al. The effects of the “0.9 Ma event” on the Mediterranean ecosystems during the Early-Middle Pleistocene transition as revealed by dental wear patterns of fossil ungulates. Quat. Sci. Rev. 210, 80–89 (2019).
Head, M. J. & Gibbard, P. L. Early-Middle Pleistocene transitions: An overview and recommendation for the defining boundary. Geol. Soc. Spec. Publ. 247(1), 1–18 (2005).
Crippa, G. et al. Seasonality fluctuations recorded in fossil bivalves during the early Pleistocene: Implications for climate change. Palaeogeogr. Palaeoclimatol. Palaeoecol. 446, 234–251 (2016).
Rook, L. et al. The Upper Valdarno Plio-Pleistocene vertebrate record: An historical overview, with notes on palaeobiology and stratigraphic significance of some important taxa. Ital. J. Geosci. 132(1), 104–125 (2013).
Madurell-Malapeira, J. et al. The Vallparadís section (Terrassa, Iberian peninsula) and the latest Villafranchian faunas of Europe. Quat. Sci. Rev. 29(27–28), 3972–3982 (2010).
Kahlke, R. D. et al. Western Palaearctic palaeoenvironmental conditions during the Early and early Middle Pleistocene inferred from large mammal communities, and implications for hominin dispersal in Europe. Quat. Sci. Rev. 30(11–12), 1368–1395 (2011).
Mazo, A. V. Los hipopótamos del Pleistoceno medio de Huescar-1, Granada. Trab. neog. cuantern. 11, 317–325 (1989).
Mancini, M. et al. Coupling basin infill history and mammal biochronology in a Pleistocene intramontane basin: The case of western L’Aquila Basin (central Apennines, Italy). Quat. Int. 267, 62–77 (2012).
Cicia, V. et al. Nuove segnalazioni di Hippopotamus Linnaeus, 1758 nel Pleistocene inferiore e medio dell'Italia peninsulare. XVI Edizione delle Giornate di Paleontologia, Faenza, 25–27 May (2016).
Acknowledgements
We would especially like to thank the Editor Annalisa Ferretti, and the two anonymous reviewers for their helpful comments, which have significantly improved this paper. J. M.-M. would like to thank the comments and suggestions by Faysal Bibi on an early version of the manuscript. We would like to thank the facilities provide by different curators namely, Dr. J. M. Robles, Dr. A. Savorelli, Dr. L. Bellucci, Dr. E. Cioppi, Dr. L. Costeur, Dr. J. Quesada, Dr. D. Berthet, Dr. R. Portela-Miguez, Dr. J. Lesur, Dr. P. Pastor, Dr. Dr. A. Gravía, Dr. E. Robert and Dr. J. Rustioni. Research activity of D.F. and A.R. has been funded by Ministerio de Ciencia e Innovación, PID2021-122356NB-I00. D.F. is supported by the Ayuda del Programa de Formación de Profesorado Universitario (FPU20/03389) and PhD student at the Programa de Doctorado en Biología at the Universidad Complutense de Madrid. B. M.-N. is founded by the Generalitat de Catalunya (2021 SGR 01238). L.R. acknowledges the support of Fondi di Ateneo from the University of Florence (Earth Sciences Department). Finally, J.M.-M would like to thank L. Sorbelli and M. Rull for their help on the design of Figure 1.
Author information
Authors and Affiliations
Contributions
D.F. and J.M.-M. conceived and designed the experiments. D.F., S.B.-L. and J.M.-M. wrote the paper and prepared the figures and tables. All authors analyzed the data and reviewed several drafts of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fidalgo, D., Rosas, A., Bartolini-Lucenti, S. et al. Increase on environmental seasonality through the European Early Pleistocene inferred from dental enamel hypoplasia. Sci Rep 13, 16941 (2023). https://doi.org/10.1038/s41598-023-42936-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42936-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.