Abstract
Nearly a century after the first reports of Rift Valley fever (RVF) were documented in Kenya, questions on the transmission dynamics of the disease remain. Specifically, data on viral maintenance in the quiescent years between epidemics is limited. We implemented a cross-sectional study in northern Kenya to determine the seroprevalence, risk factors, and ecological predictors of RVF in humans and livestock during an interepidemic period. Six hundred seventy-six human and 1,864 livestock samples were screened for anti-RVF Immunoglobulin G (IgG). Out of the 1,864 livestock samples tested for IgG, a subset of 1,103 samples was randomly selected for additional testing to detect the presence of anti-RVFV Immunoglobulin M (IgM). The anti-RVF virus (RVFV) IgG seropositivity in livestock and humans was 21.7% and 28.4%, respectively. RVFV IgM was detected in 0.4% of the livestock samples. Participation in the slaughter of livestock and age were positively associated with RVFV exposure in humans, while age was a significant factor in livestock. We detected significant interaction between rainfall and elevation's influence on livestock seropositivity, while in humans, elevation was negatively associated with RVF virus exposure. The linear increase of human and livestock exposure with age suggests an endemic transmission cycle, further corroborated by the detection of IgM antibodies in livestock.
Similar content being viewed by others
Introduction
Rift Valley fever (RVF), a zoonotic infection caused by a vector-borne Phlebovirus, is an important disease in Sub-Saharan Africa1,2. Cyclic, explosive outbreaks associated with significant morbidity in humans and livestock and restrictions on livestock trade are some of the primary reasons RVF is considered a regional priority3,4,5. Rift Valley fever virus (RVFV) was first identified in Kenya in 19316. Since then, RVF outbreaks have been reported in the Arabian peninsula, North, West, and Southern Africa, and on islands off the East African coast, a testament to RVF's potential for intercontinental spread7,8,9,10,11.
In Eastern Africa, RVF occurs in two distinct ecological phases; an endemic phase lasting between five to ten years and an epidemic cycle lasting up to four months12,13. The epidemic phase is associated with above-average rainfall, usually linked to the El Niño-Southern Oscillation, which results in widespread flooding in low-lying areas14,15,16. Flooding and high ambient temperatures provide the requisite conditions for increased mosquito populations (the primary vectors), resulting in amplified disease transmission and consequent outbreaks in humans and animals17,18,19. Besides rainfall, other environmental factors that impact vector habitat suitability, such as soil type, vegetation cover, and elevation, may influence the magnitude of outbreak events during this phase20,21. The drivers of the epidemic phase are relatively well documented. However, there is comparatively less understanding of the viral maintenance dynamics during the quiescent years of the endemic phase. Two main hypotheses have been advanced on the same. The first is that RVFV is transmitted transovarially to Aedes mosquito eggs, which are laid in low-lying plains where they desiccate but remain viable for many years, explaining the long interepidemic periods (IEP)22,23. Flooding and warm temperatures provide ideal conditions for the eggs to rehydrate and hatch, giving rise to a large population of infected Aedes mosquitoes that transmit the RVFV to susceptible species, resulting in viral amplification and epidemics24,25,26. The second theory is based on the supposition that the virus is primarily maintained by a cryptic mosquito-host infection cycle that causes sub-clinical disease in susceptible livestock and wildlife species during IEP. The transition from the endemic phase to epidemic occurs when ecological conditions support a surge in the vector population and subsequent infection of a critical mass of naïve animals27,28. The latter hypothesis has gained credence over time due to increasing evidence of active infections in apparently healthy humans and livestock during IEP and the detection of RVFV antibodies in countries with no history of RVF outbreaks, such as the French Island of Mayotte, Chad, Tunisia, and Turkey27,28,29,30,31.
In Kenya, RVF outbreaks have been reported every five to ten years since the early 1930s32. However, there has been a marked increase in the frequency of detections in the last decade33,34. A few studies in the country provide evidence of IEP infections in humans and livestock; however, questions around the primary reservoirs, effects of herd immunity on RVF emergence, species susceptibility, and ecological drivers of RVFV infection during IEP still persist35,36,37,38,39. Routine sero-monitoring is vital in answering these questions and generating valid data for risk assessments40. Furthermore, the importance of prevalence data in complementing climatic and ecological parameters to improve the spatial specificity of RVF prediction models is well described in literature21,41. Our study sought to establish the IEP prevalence of RVF, and factors associated with RVFV exposure in Isiolo County, an RVF epidemic-prone region, through a livestock-human-linked study to generate empirical data to guide early detection and response.
Materials and methods
Ethics statement
Ethical approval for the study was provided by the International Livestock Research Institute Institutional Research Ethics Committee (Reference number: ILRI-IREC2020-07) to sample livestock and humans. Livestock owners and household heads provided written consent before sampling. For individuals under 13 years, written permission was obtained from their parents or legal guardians. For those between the ages of 13 and 17, written assent and written parental/legal guardian permission were obtained. Adults over the age of 18 provided written consent before sampling. The human and animal, sampling, and data collection were performed in accordance with the standard operating procedures and guidelines outlined in the ethical approval.
Study area
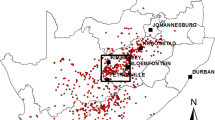
We conducted the study in three wards (administrative sub-units) in Isiolo South: Sericho, Kinna, and Garbatula (Fig. 1). Isiolo County was selected for the study because the last RVF outbreak in Kenya (November 2020 to January 2021) primarily affected the county, although Isiolo is considered a medium-risk area42. The area is a low-lying region with a mean annual rainfall of 300 to 350 mm distributed between two wet seasons: March to April and October to December43. Precipitation is usually erratic, and droughts are frequent. Although the primary economic activity is nomadic pastoralism, there are limited farming activities on the flood plains of the Ewaso-Ngiro, which is the only permanent river in the region43. The main livestock species in the area are cattle, camels, sheep, and goats, with the latter having the highest population. In addition to livestock, the area has a high population of free-roaming wildlife herbivores: African buffalo (Syncerus caffer), plains zebra (Equus quagga), and waterbucks (Kobus ellipsiprymnus) which provides an opportunity to study the epidemiology of zoonotic pathogens at the human-livestock-wildlife interphase. The Borana people, around 100,000, are sparsely distributed across the study area, which covers approximately 9,902 km244. The mean elevation of the study area ranges from 282 m in Sericho to 459 m in Garbatulla to a high of 589 m in Kinna.
Map of Africa showing the location of Kenya, the study site in green (Isiolo County), and specific wards and distribution of randomly sampled households. Shapefile used to create the map was obtained from: https://gadm.org/download_country.html.
Study design and sample size determination
The study was a household-based cross-sectional survey of humans and livestock (sheep, goats, and camels), conducted between July and August 2021. Cattle were not sampled because there was a vaccination campaign targeting cattle in part of the study area, six months before the study. The sample sizes for both livestock and humans were determined using the formula; \(n=\frac{{z}^{2}P\left(1-P\right)}{{d}^{2}}\) where the \(z\) is the standard normal distribution value corresponding to a 95% confidence interval, \(P\) is the expected prevalence, \(d\) is the precision level set at 0.05, and \(n\) is the minimum sample size45. A priori prevalence of 50% was used to estimate a sample size of 385 in humans and livestock because current exposure estimates in the study area were unavailable. The minimum sample size in humans and livestock was multiplied by a design effect to account for variance in precision because of multistage sampling. The design effect (Deff) was estimated using the formula; \(Deff=1+(b-1)\rho\) where \(b\) was the number of projected samples per herd/household and \(\rho\) is the intra-cluster correlation coefficient (ICC), which is the measure of the rate of homogeneity. In both humans and livestock, a value of 0.2 was used for ICC based on evidence that the upper estimates for this parameter do not usually exceed 0.2 except for highly infectious viral diseases46,47. Based on the fact that average herd sizes are highly variable in pastoral set-ups and we had limited data on herd sizes and composition, in the study area, we used 20 animals as the cluster size (b); this is based on a similar study done in a comparable pastoral setting in a neighboring county to our study area48. This gave a design effect of 4.8 and an adjusted sample size of 1,843 for livestock. In the human sample size estimation, we used an average cluster size of three, deduced from a similar study giving a design effect of 1.4 and a minimum sample size of 538 humans48.
Sampling design and data collection
The study was designed as a linked human-livestock study where livestock samples were collected from households from which humans were sampled. Two-stage sampling was used, with households and animals/human subjects being the primary and secondary sampling unit, respectively. A map of the study area was developed in Arc-GIS, and 250 random geographical coordinates (including additional points for replacement) were generated to identify households for sampling. A household closest to a coordinate was selected for inclusion. Inclusion criteria for livestock sampling were herds where at least five clinically healthy animals of one of three study species (camels, sheep, and goats) species were available for sampling, herds with no history of RVF vaccination, and livestock owned by households that consent to human sampling. Information on livestock species distribution in herds in the study area was unavailable, so a maximum of five animals of each available species were sampled per herd using systematic random sampling. For systematic random sampling, we calculated the sampling interval using the formula \(i=N/n\) where \(i\) was the sampling interval, \(N\) was the herd size, and \(n\) was five. A trained veterinarian collected up to 10 mL of blood from each animal using jugular venipuncture.
All consenting humans, excluding children under two years old, were enrolled in households with or without livestock. A nurse collected five mL of blood using non-heparinized vacutainer tubes. Human and livestock blood samples were transported to a field laboratory using cool boxes that maintained a temperature of 4 °C to 8 °C for processing. Sera were collected through centrifugation at 2500 g for 15 min and transferred into barcoded cryovial tubes, which were stored and transported at −20 °C to the International Livestock Research Institute (ILRI), Nairobi, using a motorized freezer.
Data on potential risk factors for RVFV infection, like demographic information in livestock and humans, livestock husbandry practices, livestock reproductive losses (including cattle), and human contact with livestock, were collected and managed electronically through structured questionnaires in Open Data Kit (ODK) database systems version 1.30.1.
Laboratory testing
Serological analysis of human and livestock sera samples for anti-RVFV Immunoglobulin G (IgG) antibodies was done using a multi-species competitive enzyme-linked immunosorbent assay (cELISA) from ID.vet (Innovative Diagnostics, Grabels, France). Livestock testing for anti-RVFV Immunoglobulin M (IgM) antibodies was done using IgM antibody capture ELISA for the detection of anti-nucleoprotein IgM antibodies in ruminant serum and plasma from ID.vet. These kits were selected due to their reported high sensitivity and specificity values49. A total of 1,864 livestock samples and 676 human samples were tested using the anti-RVFV Immunoglobulin G (IgG). A subset of the livestock samples, 1,103 randomly selected from the 1,864 samples tested for IgG, were additionally tested for anti-RVFV Immunoglobulin M (IgM). Human samples were not tested for IgM because, unlike the IgG kit, the IgM kit is only validated for use in ruminants. All testing was conducted as per the manufacturer's instructions. Briefly, 100 µl of diluted serum samples and kit positive and negative controls were loaded onto precoated microtiter plates and incubated at 37 °C for 1 h. The plates were then washed three times using 1X wash solution followed by adding 100 µL 1X conjugate solution. The reaction was incubated at room temperature for 30 min and then washed thrice. The substrate solution (100 µL) was added to each well, and the test plate was incubated at room temperature for 15 min. The reaction was stopped by adding 100 µL of the stop solution, and the optical densities (O.D.s) were read at 450 nm wavelength using a BioTek ELISA reader (Synergy HT, United States). The sample competition percent (S/N%) was determined by dividing the mean O.D. of each sample by the mean O.D. for the negative control and multiplying by 100%. An animal was considered positive if S/N% was ≤ 40%, negative if > 50%, and borderline if the value was between 40 and 50% as per the manufacturer's recommended cut-off values. Validation of the test results was done by duplicate testing of all assays. Serological analysis for all samples was done at the ILRI in Nairobi.
Data analysis methods
Data analysis was done using R statistical environment, version 4.1.250. After data merging and cleaning, initial descriptive statistical analyses included the estimation of the overall seroprevalence of RVF with 95% confidence intervals (CI) in livestock and humans at the animal/individual and herd/household levels using RVF IgG data (we defined a seropositive household or herd as any household with at least one seropositive human or animal, respectively) generated through cross-tabulations. These results were generated through cross-tabulations using the propCI function in the prevalence package. Prevalence was tabulated for each categorical variable. In livestock, the categorical variables considered included animal sex (male and female), species (camels, goats, and sheep), and age (less than one year and above one year). The above descriptive statistical analyses were further calculated for each livestock species. Human seroprevalence estimates were also stratified by demographic characteristics.
Household size was categorized into two levels (≤ five and > five household members), while herd size was categorized into three levels (≤ 100, 101–200, 201–300, and > 300 animals). The Chi-square (χ2) was performed to determine if differences between variables were significant and to assess the relationships between categorical variables and seropositivity of RVF IgG in livestock and humans. Positive samples by IgM ELISA were classified as recent exposures because these antibodies are known to last between four to 72 days post-exposure and are usually undetectable six months post-exposure51,52. Samples that remained borderline after retesting were removed from the analysis.
Statistical modeling
Two models were used, one for human and another for animal-level data, with the level of IgG RVF exposure at the human subject/animal level being used as the outcome variable in both models. We used a hierarchical Bayesian modeling algorithm that applies both Integrated Nested Laplace approximation (INLA) to account for the clustering of data and stochastic partial differential equation (SPDE) to estimate model parameters and account for spatial effects. These models were fitted using the R-INLA function in R (R-INLA version 4.1.1.)53. A hierarchical model was preferred to the standard analysis based on evidence that showed RVFV exposure is influenced to a large extent by spatial variability54,55. A mesh was created to help capture relative locations of the sampling points and herds (Supplementary Figure S1).
For both models, subject-level, herd, and ecological-level variables were used as predictors in the model. The animal-level variables considered were age, sex, and species. For humans, the variables considered were sex, age, and risk practices. The herd level variable was herd size. The ecological predictor variables for RVF transmission were selected based on their possible role in RVF transmission, as described in the literature. These included soil type, slope, elevation, soil texture, distance to a river, normalized difference vegetation index (NDVI), land surface temperature(LST), land use, and land cover20,55,56,57. Table 1 details predictors of RVFV transmission and their sources.
Model building
For both models, progressive forward and backward variable selection procedures were used to identify and select fixed factors that could be retained in the model. For the first model, a variable or interaction terms were retained in the model if their inclusion minimized the Deviance Information Criterion (DIC). For continuous variables, the linearity assumption was evaluated by fitting and evaluating the significance of their quadratic terms. The significance of the spatial effect in both models was tested using DIC statistics.
Results
We collected samples from 676 humans in 237 households and 1,864 livestock sera samples (96 camels, 828 sheep, and 940 goats) from 201 households. The median age of the humans sampled was 37.1 years old (range 4–82). The majority of the study participants were male (83%). On average, we sampled three humans per household (range 1–8), and the median household size was four (1–15). Most study participants had no formal education (75%); less than 10% had completed high school education. The median herd size of all livestock combined was 155 (ranging from 8 to 1,113), while the median herd/flock sizes for camels, goats, and sheep were 40 (range: 19–100), 79 (range: 8–546), and 73 (range: 4–626), respectively.
Of the total number of livestock-keeping households surveyed, 62% owned two different types of livestock, 21.16% owned only one species, and only 15% owned three different types of livestock. The median number of animals sampled in each household was 10 (range: 1–15). Most animals sampled were female (77%), and 86% of livestock samples were from animals above one year (Table 2).
We evaluated the prevalence of livestock abortion in the three months preceding the study as it is considered one of the primary syndromes indicative of RVFV infection. We used adult female animals as the denominator in our calculations; 131 cattle, 60 camels, 570 sheep, and 655 goats. The prevalence of abortion was observed in goats at 37.1% (95% CI 33.4–40.9), followed by camels at 15% (95% CI 7.1–26.6%), and cattle at 19.8% (95% CI 13.4–27.7). Sheep had the lowest reported abortion rate at 10.9% (95% CI 8.4–13.7). Overall, the herd-level prevalence of abortion in the three months preceding our study was 85.9%.
Livestock Rift Valley fever virus seroprevalence by cELISA
Overall, livestock anti-RVFV IgG seropositivity was 21.7% (95% CI 19.9–23.7%), and the livestock household positivity (households with at least one seropositive animal) was 64.2% (95% CI 58.9–71.4). Among livestock, the prevalence was highest in goats (Table 2). RVF seropositivity in animals significantly differed by age, animal sex, and location (Table 2).
Livestock Rift Valley fever virus seroprevalence by IgM ELISA
Four of 1,103 randomly selected livestock samples (535 sheep, 471 goats, 97 camels) tested positive using anti-RVFV IgM antibody Capture (MAC) ELISA giving an overall positivity rate of 0.4% (95% CI 0.1–0.9%). All the IgM-positive livestock were sheep; of these, only one was positive for both IgG and IgM.
Human Rift Valley fever virus seroprevalence by cELISA
The individual human seroprevalence was 28.4% (95% CI 24.9–31.90), while the human household seroprevalence was 53.5% (95% CI 46.60–59.65). Although females had a lower seropositivity of 23.3% compared to males at 29.5%, the difference was not statistically significant. Age, location, and education level were, however, significant (Table 2).
Multivariable analysis
Results from the multivariable analysis of animal and human data are shown in Tables 3 and 4, respectively. The animal-level factors that were significantly associated with RVFV seroprevalence included sex and age. Female livestock had significantly lower odds of RVFV infection compared to males. Younger livestock, including suckling animals and weaners, had significantly lower odds of RVF exposure compared to adults (Table 3). Out of the eight ecological variables, elevation was found to negatively influence RVF exposure in livestock while rainfall positively influences RVF exposure. However, further analysis reveals significant interaction between the two variables because the rate of decline of RVFV exposure with elevation was faster in areas with lower rainfall than in areas with higher rainfall (Fig. 2). The presence of a significant interaction between mean rainfall and elevation implies that the effects of these two variables on RVFV seroprevalence are interdependent and should not be evaluated in isolation. Household herd sizes were not associated with RVF exposure in livestock and were therefore removed from the final multivariable model. DIC estimates of models with and without the spatial random effect were 2028.23 and 2193.27, respectively, indicating a significant spatial autocorrelation in the data.
Human data
In humans, age, participation in animal slaughter, and elevation were found to be significantly associated with RVFV exposure (Table 4). Younger people (< 18 years) had lower odds of RVFV exposure compared to middle-aged individuals (18–40 years). Participation in the slaughter of animals was associated with higher odds of exposure. Similarly, people who lived in lower altitude areas had higher odds of exposure compared to those who lived in elevated areas. DIC estimates of models with and without the spatial random effect were 736.61 and 737.47, respectively, indicating that the spatial component substantially improves the fit of the model.
Discussion
This study provides evidence of RVFV exposure in humans and livestock and RVFV circulation during the IEP period. We also provide data quantifying the influence of spatial variability in RVFV infections in a defined ecosystem that has had repeated outbreaks of the disease. The detection of RVFV IgM antibodies in non-clinical study animals provides evidence of recent exposure. This supports the hypothesis that RVF could be primarily maintained through cryptic transmission in-between outbreaks because these antibodies are known to persist for four days to two months post-exposure in sheep while in cattle, the highest mean level of IgM detection is within the first 30 days although antibodies could be detected in a small proportion of cattle (3.6%) up to five months post-exposure51,52. Similar studies have also reported low rates of subclinical, active infection during IEP in Kenya and Tanzania, not only building evidence of the role of cryptic transmission in RVF maintenance but also reaffirming the challenges of RVF passive surveillance during non-outbreak periods39,58. An interesting data point from our study was that all the IgM-positive animals were sheep. Although we cannot explicitly infer this to mean sheep are more susceptible to RVFV exposure during IEP, multiple other studies have reported the same finding59,60,61,62.
The high IgG RVF prevalence in livestock was not unexpected; Isiolo has had a history of RVF outbreaks (the last outbreak reported was in December 2021), and the area has suitable habitat for RVFV vector survival20,32,63. Transhumance pastoralism, the primary livestock production system practiced in the region, could also contribute to the high exposure rates because livestock movement is a primary pathway for the introduction of RVFV into susceptible populations64,65. Interspecies differences between goats, sheep, and camels were not significant, which we postulate to be a factor of similarities in exposure for animals living in the same environment. We found no published data on the IEP prevalence of RVF in small ruminants in Isiolo. Our results are, however, comparable to livestock prevalence of 25% and 28% reported in the neighboring counties of Tana-River and Garissa, respectively, providing further insight into the endemicity of RVF in northeastern Kenya66,67. Our data on RVF exposure in camels makes for an interesting discussion point because studies providing evidence of camel exposure to RVFV in Kenya are very few and far between. We found a seroprevalence of 14% in camels, the same as we reported in a 2020 study68, corroborating previous assertions that camels could play a significant role in RVF maintenance. Other studies in East Africa have reported varying seroprevalences, 28% in Tanzania69 and 43% in Ethiopia70, further justifying the urgent need to invest in RVF active surveillance studies in Kenyan camels.
Surveillance for livestock abortion is essential in RVF detection and response because abortions are one of the earliest observable signs of RVF12. As such, establishing baseline abortion levels in livestock is critical in determining thresholds for event-based RVF early warning systems34. However, there is very limited data on abortion surveillance in Kenya, and we believe this is the first report on the prevalence of abortion in pastoral herds in Kenya. A recent study in Tanzania, however, reported comparable results; the prevalence of abortion was highest in goats, followed by cattle, and least in sheep. The Tanzanian study detected an outbreak of RVF during an interepidemic period, exemplifying the utility of abortion surveillance as a tool in RVF detection71.
The significant association between livestock seropositivity and age, a finding commonly reported by similar studies in and outside Kenya, implies continuous viral exposure over time, a defining characteristic of enzootic transmission cycles66,67,72,73. Our data shows female livestock had lower odds of exposure. Literature on the association between sex and RVF positivity in livestock is contradictory; several studies report higher odds of infection in females66,72,74,75, some report no association58,73,76, and others, like ours, find higher odds of exposure in males77. The varying statistics indicate that it is improbable that sex has a significant association with RVF positivity. The differences could be attributed to differences in study sites and techniques, or as we hypothesize in our study, differences in livestock management practices such as animal movement and vector control between male animals and the more valued female livestock.
As is the case in livestock, the high individual and household prevalence in humans indicates the endemicity of RVF. A survey done in the same study area six years prior reported a human prevalence of 26.7%, closely mirroring our 24% finding, reaffirming the almost hyperendemic status of RVF in Isiolo78. Other studies in Kenya and Tanzania have reported widely varying positivity ranges, from 4.5% in Serengeti to 39% in Kenya, which could be primarily a factor of spatial variation or risk practices78,79. We attribute the high human seroprevalence to the pastoral lifestyle, characterized by regular contact with potentially-infected livestock and livestock products and the abundance of potential RVFV vectors in the study area63,78,80,81.
Risk factor analysis revealed slaughtering and butchering animals and age was associated with higher odds of RVFV exposure. Direct human contact with infected animal tissues has long been reported to be one of the main pathways for zoonotic transmission of RVFV, explaining why slaughtering and butchering is a common risk factor in many studies78,82,83. This underlines the importance of awareness creation on infection prevention control practices among high-risk groups like pastoralists, meat handlers, butchers, and slaughterhouse workers. We found age has a positive effect on seropositivity; this demonstrates continuous viral exposure over time. The fact that older populations are more likely to be involved in risky practices like slaughtering and butchering animals could also contribute to the age-dependent variance66,78. In our study, there was no association between sex and RVFV seropositivity. Reports on the association between sex and human exposure are heterogeneous. Some studies report males and females have different odds of infection due to cultural-gender roles predisposing them to distinct high-risk practices83. In contrast, others do not report significant associations. In our study, we did not find a significant association between sex and RVFV exposure, a finding reported by another RVF cross-sectional study in Kenya37. We postulate this could be due to similarities in viral exposure between males and females in our study area.
Our analysis shows humans and livestock in Sericho ward had significantly higher RVFV seropositivity, leading us to investigate the possible role of ecological variability on RVFV exposure in humans and livestock. We found a significant interaction between rainfall and elevation in their influence on livestock seropositivity meaning areas with low rainfall densities (e.g., 20 mm/year) and low altitude ~ 200 m above sea level will generally have high baseline RVFV seroprevalences. These areas are likely to be flood plains in areas like Sericho ward which often suffer high infection pressures during the wet season. It would therefore be expected that the prevalence of the disease would drop considerably with increasing altitude because, at higher altitudes, incidences of flooding will be minimal. Conversely, areas with higher rainfall levels (e.g., 60 mm) would be able to sustain RVFV infections regardless of whether they are in lower or higher altitudes. This is because the seasonal rains received would be able to support mosquito populations. Elevation influences the amount of precipitation, possibly explaining the correlation between the two variables. Additionally, rainfall provides water pools required for mosquito vector larvae development and eventual survival in the dry season, which could explain the significant linkage between elevation, rainfall, and seropositivity25,57. Elevation was a significant predictor variable for seropositivity in humans. The impact of elevation alone on RVF can vary based on local ecological and climatic conditions, but lower elevation areas below 1000 m are generally warmer and permissive of vector survival compared to high altitude areas explaining the inverse relationship between RVFV seropositivity and elevation10,27. Other ecological determinants like soil type investigated were not significant, which could be a factor of their homogeneity in our small study area compared to studies done over vast regions.
There were several limitations to our study. One was insecurity in parts of our study area, making it impossible to sample the entire target area. The other limitation was we did not perform a confirmatory plaque reduction neutralization test to rule out false positives and cross-immunoreactivity with viruses in the same family. An inherent weakness of crossectional studies is that study participants are expected to recall and report exposures subject to bias. The results suggest cryptic transmission of RVFV with spatial variability in exposure driven by elevation and rainfall in northern Kenya. Our data did illustrate the association of demographic and behavioral risk practices to RVFV, which can be used to define most at-risk groups. The data demonstrates the need for more extensive studies to elucidate further the true burden of RVF and the drivers of infection in high-risk regions. Our findings demonstrate the need for multi-species, prospective, active surveillance studies to better understand transmission dynamics during IEP. This data will guide the design of effective interventions to minimize future outbreaks' impact on vulnerable populations.
Data availability
All data is completely available without any restrictions. Please contact the corresponding author at muturimathew@gmail.com for any data requests.
References
Munyua, P. et al. Prioritization of zoonotic diseases in Kenya, 2015. PLoS ONE 11, e0161576 (2016).
Cavalerie, L. et al. One hundred years of zoonoses research in the horn of Africa: A scoping review. PLoS Negl. Trop. Dis. 15, e0009607 (2021).
Peyre, M. et al. A systematic scoping study of the socio-economic impact of Rift Valley fever: Research gaps and needs. Zoonoses Public Health 62, 309–325 (2015).
Muga, G. O., Onyango-Ouma, W., Sang, R. & Affognon, H. Sociocultural and economic dimensions of Rift Valley fever. Am. Soc. Trop. Med. Hyg. 92, 730–738 (2015).
Rich, K. M. & Wanyoike, F. An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley fever outbreak in Kenya. Am. J. Trop. Med. Hyg. 83, 52–57 (2010).
Daubney, R. & Hudson, J. R. Rift Valley fever. Lancet 219, 611–612 (1932).
Imam, I. Z. E., Darwish, M. A. & El-Karamany, R. An epidemic of Rift Valley fever in Egypt. I. Diagnosis of Rift Valley fever in man. Bull. World Health Organ. 57, 437–439 (1979).
Madani, T. et al. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin. Infect. Dis. 37, 1084–1092 (2003).
Hui, D. S. et al. Middle east respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet. Infect. Dis. 18, e217–e227 (2018).
Seck, I. et al. Identification of drivers of Rift Valley fever after the 2013–14 outbreak in Senegal using serological data in small ruminants. PLoS Negl. Trop. Dis. 16, e0010024 (2022).
Meegan, J. M. The Rift Valley fever epizootic in Egypt 1977–1978 1. Description of the epizootic and virological studies. Trans. R. Soc. Trop. Med. Hyg. 73, 618–623 (1979).
Hartman, A. Rift Valley fever. Clin. Lab. Med. 37, 285–301 (2017).
Nguku, P. M. et al. An investigation of a major outbreak of Rift Valley fever in Kenya: 2006–2007. Am. J. Trop. Med. Hyg. 83, 5–13 (2010).
Linthicum, K. J. et al. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 285, 397–400 (1999).
Anyamba, A. et al. Climate teleconnections and recent patterns of human and animal disease outbreaks. PLoS Negl. Trop. Dis. 6, e1465 (2012).
Davies, F. G., Koros, J. & Mbugua, H. Rift Valley fever in Kenya: The presence of antibody to the virus in camels (Camelus dromedarius). J. Hyg. (Lond) 94, 241–244 (1985).
Pepin, M., Bouloy, M., Bird, B. H., Kemp, A. & Paweska, J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 41, 61 (2010).
Turell, M. J., Dohm, D. J., Geden, C. J., Hogsette, J. A. & Linthicum, K. J. Potential for stable flies and house flies (Diptera: Muscidae) to transmit Rift Valley fever virus. J. Am. Mosq. Control Assoc. 26, 445–448 (2010).
Rolin, A. I., Berrang-Ford, L. & Kulkarni, M. A. The risk of Rift Valley fever virus introduction and establishment in the United States and European union. Emerg. Microbes Infect. 2, e81–e81 (2013).
Mosomtai, G. et al. Association of ecological factors with Rift Valley fever occurrence and mapping of risk zones in Kenya. Int. J. Infect. Dis. 46, 49–55 (2016).
Glancey, M. M., Anyamba, A. & Linthicum, K. J. Epidemiologic and environmental risk factors of Rift Valley fever in Southern Africa from 2008 to 2011. Vector-Borne Zoonotic Dis. 15, 502–511 (2015).
Linthicum, K. J., Bailey, C. L., Davies, F. G. & Kairo, A. Observations on the dispersal and survival of a population of Aedes lineatopennis (Ludlow) (Diptera: Culicidae) in Kenya. Bull. Entomol. Res. 75, 661–670 (1985).
Gargan, T. P., Jupp, P. G. & Novak, R. J. Panveld oviposition sites of floodwater Aedes mosquitoes and attempts to detect transovarial transmission of Rift Valley fever virus in South Africa. Med. Vet. Entomol. 2, 231–236 (1988).
Linthicum, K. J. et al. Towards real-time prediction of Rift Valley fever epidemics in Africa. Prev. Vet. Med. 11, 325–334 (1991).
Davies, F. G., Linthicum, K. J. & James, A. D. Rainfall and epizootic Rift Valley fever. Bull. World Health Organ. 63, 941 (1985).
Linthicum, K. J., Britch, S. C. & Anyamba, A. Rift Valley fever: An Emerging mosquito-borne disease. Annu. Rev. Entomol. 61, 395–415 (2016).
Kariuki Njenga, M. & Bett, B. Rift Valley fever virus—how and where virus is maintained during inter-epidemic periods. Curr. Clin. Microbiol. Rep. 6, 18–24 (2019).
Lumley, S. et al. Rift Valley fever virus: strategies for maintenance, survival and vertical transmission in mosquitoes. J. Gen. Virol. 98, 875–887 (2017).
Ringot, D., Durand, J.-P., Tolou, H., Boutin, J.-P. & Davoust, B. Rift Valley fever in chad. Emerg. Infect. Dis. J. 10, 945 (2004).
Bosworth, A. et al. Serologic evidence of exposure to Rift Valley fever virus detected in Tunisia. New Microbes New Infect. 9, 1–7 (2016).
Yılmaz, A. et al. Presence of antibodies to Rift Valley fever virus in children, cattle and sheep in Turkey. J. Virol. Antivir. Res. 6, 29 (2017).
Murithi, R. M. et al. Rift Valley fever in Kenya: History of epizootics and identification of vulnerable districts. Epidemiol. Infect. 139, 372–380 (2011).
Hassan, A. et al. Epidemiological investigation of a Rift Valley fever outbreak in humans and livestock in Kenya, 2018. Am. J. Trop. Med. Hyg. https://doi.org/10.4269/ajtmh.20-0387 (2020).
Oyas, H. et al. Enhanced surveillance for Rift Valley fever in livestock during El Niño rains and threat of RVF outbreak, Kenya, 2015–2016. PLoS Negl. Trop. Dis. 12, e0006353 (2018).
Mbotha, D. et al. Inter-epidemic Rift Valley fever virus seroconversions in an irrigation scheme in Bura, south-east Kenya. Transbound. Emerg. Dis. 65, e55–e62 (2018).
Lichoti, J. K. et al. Detection of Rift Valley fever virus interepidemic activity in some hotspot areas of Kenya by sentinel animal surveillance, 2009–2012. Vet. Med. Int. 2014, 379010 (2014).
Gray, G. C. et al. Seroepidemiological study of interepidemic Rift Valley fever virus infection among persons with intense ruminant exposure in Madagascar and Kenya. Am. Soc. Trop. Med. Hyg. 93, 1364–1370 (2015).
Wekesa, F. C., Wamalwa, M. & Oduor, R. Serological evidence of inter-epidemic circulation of Rift Valley fever virus in livestock in Kenya. East Afr. Agric. For. J. 85, 13–13 (2022).
Sang, R. et al. Tracking the inter-epidemic activity of Rift Valley fever (RVFV) virus in RVF outbreak hotspots in Kenya: Determination of biotic and socio-economic drivers. Int. J. Infect. Dis. 21, 177 (2014).
Gerken, K. N. et al. Paving the way for human vaccination against Rift Valley fever virus: A systematic literature review of RVFV epidemiology from 1999 to 2021. PLoS Negl. Trop. Dis. 16, e0009852 (2022).
Métras, R. et al. Rift Valley fever epidemiology, surveillance, and control: What have models contributed?. Vector-Borne Zoonotic Dis. 11, 761–771 (2011).
Munyua, P. M. et al. Predictive factors and risk mapping for Rift Valley fever epidemics in Kenya. PLoS ONE 11, e0144570 (2016).
De Leeuw, J. et al. Benefits of riverine water discharge into the lorian swamp, Kenya. Water 4, 1009–1024 (2012).
2019 Kenya Population and housing census-Kenya National Bureau of Statistics. https://www.knbs.or.ke/?wpdmpro=2019-kenya-population-and-housing-census-volume-i-population-by-county-and-sub-county (2019).
Thrusfield, M. Veterinary epidemiology (John Wiley & Sons, 2018).
Otte, M. J. & Gumm, I. D. Intra-cluster correlation coefficients of 20 infections calculated from the results of cluster-sample surveys. Prev. Vet. Med. 31, 147–150 (1997).
Rutterford, C., Copas, A. & Eldridge, S. Methods for sample size determination in cluster randomized trials. Int. J. Epidemiol. 44, 1051–1067 (2015).
Kairu-Wanyoike, S. et al. Positive association between Brucella spp. seroprevalences in livestock and humans from a cross-sectional study in Garissa and Tana river counties. Kenya. PLoS Negl. Trop. Dis. 13, e0007506 (2019).
de Bronsvoort, B. M. C. et al. Comparison of two Rift Valley fever serological tests in cameroonian cattle populations using a Bayesian latent class approach. Front. Vet. Sci. 6, 258 (2019).
Hijmans, R. J. et al. Package ‘raster’. R Packag. 734, (2015).
Paweska, J. T. et al. IgG-sandwich and IgM-capture enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in domestic ruminants. J. Virol. Methods 113, 103–112 (2003).
Morvan, J., Rollin, P. E., Laventure, S. & Roux, J. Duration of immunoglobulin M antibodies against Rift Valley fever virus in cattle after natural infection. Trans. R. Soc. Trop. Med. Hyg. 86, 675 (1992).
Rue, H. & Martino, S. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Ser. B: Stat. Methodol. 71(2), 319–392 (2009).
Redding, D. W., Tiedt, S., Lo Iacono, G., Bett, B. & Jones, K. E. Spatial, seasonal and climatic predictive models of Rift Valley fever disease across Africa. Philos. Trans. R. Soc. B: Biol. Sci. 372, 20160165 (2017).
Lo Iacono, G. et al. Environmental limits of Rift Valley fever revealed using ecoepidemiological mechanistic models. Proc. Natl. Acad. Sci. 115, E7448–E7456 (2018).
Britch, S. C. et al. Rift Valley fever risk map model and seroprevalence in selected wild ungulates and camels from Kenya. PLoS ONE 8, e66626 (2013).
Assaf, A. et al. Prediction of a Rift Valley fever outbreak. Proc. Natl. Acad. Sci. 106, 955–959 (2009).
Kumalija, M. S. et al. Detection of Rift Valley fever virus inter-epidemic activity in Kilimanjaro region, North Eastern Tanzania. Glob. Health Act. 14, 1957554 (2021).
Ikegami, T. & Makino, S. The pathogenesis of Rift Valley fever. Viruses 3, 493–519 (2011).
Wright, D., Kortekaas, J., Bowden, T. A. & Warimwe, G. M. Rift Valley fever: Biology and epidemiology. J. Gen. Virol. 100, 1187–1199 (2019).
Sado, F. Y. et al. Seroprevalence of Rift Valley fever virus in domestic ruminants of various origins in two markets of Yaoundé. Cameroon. PLoS Negl. Trop. Dis. 16, e0010683 (2022).
Clark, M. H. A., Warimwe, G. M., Di Nardo, A., Lyons, N. A. & Gubbins, S. Systematic literature review of Rift Valley fever virus seroprevalence in livestock, wildlife and humans in Africa from 1968 to 2016. PLoS Negl. Trop. Dis. 12, e0006627 (2018).
Sang, R. et al. Distribution and abundance of key vectors of Rift Valley fever and other arboviruses in two ecologically distinct counties in Kenya. PLoS Negl. Trop. Dis. 11, e0005341 (2017).
Nicolas, G., Chevalier, V., Tantely, L. M., Fontenille, D. & Durand, B. A Spatially explicit Metapopulation model and cattle trade analysis suggests key determinants for the recurrent circulation of Rift Valley fever virus in a pilot area of Madagascar highlands. PLoS Negl. Trop. Dis. 8, e3346 (2014).
Durand, B. et al. Rift Valley fever in northern Senegal: A modelling approach to analyse the processes underlying virus circulation recurrence. PLoS Negl. Trop. Dis. 14, e0008009 (2020).
Bett, B. et al. Association between Rift Valley fever virus seroprevalences in livestock and humans and their respective intra-cluster correlation coefficients, Tana river county, Kenya. Epidemiol. Infect. 147, 1–9 (2018).
Nanyingi, M. O. et al. Seroepidemiological survey of Rift Valley fever virus in ruminants in Garissa, Kenya. Vector-Borne Zoonotic Dis. 17, 141–146 (2016).
Muturi, M. et al. Serological evidence of single and mixed infections of Rift Valley fever virus, Brucella spp. and Coxiella burnetii in dromedary camels in Kenya. PLoS Negl. Trop. Dis. 15, e0009275 (2021).
Swai, E. S. & Sindato, C. Seroprevalence of Rift Valley fever virus infection in camels (dromedaries) in northern Tanzania. Trop. Anim. Health Prod. 47, 347–352 (2015).
Ibrahim, M. et al. Sero-prevalence of brucellosis, Q-fever and Rift Valley fever in humans and livestock in Somali Region. Ethiopia. PLoS Negl. Trop. Dis. 15, e0008100 (2021).
Thomas, K. M. et al. Prospective cohort study reveals unexpected aetiologies of livestock abortion in northern Tanzania. Sci. Rep. 12, 11669 (2022).
Sumaye, R. D., Geubbels, E., Mbeyela, E. & Berkvens, D. Inter-epidemic transmission of Rift Valley fever in livestock in the Kilombero River valley, Tanzania: A cross-sectional survey. PLoS Negl. Trop. Dis. 7, e2356 (2013).
Salekwa, L. P., Wambura, P. N., Matiko, M. K. & Watts, D. M. Circulation of Rift Valley fever virus antibody in cattle during Inter-epizootic/epidemic periods in selected regions of Tanzania. Am. J. Trop. Med. Hyg. 101, 459–466 (2019).
Owange, N. O. et al. Occurrence of Rift Valley fever in cattle in Ijara district, Kenya. Prev. Vet. Med. 117, 121–128 (2014).
Kainga, H. et al. Seroprevalence and associated risk factors of Rift Valley fever in livestock from three ecological zones of Malawi. Pathogens 11, 1349 (2022).
Matiko, M. K. et al. Serological evidence of inter-epizootic/inter-epidemic circulation of Rift Valley fever virus in domestic cattle in Kyela and Morogoro, Tanzania. PLoS Negl. Trop. Dis. 12, e0006931 (2018).
Jeanmaire, E. M. et al. Prevalence of Rift Valley fever infection in ruminants in Madagascar after the 2008 outbreak. Vector-Borne Zoonotic Dis. 11, 395–402 (2011).
Tigoi, C. et al. High risk for human exposure to Rift Valley fever virus in communities living along livestock movement routes: A cross-sectional survey in Kenya. PLoS Negl. Trop. Dis. 14, e0007979 (2020).
Ahmed, A., Makame, J., Robert, F., Julius, K. & Mecky, M. Sero-prevalence and spatial distribution of Rift Valley fever infection among agro-pastoral and pastoral communities during Interepidemic period in the Serengeti ecosystem, northern Tanzania. BMC Infect. Dis. 18, 276 (2018).
Anyangu, A. S. et al. Risk factors for severe Rift Valley fever infection in Kenya, 2007. Am. J. Trop. Med. Hyg. 83, 14–21 (2010).
Ng’ang’a, C. M., Bukachi, S. A. & Bett, B. K. Lay perceptions of risk factors for Rift Valley fever in a pastoral community in northeastern Kenya. BMC Public Health 16, 32 (2016).
McMillen, C. M. & Hartman, A. L. Rift Valley fever in animals and humans: Current perspectives. Antiviral Res. 156, 29–37 (2018).
Woods, C. W. et al. An outbreak of Rift Valley fever in Northeastern Kenya, 1997–98. Emerg. Infect. Dis. 8, 138–144 (2002).
Acknowledgements
We express our gratitude to our study participants for willingly participating in our research. We would also like to thank the study team for their diligent efforts in carrying out the study. Additionally, we would like to acknowledge the human and animal health officials from the national ministries of Health and Agriculture and the County government of Isiolo for approving the study. This study was conducted as part of the project titled "Co-infection with Rift Valley fever virus, Brucella spp., and Coxiella burnetii in humans and animals in Kenya: Disease burden and ecological factors," which received funding from the Defense Threat Reduction Agency under Grant No. HDTRA11910031. It is important to note that the information presented does not necessarily reflect the position or policy of the federal government, and no official endorsement should be assumed. Furthermore, we would like to acknowledge the donors of the CGIAR Fund, whose contributions can be found at https://www.cgiar.org/funders.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Design of the work: M.M., A.M., A.M.N., J.A., R.N., K.R., L.W., B.B. Data analysis and interpretation: M.M., M.N., A.M., M.N., D.N., B.B., S.M.T. Manuscript review and approval: M.M., A.M., A.M.N., J.A., R.N., A.M., M.N., D.N., L.W., K.R., S.M.T., B.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muturi, M., Mwatondo, A., Nijhof, A.M. et al. Ecological and subject-level drivers of interepidemic Rift Valley fever virus exposure in humans and livestock in Northern Kenya. Sci Rep 13, 15342 (2023). https://doi.org/10.1038/s41598-023-42596-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42596-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.