Abstract
Cancer-related anorexia/cachexia syndrome (CACS) is characterized by anorexia and loss of body weight. Evidence is insufficient to strongly endorse any pharmacologic agent for the treatment of CACS. In this systematic review, we assessed the efficacy of oral anamorelin treatment for patients with CACS. On July 6, 2022, we systematically searched the following databases for randomized controlled trials (RCTs) of adults with CACS comparing oral anamorelin versus placebo: CENTRAL, PubMed, EMBASE, and ICHUSHI. The primary outcomes were total body weight (TBW), patient-reported quality of life (QOL), and adverse events (AEs). Secondary outcomes included lean body mass (LBM), overall survival (OS), non-dominant hand grip strength (HGS), and appetite. We included seven RCTs with a total of 1944 CACS patients. Anamorelin significantly increased TBW (mean difference (MD) 1.73, 95% confidence interval (CI) 1.34–2.13, p < 0.00001), LBM (MD 1.06, 95% CI 0.30–1.81, p = 0.006), and QOL (standardized mean difference (SMD) 0.16, 95% CI 0.04–0.27, p = 0.006) compared with placebo without a significant difference in all AEs, severe AEs, OS, HGS or appetite. Anamorelin may be an effective treatment for CACS patients; however, further studies are needed to confirm the efficacy and safety of this drug.
Similar content being viewed by others
Introduction
Cancer-related anorexia/cachexia syndrome (CACS) is a multifocal disease with anorexia and body weight loss associated with reduced adipose tissue and muscle mass1. It is frequently seen in patients with advanced cancer, and is estimated to occur in more than 50% of all cancer patients2. The criteria for cancer cachexia are loss of body weight of > 5%, or loss of body weight of > 2% in individuals already having depletion based on a current body mass index (BMI) of < 20 kg/m2 or skeletal muscle mass3. It is related to increased mortality, poor performance status and quality of life (QOL), and poor treatment outcomes, therefore various pharmacological and non-pharmacological treatments are applied4. CACS treatment is expected to improve appetite, body weight, QOL, performance status (PS), and overall survival. However, even now there is no effective or standard treatment.
Ghrelin is a 28-amino-acid peptide, and is the natural ligand for the growth hormone secretagogue receptor5. Ghrelin is produced in the stomach and stimulates growth hormone secretion and induction of insulin-like growth factor-1 secretion, leading to increased food intake and weight gain6. Since previous small randomized trials have shown that ghrelin therapy can be safely administered to patients with advanced cancer, ghrelin-related medicines have recently been studied as a promising approach to CACS7,8,9.
Anamorelin is an oral ghrelin mimetic and selective agonist that exerts its action at the ghrelin receptor10. Anamorelin is well tolerated and multiple randomized controlled trials (RCTs) have shown an improvement in total body weight (TBW), lean body mass (LBM), QOL, and appetite in patients with incurable cancer compared with placebo11,12,13,14. In December 2020, anamorelin 100 mg has been approved for CACS in Japan, largely based on its positive effect on patients’ weight observed in phase II trials; however, anamorelin has not been approved in the United States or Europe, with the Committee for Medicinal Products for Human Use noting its limited effect on lean body mass and no proven impact on hand grip strength or quality of life; additionally, its safety data was inadequately recorded15. Therefore, several phase III trials are currently conducted in the United States and Europe.
Two previous systematic reviews from 2017 reported improvement in TBW, LBM, and QOL for cancer patients receiving anamorelin16,17. However, limitations such as the lack of a comprehensive search strategy, the small number of eligible studies for inclusion, and heterogeneity of studies have been noted. Several RCTs have also been published since these systematic reviews have been conducted, making it necessary to update the evidence on the effectiveness of anamorelin on TBW, QOL, and adverse events (AEs) in all cancer patients.
The aim of this systematic review is to evaluate the efficacy and limitations of anamorelin on patients with CACS.
Methods
This systematic review and meta-analysis was carried out according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) Statement18. The protocol was registered in PROSPERO (CRD42022340705).
Types of studies
We included individually randomized controlled trials. We excluded animal, in vitro, observational studies, and narrative and systematic reviews.
Types of participants
We included trials in which the participants were older than 18 years and were patients with any type and stage of cancer.
Types of interventions and comparison
We compared oral anamorelin (any dosage) with placebo.
Types of outcome measures
Primary outcomes
The primary outcomes were total body weight (TBW), patient-reported quality of life (QOL), and adverse events (AEs). Adverse events were tabulated as all AEs, severe AEs, and drug-related AEs. Adverse events reported as higher than Grade 3 in the Common Terminology Criteria for Adverse Events (CTCAE) score were counted as severe adverse events.
Secondary outcomes
Secondary outcomes included lean body mass (LBM), overall survival (OS), non-dominant hand grip strength (HGS), and appetite.
Search methods for identification of studies
We conducted literature searches for studies in the following electronic databases on July 6, 2022: PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, and ICHUSHI.
The search strategy was based on discussions with an information specialist and was adapted for each database. The full search strategies are detailed in Supplement 1. We only included peer-reviewed articles with human participants, and excluded any abstracts and meeting presentations. No language restrictions were applied.
Data collection
Selection of studies
After database searches and removal of duplicates, JT and MS independently screened and reviewed the title and abstracts of all references identified. The full text of all relevant articles was then retrieved to assess eligibility. Any disagreements were resolved through discussion.
Data extraction
JT extracted the following information on each study into a pre-specified data extraction form and SM checked for accuracy: (1) study characteristics: the first author’s name, publication year, type of study, country, and source of funding; (2) patients’ characteristics: cancer type, age, sex, race; (3) intervention and comparison: dose and duration; (4) relevant outcomes.
Assessment of risk of bias in included studies
JT and SM independently assessed risk of bias for each study using the revised Cochrane risk of bias tool 2 for randomized trials (RoB 2 tool)19. We assessed the following domains: randomization process (D1), deviation from intended interventions (D2), missing outcome data (D3), measurement of outcome (D4), selection of the reported result (D5), and overall bias. Each domain was judged for low or high risk of bias, or any concerns. In accordance with RoB 2, if an individual domain was at a particular level of risk of bias, the overall risk of bias was at least that severe. Therefore, if the risk of bias was “high” in any domain, we judged the overall risk of bias as “high.” If there were “some concerns” in multiple domains, we judged the overall risk of bias as “high” risk for that outcome. We resolved any disagreements through discussion.
Assessment of the certainty of evidence
We used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach20 to assess the certainty of the evidence for our outcomes, TBW, QOL, AEs, and LBM using the following five domains: risk of bias, inconsistency, imprecision, indirectness, and publication bias. We judged them to be either of high, moderate, low, or very low certainty. We created a ‘Summary of findings’ table using GRADEpro21.
Meta-analysis and statistical analysis
Statistical analyses and meta-analysis were performed using Review Manager (RevMan) 5.4 software22. For continuous outcomes the mean difference (MD) or standardized mean difference (SMD) was calculated with 95% confidence interval (CI). When the standard deviation (SD) was not available, SD was calculated from the standard error (SE) using the RevMan calculator. Hazard ratio (HR) was calculated for expressing OS. Pooled HRs for outcomes were calculated using the inverse variance method. Risk ratio (RR) was used to assess AEs.
We assessed heterogeneity in meta-analysis using Tau2, I2, and Chi2 statistics. If there was no statistical heterogeneity (p > 0.1, I2 ≤ 50%), we used the fixed-effects model. We used the random-effects model when heterogeneity was detected (p < 0.1, I2 > 50%).
We performed subgroup analysis according to the dosage of anamorelin (100 mg and 50 mg) to determine whether anamorelin 100 mg, as approved in Japan, is appropriate in terms of efficacy and safety.
Results
Study selection
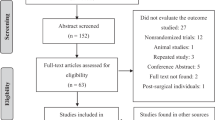
A total of 2176 records were identified by searching four electronic databases. After the exclusion of 619 duplications, we screened 1557 records. We then assessed the full text of 24 articles for inclusion. Ultimately, seven RCTs from six articles were included. The study selection process is shown in Fig. 1.
Characteristics of included studies
The characteristics of the included studies and patients are presented in Table 1. Among the included RCTs, one RCT was a crossover pilot study11, three were phase II clinical trials12,13,23, and the remaining three were phase III clinical trials14,24. Two RCTs were conducted in the United States11,12, two in Japan13,23, and the other RCTs were international and multicenter clinical trials conducted in several countries14,24. The mean or median age was 60–65 years, with more men in all trials. RCTs conducted in the U.S. or multicounty trials had a higher percentage of white participants, while the RCTs conducted in Japan included only Asians (Japanese). Five RCTs included only NSCLC (non-small cell lung carcinoma) patients13,14,23,24 and two RCTs targeted patients with any incurable cancer type11,12. The results of two RCTs, Temel 2016 (ROMANA I) and Temel 2016 (ROMANA II), were reported in one article14. Currow 2017 (ROMANA III) was a safety extension study of the international phase 3 ROMANA I and ROMANA II trials24. The treatment period was 12 weeks in six trials12,13,14,23,24 and only 3 days in the crossover trial11. The dose of anamorelin was 50 mg in 3 trials11,12,13 and 100 mg in 5 trials13,14,23,24. All RCTs were double-blinded and the intervention was compared to placebo. All outcomes were reported as changes from baseline. Five RCTs were funded by Helsinn Therapeutics (US)11,12,14,24and two RCTs by Inc. or Ono Pharmaceutical Co. (Japan), Ltd13,23.
Risk of bias of included studies
We assessed study quality using RoB 2 tool.
Two trials were judged to be at an overall low risk of bias14, three trials were evaluated at a high risk12,13,23, and two trials were judged to have some concerns11,24. All RCTs employed the random number method according to a computer-generated randomization schedule. Allocation concealment was adequate in all trials. All trials were double-blinded (participants and personnel); however, no RCT reported blinding of the outcome assessor. Three studies had missing outcome data after randomization and were considered at high risk of bias for domain 312,13,23. One study also had moderate outcome data missing, therefore domain 3 was judged to be of some concern24. One study was judged as high risk of bias for domain 5 since it was unclear whether the data was analyzed in accordance with a pre-specified analysis plan and there were multiple eligible outcome measurements within the outcome domain23. Similarly, two studies did not indicate a data analysis plan, therefore we judged domain 5 to have some concerns11,13. The risk of bias assessment for each RCT is summarized in Table 2.
Effects of intervention
We summarized the certainty of the evidence for TBW, LBM, QOL, and AEs using the GRADE approach, as shown in Table 3. We downgraded by one or two levels because of high risk of bias due to missing outcome data and reporting bias or the small number of studies and wide CIs. The certainty of the evidence was moderate for TBW, all AEs, severe AEs, and low for LBM, QOL, and drug-related AEs.
Primary outcomes
Total body weight
Five RCTs with 1190 patients were included in the analysis of TBW change from the baseline (Fig. 2)12,13,14,23. There was a significant increase in TBW from baseline among participants treated with anamorelin (MD 1.73, 95% CI 1.34–2.13, p < 0.00001; moderate-certainty evidence). Subgroup analysis according to dosage revealed no differences between 50 and 100 mg of anamorelin. We did not detect heterogeneity (Chi2 = 2.57, p = 0.77, I2 = 0%). Garcia 2013 reported TBW from baseline at 3 days; therefore, the study was not included in the meta-analysis11. The study reported a statistically significant average weight gain of 1.0 kg in the anamorelin group compared with the placebo control (95% CI 0.30–1.9, p = 0.016). Currow 2017 was a safety 12 weeks extension study of Temel 2016 ROMANA I and ROMANA II; therefore, we did not include this trial in this meta-analysis24. Over the 0–24 weeks treatment period, TBW significantly increased in the anamorelin group versus the placebo group (MD 2.1 kg, 95% CI 1.3–3.0, p < 0.001).
Quality of life
Five studies with a total of 1340 participants reported QOL (Fig. 3)12,13,14,23. The results showed anamorelin significantly improved QOL compared with placebo (SMD 0.16, 95% CI 0.04–0.27, p = 0.006; low-certainty evidence). There were no differences between subgroups. Quality of life was assessed with the adjusted Anderson symptom assessment scale (ASAS) in one RCT12, the M.D. Anderson Symptom Inventory (MDASI) scale in one RCT13, the anorexia/cachexia scale of functional assessment of anorexia/cachexia therapy (A/CS of FAACT) in two RCTs14, and quality of life questionnaire for patients treated with anticancer drugs (QOL-ACD) scale in one RCT23. Heterogeneity was low (Chi2 = 2.53, p = 0.77, I2 = 0%). Garcia 2013 reported the mean-adjusted ASAS total scores from baseline at 3 days; therefore, we did not include this study in the meta-analysis11. The results of this study showed the mean-adjusted ASAS total scores significantly increased from 66.44 to 73.80 for the intervention group compared with 66.44–67.44 for the placebo group (95% CI 3.5–10.3, p < 0.002). Currow 2017 reported QOL as the Anorexia/Cachexia Subscale score24. However, there were no significant differences between the two groups (MD 1.2, 95% CI − 0.1 to 2.5) over the period of 24 weeks.
Adverse events and drug-related adverse events
A total of 1405 patients from five RCTs were included in this analysis (Figs. 4 and 5)12,13,14,23. The frequency of all AEs did not differ significantly between the anamorelin and placebo group (RR 1.03, 95% CI 0.96–1.10, p = 0.48; moderate-certainty evidence). The frequency of severe AEs also did not differ significantly between the two groups (RR 1.01, 95% CI 0.72–1.41, p = 0.95; moderate-certainty evidence). We detected high heterogeneity for all AEs and severe AEs (Chi2 = 13.35, p = 0.02, I2 = 63%; Chi2 = 11.21, p = 0.05, I2 = 55%, respectively).
A total of 433 patients with 142 drug-related adverse events were reported in three RCTs (Fig. 6)12,13,23. The frequency of drug-related adverse events was significantly higher in the anamorelin group compared with the placebo group (RR 1.84, 95% CI 1.34–2.53, p = 0.0002; low-certainty evidence). There was no heterogeneity (Chi2 = 1.80, p = 0.61, I2 = 0%). Garcia 2013 was not included in this meta-analysis because the total duration of anamorelin and placebo treatment was 6 days11. Twelve of the 16 patients (75%) reported more than one AE while receiving anamorelin and eight (50%) while receiving placebo. There were no serious AEs while receiving anamorelin, and there was one severe AE while receiving placebo. Adverse events in four patients were assessed to be possibly or probably related to anamorelin. Currow 2017 was a safety extension study of the international phase 3 Temel trials) and therefore, we did not include this study in the meta-analysis24. Adverse events were reported as treatment-emergent AEs (TEAEs). In this study, 179 of 343 patients (52.2%) in the anamorelin group and 93 of 167 patients (55.7%) in the placebo group reported any TEAEs, with 44 (12.8%) and 21 (12.6%) serious TEAEs. Of these, 12 (3.5%) and 2 (1.2%) were determined to be drug-related AEs, respectively. Overall, authors concluded that there are no major safety concerns.
Secondary outcomes
Lean body mass
We included three RCTs with 360 patients in this meta-analysis of LBM (Fig. 7)12,13,23. There was a significant increase in LBM for patients receiving anamorelin compared with placebo (MD 1.06, 95% CI 0.30–1.81, p = 0.006; low-certainty evidence). We detected high heterogeneity between included studies (Chi2 = 10.65, p = 0.01, I2 = 72%). ROMANA I and ROMANA II reported LBM as median values and were not included in this meta-analysis14. However, anamorelin revealed a significant increase in LBM over 12 weeks in both ROMANA I (anamorelin group 0.99 kg (median), 95% CI 0.61–1.36 vs placebo group -0.47 kg (median), 95% CI − 1.00 to 0.21) and ROMANA II (anamorelin group 0.65 kg (median), 95% CI 0.38–0.91 vs placebo group -0.98 kg (median), 95% CI − 1.49 to − 0.41).
Non-dominant hand grip strength
We included three RCTs with 361 patients for HGS in this meta-analysis (Fig. 8)12,13,23. There were no significant differences between the anamorelin group and the placebo group (MD 0.64, 95% CI − 0.01 to 1.28, p = 0.05), and there were no differences between subgroups. Heterogeneity was moderate (Chi2 = 4.56, p = 0.21, I2 = 34%). ROMANA I and ROMANA II reported HGS as median values and therefore, were not included in this meta-analysis14. Anamorelin 100 mg did not show any benefits in ROMANA I (anamorelin group − 1.10 kg, 95% CI; − 1.69 to − 0.40 vs placebo group − 1.58 kg, 95% CI − 2.99 to − 1.14) and ROMANA II (anamorelin group − 1.49 kg, 95% CI − 2.06 to − 0.58 vs placebo group − 0.95 kg, 95% CI − 1.56 to 0.04). The extension study of ROMANA I and ROMANA II also did not show any benefits at 24 weeks24.
Overall survival
We included two RCTs in this analysis (Fig. 9)13,14. Both of these two RCTs evaluated OS at 1 year after 12 weeks of anamorelin treatment. The pooled HR was not significantly different between the intervention group and the placebo group (HR 0.99, 95% CI 0.85–1.14, p = 0.84). Heterogeneity was moderate between included studies (Chi2 = 2.89, p = 0.24, I2 = 31%).
Appetite
Data on appetite were available for 361 individuals in three RCTs (Fig. 10)12,13,23. Patients receiving 100 mg of anamorelin showed a significant improvement in appetite compared with control (SMD, 0.44, 95% CI 0.18–0.71, p = 0.001). However, there were no differences between the groups when patients received 50 mg of anamorelin (SMD, − 0.09, 95% CI − 0.44 to 0.26, p = 0.62). There was no significant difference between subgroups according to the dose of anamorelin. Overall, we detected high heterogeneity between studies (Chi2 = 6.23, p = 0.10, I2 = 52%).
Discussion
We conducted a systematic review and meta-analysis of RCTs to determine the clinical benefit of anamorelin for CACS patients. Our results suggest that anamorelin improves TBW, LBM, and QOL without increasing systemic AEs from soon after the start to 12 weeks of treatment. There were no significant differences between subgroups (50 mg and 100 mg) for all outcomes. Anamorelin significantly increased drug-related adverse events; however, there were no significant dose-dependent differences between subgroups. No significant improvement in OS or HGS was identified; however, results are derived from a small number of trials.
We assessed the certainty of evidence for the outcomes TBW, AEs, LBM, and QOL using GRADE (Table 3). None of the included studies had concerns about random allocation, blinding, or outcome measures. However, some studies were of high risk of bias due to missing outcome data and reporting bias. We also had a limited number of studies for the outcomes LBM and drug-related AEs, so the small number of participants may have resulted in imprecise effect estimates, as indicated by wide CIs. Therefore, we downgraded by one or two levels. Overall, the certainty of the evidence was moderate for TBW, all AEs, severe AEs, and low for LBM, QOL and drug-related AEs.
A previous systematic review from 2017 compared anamorelin versus placebo and included four RCTs17. The review showed an increase in LBM (SMD 0.34, 95% CI 0.21–0.46, p < 0.00001) and TBW (SMD 1.91, 95% CI 0.53–3.29, p = 0.007) from baseline, and improved the total ASAS score (MD 8.05, 95% CI 5.97–10.12, p < 0.00001). However, high heterogeneity was observed because of the inclusion of Garcia 2013 in the meta-analysis, a trial where patients received anamorelin for only 3 days. Notably, the review also focused on the molecular mechanisms, presenting increases in IGF-1, which has anabolic and appetite-stimulating effects, and IGFBP-3, which indicates an increase in protein synthesis. The positive effects of anamorelin on TBW, LBM might be mediated through these molecular mechanisms. Since all four RCTs in the review were included in our analysis, the results of the review were similar to our results. Our review further reported about QOL, OS, and drug-related AEs by adding three additional RCTs.
Another systematic review conducted in the same year also compared anamorelin to placebo16. This review included six RCTs; four RCTs from peer-reviewed articles and two reports from annual meetings. Anamorelin did not cause an increase in AEs compared with placebo, significantly increased TBW (MD 1.78, 95% CI 1.28–2.28, p < 0.00001) and LBM (MD 1.10, 95% CI 0.35–1.85, p = 0.004), and improved QOL (SMD 0.19, 95%CI 0.08–0.30, p = 0.0006). No significant improvement in OS was observed. The authors suggested that anamorelin 100 mg was the appropriate dose mainly based on the subgroup analysis of appetite that 100 mg significantly improved appetite compared to 50 mg. The results of this review were generally consistent with our results, because all four RCTs from peer-reviewed articles were included in our analysis, and two reports from annual meetings were presumed to be prior reports of the RCTs included in our analysis. However, our study did not show significant differences between subgroups in all outcomes, including appetite. Therefore, it is still unclear whether 100 mg of anamorelin is an appropriate dose. In comparison to this systematic review, we included an additional trial and the two reports from annual meetings which have now been published in peer-reviewed journals. Moreover, we assessed the certainty of the evidence using the GRADE approach20.
There is still no established, high-quality, evidence-based drug treatment for patients with CACS. Loss of body weight in CACS is a marker of both progression of the syndrome and prognosis, and there has also been a trend towards lower response rates when using chemotherapy among loss of body weight patients with CACS1. Therefore, various drug treatments have been studied in the hope of increasing appetite and weight gain. Two relatively well-studied options are available: progesterone analogs and glucocorticoids25. Progesterone analogs improve appetite and body weight in patients with CACS, but the type of weight gain is primarily due to gain in adipose tissue rather than skeletal muscle26. They have also been associated with serious AEs such as thromboembolic events, edema, adrenal suppression, and increased mortality, suggesting that their administration must balance potential benefits and risks27. Glucocorticoids have been suggested to improve appetite to a similar degree to that seen with progesterone analogs28. However, it has not been shown to increase body weight, and given a wide variety of other side effects such as myopathy, infections, and hyperglycemia, and decline in efficacy associated with long-term use, the role of glucocorticoids as an appetite stimulant is sometimes limited in patients with a life expectancy of a few weeks to several months25. Moderate to low evidence indicates that, anamorelin increased TBW, LBM, and QOL without increasing overall AEs over a 12-week treatment period. The mechanism of action may be related to a dose-dependent increase in growth hormone, IGF-1, and IGFBP-3 concentrations after treatment with anamorelin as shown in previous research in healthy volunteers13.
Our results have important clinical implications for healthcare providers. Anamorelin may be a valuable treatment option for patients suffering from CACS.
Limitations
We thoroughly assessed the current literature to study the efficacy of anamorelin for CACS. However, our study has several limitations. First, the number of studies for inclusion and available data was limited. We identified seven RCTs from six articles, one of which was a pilot study completed within 3 days of the observation period11, and one of which was an extension of two RCTs that were reintegrated24. Therefore, we could not include them in this meta-analysis. ROMANA I and ROMANA II, which were considered to have the lowest risk of bias and highest reliability, reported results of LBM and HGS in a way that could not be used for meta-analysis14. Regarding OS, which is the most important outcome, only two RCTs reported results at 12 months after treatment13,14. Therefore, long-term benefits of treating CACS with anamorelin is still unclear.
Second, although this study demonstrated significant improvements in TBW and QOL, it is not yet clear whether these changes were clinically meaningful. For example, patients had increased TBW with anamorelin, however, caloric intake or food diaries were not reported. This may limit our ability to estimate the effect of anamorelin on food intake. Although each study reported patients’ QOL, comparison was limited because studies used different tools to assess QOL. Furthermore, several studies measured multiple QOL outcomes and gave priority to reporting those that showed significant improvement13,14,23.
Third, due to the characteristic of CACS, there are some missing data that may be due to death or changes in physical condition, causing selection bias. In addition, included studies focused mainly on NSCLC. The efficacy of anamorelin for other types of cancer is still unclear, therefore limiting the external validity of the intervention.
Despite these limitations, we were able to show that anamorelin improved TBW, LBM, and QOL without increasing AEs in CACS patients.
Implications for practice
In this systematic review and meta-analysis of RCTs on anamorelin, we showed that anamorelin improves TBW, LBM, and QOL in patients with CACS without increasing AEs. Although anamorelin 100 mg is currently approved, there is room for further study regarding the appropriate dose, as subgroup analyses of all outcomes showed no significant differences.
We showed that anamorelin would be a valuable option for CACS, for which few effective treatments are available. The evidence is still limited, but several international clinical trials are currently in progress (NCT04844970, NCT03637816, NCT03743064, NCT03743051), which will provide us with more robust evidence in the future. In addition to weight gain and QOL, future studies should ideally also assess the appropriate dose, chemotherapy response, and long-term OS. Since we were only able to demonstrate safety until 12 weeks in this analysis, it is necessary to conduct studies to confirm long-term safety. Since many RCTs only included NSCLC patients, it would also be necessary to confirm whether similar results can be obtained in populations with other types of cancer.
Conclusions
Moderate to low evidence suggests that anamorelin increases TBW, LBM, and QOL in patients with CACS without increasing overall AEs. Anamorelin may be one of the effective options for CACS patients; however, further studies are needed to confirm the efficacy and safety of this drug.
Data availability
Data used and analyzed was extracted from primary studies included in this review. The data analyzed is available from the review authors on request.
References
Dewys, W. D. et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 69, 491–497. https://doi.org/10.1016/s0149-2918(05)80001-3 (1980).
Esposito, A. et al. Mechanisms of anorexia-cachexia syndrome and rational for treatment with selective ghrelin receptor agonist. Cancer Treat. Rev. 41, 793–797. https://doi.org/10.1016/j.ctrv.2015.09.002 (2015).
Fearon, K. et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 12, 489–495. https://doi.org/10.1016/s1470-2045(10)70218-7 (2011).
Reuben, D. B., Mor, V. & Hiris, J. Clinical symptoms and length of survival in patients with terminal cancer. Arch. Intern. Med. 148, 1586–1591 (1988).
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660. https://doi.org/10.1038/45230 (1999).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198. https://doi.org/10.1038/35051587 (2001).
Strasser, F. et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: A randomised, placebo-controlled, double-blind, double-crossover study. Br. J. Cancer 98, 300–308. https://doi.org/10.1038/sj.bjc.6604148 (2008).
Lundholm, K. et al. Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: A randomized double-blind study. Cancer 116, 2044–2052. https://doi.org/10.1002/cncr.24917 (2010).
Neary, N. M. et al. Ghrelin increases energy intake in cancer patients with impaired appetite: Acute, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 89, 2832–2836. https://doi.org/10.1210/jc.2003-031768 (2004).
Currow, D. C. & Abernethy, A. P. Anamorelin hydrochloride in the treatment of cancer anorexia-cachexia syndrome. Future Oncol. 10, 789–802. https://doi.org/10.2217/fon.14.14 (2014).
Garcia, J. M., Friend, J. & Allen, S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: A multicenter, randomized, double-blind, crossover, pilot study. Support. Care Cancer 21, 129–137. https://doi.org/10.1007/s00520-012-1500-1 (2013).
Garcia, J. M. et al. Anamorelin for patients with cancer cachexia: An integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 16, 108–116. https://doi.org/10.1016/S1470-2045(14)71154-4 (2015).
Takayama, K. et al. Anamorelin (ONO-7643) in Japanese patients with non-small cell lung cancer and cachexia: Results of a randomized phase 2 trial. Support. Care Cancer 24, 3495–3505. https://doi.org/10.1007/s00520-016-3144-z (2016).
Temel, J. S. et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 17, 519–531. https://doi.org/10.1016/s1470-2045(15)00558-6 (2016).
Wakabayashi, H., Arai, H. & Inui, A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: Facts and numbers. J. Cachexia Sarcopenia Muscle 12, 14–16. https://doi.org/10.1002/jcsm.12675 (2021).
Nishie, K., Yamamoto, S., Nagata, C., Koizumi, T. & Hanaoka, M. Anamorelin for advanced non-small-cell lung cancer with cachexia: Systematic review and meta-analysis. Lung Cancer 112, 25–34. https://doi.org/10.1016/j.lungcan.2017.07.023 (2017).
Bai, Y. et al. Anamorelin for cancer anorexia-cachexia syndrome: A systematic review and meta-analysis. Support. Care Cancer 25, 1651–1659. https://doi.org/10.1007/s00520-016-3560-0 (2017).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ https://doi.org/10.1136/bmj.n71 (2021).
Sterne, J. A. C. et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ https://doi.org/10.1136/bmj.l4898 (2019).
Schünemann, H. J. GRADE: From grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice. Z Evidenz Fortbild. Qual. Gesundh. 103, 391–400. https://doi.org/10.1016/j.zefq.2009.05.023 (2009).
[Software]., G. G. G. G. D. T. GRADEpro GDT. Version accessed 1 April 2023. McMaster University and Evidence Prime (2022).
Review Manager (RevMan) [Computer program]. Version 5.4. . The Cochrane Collaboration, 2020 (2020).
Katakami, N. et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 124, 606–616. https://doi.org/10.1002/cncr.31128 (2018).
Currow, D. et al. ROMANA 3: A phase 3 safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann. Oncol. 28, 1949–1956. https://doi.org/10.1093/annonc/mdx192 (2017).
Yavuzsen, T., Davis, M. P., Walsh, D., LeGrand, S. & Lagman, R. Systematic review of the treatment of cancer-associated anorexia and weight loss. J. Clin. Oncol. 23, 8500–8511. https://doi.org/10.1200/jco.2005.01.8010 (2005).
Loprinzi, C. L., Schaid, D. J., Dose, A. M., Burnham, N. L. & Jensen, M. D. Body-composition changes in patients who gain weight while receiving megestrol acetate. J. Clin. Oncol. 11, 152–154. https://doi.org/10.1200/jco.1993.11.1.152 (1993).
Madeddu, C., Macciò, A., Panzone, F., Tanca, F. M. & Mantovani, G. Medroxyprogesterone acetate in the management of cancer cachexia. Expert Opin. Pharmacother. 10, 1359–1366. https://doi.org/10.1517/14656560902960162 (2009).
Moertel, C. G., Schutt, A. J., Reitemeier, R. J. & Hahn, R. G. Corticosteroid therapy of preterminal gastrointestinal cancer. Cancer 33, 1607–1609. https://doi.org/10.1002/1097-0142(197406)33 (1974).
Acknowledgements
I received excellent assistance from the librarians at St. Luke's International University with literature searches.
Author information
Authors and Affiliations
Contributions
J.T. and K.L. contributed to formulating the eligibility criteria. J.T. and S.M. performed study selection, data extraction, and assessing risk of bias. J.T. analyzed the data. J.T. and K.L. contributed to the writing and revision of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taniguchi, J., Mikura, S. & da Silva Lopes, K. The efficacy and safety of anamorelin for patients with cancer-related anorexia/cachexia syndrome: a systematic review and meta-analysis. Sci Rep 13, 15257 (2023). https://doi.org/10.1038/s41598-023-42446-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42446-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.