Abstract
Omega-3 fatty acids are bioactive nutrients with the potential to preserve lean body mass in individuals with cancer. This study aimed to review the literature on randomized clinical trials that evaluated the effects of omega-3 supplementation on lean body mass in cancer patients. As secondary objectives, we evaluated the effects of omega-3 supplementation on body mass index (BMI) and body weight. We conducted a systematic review and meta-analysis in the following databases: Pubmed, LILACS, Scielo, Scopus, Web of Science, Cochrane, and Embase. It included randomized clinical trials that investigated the effects of omega-3 supplementation on lean body mass in cancer patients. Observational studies, animal experiments, studies carried out with healthy humans, and non-randomized clinical trials were excluded. We utilized the Cochrane scale to assess the quality of the studies. A meta-analysis was carried out to evaluate the effect of omega-3 on lean body mass, BMI, and body weight. Fourteen studies were included, of which four showed significant results from omega-3 supplementation for lean body mass. In the meta-analysis, omega-3 fatty acids increased lean body mass by 0.17 kg compared to placebo, but without significant differences between the groups [SMD: 0.17; CI 95%: −0.01, 0.35; I2 = 41%]. For body weight, omega-3 showed a statistically significant effect [SMD: 0.26; CI 95%: 0.06, 0.45; I2 = 46%], whereas for BMI the results were not significant. This systematic review and meta-analysis showed no statistically significant effect from omega-3 on lean body mass and BMI. On the other hand, there was a statistical significance for body weight.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9.
Pin F, Couch ME, Bonetto A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr Opin Support Palliat Care. 2018;12:420–6.
Mazzuca F, Onesti CE, Roberto M, Di Girolamo M, Botticelli A, Begini P, et al. Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget. 2018;9:25714–22.
Wallengren O, Iresjö BM, Lundholm K, Bosaeus I. Loss of muscle mass in the end of life in patients with advanced cancer. Support Care Cancer. 2015;23:79–86.
Aoyama T, Sato T, Segami K, Maezawa Y, Kano K, Kawabe T, et al. Risk factors for the loss of lean body mass after gastrectomy for gastric cancer. Ann Surg Oncol. 2016;23:1963–70.
Lonbro S, Dalgas U, Primdahl H, Johansen J, Nielsen JL, Overgaard J, et al. Lean body mass and muscle function in head and neck cancer patients and healthy individuals-results from the DAHANCA 25 study. Acta Oncol (Madr). 2013;52:1543–51.
Tsai S. Importance of lean body mass in the oncologic patient. Nutr Clin Pr. 2012;27:593–8.
Delpino FM, Figueiredo LM, da Silva BGC, da Silva TG, Mintem GC, Bielemann RM, et al. Omega-3 supplementation and diabetes: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021. https://doi.org/10.1080/10408398.2021.1875977.
Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9:1–9.
Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725–34.
Freitas RDS, Campos MM. Protective effects of omega-3 fatty acids in cancer-related complications. Nutrients. 2019;11. https://doi.org/10.3390/nu11050945.
Murphy RA, Yeung E, Mazurak VC, Mourtzakis M. Influence of eicosapentaenoic acid supplementation on lean body mass in cancer cachexia. Br J Cancer. 2011;105:1469–73.
Gorjao R, dos Santos CMM, Serdan TDA, Diniz VLS, Alba-Loureiro TC, Cury-Boaventura MF, et al. New insights on the regulation of cancer cachexia by N-3 polyunsaturated fatty acids. Pharm Ther. 2019;196:117–34.
Werner K, Küllenberg de Gaudry D, Taylor LA, Keck T, Unger C, Hopt UT, et al. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: marine phospholipids versus fish oil - a randomized controlled double-blind trial. Lipids Health Dis. 2017;16. https://doi.org/10.1186/s12944-017-0495-5.
Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: Results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–63.
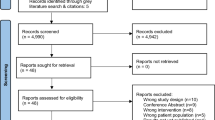
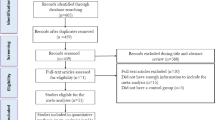
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. 2009. https://doi.org/10.1002/9780470743386.
The Cochrane Collaboration. Obtaining standard deviations from standard errors. Cochrane Handb Syst Rev Interv. Version 510 [updated March 2011] 2011.
Higgins JPT. SG Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated. 2011.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–60.
Marques DC, Stringhini MLF, Fornés NAS de. Omega-3 fatty acid supplementation, nutritional status and quality of life in patients with gastrointestinal cancer: double-blind, placebo-controlled, randomized study. Rev Médica Minas Gerais. 2013;23:39–46.
Sánchez-Lara K, Turcott JG, Juárez-Hernández E, Nuñez-Valencia C, Villanueva G, Guevara P, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr. 2014;33:1017–23.
Mocellin MC, Camargo C de Q, Fabre ME de S, Trindade EBS. Fish oil effects on quality of life, body weight and free fat mass change in gastrointestinal cancer patients undergoing chemotherapy: a triple blind, randomized clinical trial. J Funct Foods. 2017;31:113–22.
Paixão EMDS, Oliveira ACDM, Pizato N, Muniz-Junqueira MI, Magalhães KG, Nakano EY, et al. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naïve breast cancer patients: a randomized double-blind controlled trial. Nutr J. 2017;16. https://doi.org/10.1186/s12937-017-0295-9.
Golkhalkhali B, Rajandram R, Paliany AS, Ho GF, Wan Ishak WZ, Johari CS, et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia Pac J Clin Oncol. 2018;14:179–91.
Solís-Martínez O, Plasa-Carvalho V, Phillips-Sixtos G, Trujillo-Cabrera Y, Hernández-Cuellar A, Queipo-García GE, et al. Effect of eicosapentaenoic acid on body composition and inflammation markers in patients with head and neck squamous cell cancer from a public hospital in Mexico. Nutr Cancer. 2018;70:663–70.
de la Rosa Oliva F, García AM, Calzada HR, Astudillo de la Vega H, Rocha EB, Lara-Medina F, et al. Effects of omega-3 fatty acids supplementation on neoadjuvant chemotherapy-induced toxicity in patients with locally advanced breast cancer: a randomized, controlled, double-blinded clinical trial. Nutr Hosp. 2019;36:769–76.
Hossain T, Phillips BE, Doleman B, Lund JN, Williams JP. A double-blind randomized controlled trial of the effects of eicosapentaenoic acid supplementation on muscle inflammation and physical function in patients undergoing colorectal cancer resection. Clin Nutr. 2020;39:2055–61.
Fearon KCH, Von Meyenfeldt MF, Moses AGW, Van Geenen R, Roy A, Gouma DJ, et al. Effect of a protein and energy dense n-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003;52:1479–86.
Moses AWG, Slater C, Preston T, Barber MD, Fearon KCH. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90:996–1002.
Fearon KCH, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, et al. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol. 2006;24:3401–7.
Van Der Meij BS, Langius JAE, Smit EF, Spreeuwenberg MD, Von Blomberg BME, Heijboer AC, et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J Nutr. 2010;140:1774–80.
Bruera E, Strasser F, Palmer JL, Willey J, Calder K, Amyotte G, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. J Clin Oncol. 2003;21:129–34.
Gow RV, Hibbeln JR. Omega-3 fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors. Child Adolesc Psychiatr Clin N. Am. 2014;23:555–90.
Zamora Zamora F, Martínez Galiano JM, Gaforio Martínez JJ, Delgado Rodríguez M. Aceite de oliva y peso corporal. Revisión sistemática y metaanálisis de ensayos controlados aleatorizados. Rev Esp Salud Publica. 2018;92. http://www.ncbi.nlm.nih.gov/pubmed/30461730 (accessed 28 Jan 2022).
Paez A. Gray literature: an important resource in systematic reviews. J Evid Based Med. 2017;10:233–40.
Acknowledgements
During the preparation of the manuscript, FMD received a doctoral grant from the National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Contributions
All authors contributed to data interpretation and reviewed, edited, and approved the final manuscript. FMD conducted the writing, analysis, and critical review of the manuscript. LMF was the second reviewer and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Delpino, F.M., Figueiredo, L.M. Effects of omega-3 supplementation on lean body mass in cancer patients: a systematic review and meta-analysis. Eur J Clin Nutr 76, 1636–1645 (2022). https://doi.org/10.1038/s41430-022-01100-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01100-x