Abstract
The aim of this randomized clinical trial was to evaluate the impact of all-trans retinoic acid (ATRA) in combination with non-intensive chemotherapy in older unfit patients (> 60 years) with newly diagnosed NPM1-mutated acute myeloid leukemia. Patients were randomized (1:1) to low-dose chemotherapy with or without open-label ATRA 45 mg/m2, days 8–28; the dose of ATRA was reduced to 45 mg/m2, days 8–10 and 15 mg/m2, days 11–28 after 75 patients due to toxicity. Up to 6 cycles of cytarabine 20 mg/day s.c., bid, days 1–7 and etoposide 100 mg/day, p.o. or i.v., days 1–3 with (ATRA) or without ATRA (CONTROL) were intended. The primary endpoint was overall survival (OS). Between May 2011 and September 2016, 144 patients (median age, 77 years; range, 64–92 years) were randomized (72, CONTROL; 72, ATRA). Baseline characteristics were balanced between the two study arms. The median number of treatment cycles was 2 in ATRA and 2.5 in CONTROL. OS was significantly shorter in the ATRA compared to the CONTROL arm (p = 0.023; median OS: 5 months versus 9.2 months, 2-years OS rate: 7% versus 10%, respectively). Rates of CR/CRi were not different between treatment arms; infections were more common in ATRA beyond treatment cycle one. The addition of ATRA to low-dose cytarabine plus etoposide in an older, unfit patient population was not beneficial, but rather led to an inferior outcome.
The clinical trial is registered at clinicaltrialsregister.eu (EudraCT Number: 2010-023409-37, first posted 14/12/2010).
Similar content being viewed by others
Introduction
Mutations in the Nucleophosmin-1 (NPM1) gene occur in ∼ 20 to 33% of adult patients with acute myeloid leukemia (AML)1,2,3,4 and are present in all age groups and still about one fifth of patients above the age of 70 years exhibit NPM1 mutation4. AML with mutated NPM1 is recognized as a disease entity in the WHO classification5 and clinical trials focusing on this disease entity are emerging6,7.
All-trans retinoic acid (ATRA) in combination with chemotherapy or arsenic trioxide has revolutionized the treatment of acute promyelocytic leukemia (APL)8,9. Furthermore, preclinical and clinical studies also provided a rationale for the use of ATRA in non-APL AML10,11,12 and in particular in AML with mutated NPM113,14,15,16,17,18. However, results from large randomized studies evaluating ATRA in combination with intensive but also non-intensive chemotherapy have been contradictory12,17,18,19,20,21,22,23,24.
In three studies performed by the British Medical Research Council (MRC), one in younger patients receiving intensive first-line treatment (MRC AML12, n = 1097)22, one in medically unfit patients (MRC AML14, n = 207)23, and one in high-risk, refractory or relapsed patients (MRC AML-HR, n = 362)24, consistently negative results were reported overall and in genetic subgroups20. In the study by Estey et al. of 215 patients with high-risk myelodysplastic syndrome or AML older than 71 years, there was no effect of ATRA in multivariable analysis, but a significantly better overall survival was found in univariable analyses for patients treated in the ATRA arms21. In all these trials, ATRA was started simultaneously or before initiation of chemotherapy21,22,23,24. In contrast, in the trials conducted by the German-Austrian Study Group (AMLSG), ATRA was started at the end of chemotherapy6,17,18,19 in accordance with the in vitro data10,11. In these trials, older patients randomized to the ATRA arm had a significantly higher complete remission (CR) rate, better event-free (EFS) and overall survival (OS)17, whereas in younger patients only per protocol but not intention-to-treat analysis revealed a better EFS and OS for patients receiving ATRA as adjunct to intensive chemotherapy19. Similar results were found in subgroup analysis of NPM1-mutated AML indicating a benefit in older patients on an intention to treat basis and in younger patients only in a per protocol analysis18,19.
In 2011, the AMLSG initiated the randomized AMLSG 15–10 trial in older patients with newly diagnosed AML with NPM1 mutation not fit for intensive chemotherapy evaluating ATRA in combination with low-dose cytarabine plus etoposide. Here, we report the results of the upfront randomization for ATRA in 144 older adult patients.
Patients and methods
Patients
Screening for NPM1 mutations was performed in patients with newly diagnosed AML within the AMLSG BiO study4. Between May 2011 and September 2016, 144 patients were enrolled in the AMLSG 15-10 study. Patients aged > 60 years with newly diagnosed AML including de novo AML, secondary AML with a preceding history of myelodysplastic or myeloproliferative disorder (sAML), and therapy-related AML following treatment of a primary malignancy (tAML), as defined by the WHO 2008 classification were eligible for the trial25. Patients not eligible for intensive chemotherapy were included and criteria for unfitness were age ≥ 75 years, hematopoietic cell transplantation-comorbidity index (HCT-CI) > 2 and/or patient decision. Patients with the following disease entities were excluded: AML with t(8;21)(q22;q22.1), RUNX1::RUNX1T1; AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22), CBFB::MYH11; AML with t(15;17)(q24.1;q21.2), PML::RARA (or other translocations involving RARA); AML with t(9;11)(p21.3;q23.3), MLLT3::KMT2A (or other translocations involving KMT2A); AML with t(6;9)(p23.3;q34.1), DEK::NUP214; AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2), GATA2, MECOM(EVI1). Furthermore, patients with concomitant renal (creatinine > 1.5 × upper normal serum level), liver (bilirubin, AST or AP > 2.5 × upper normal serum level) or cardiac dysfunction (New York Heart Association III/IV), uncontrolled infectious disease, primary coagulation disturbance or performance status (ECOG) > 2 were excluded. Written informed consent was obtained from all patients. The protocol was approved by the lead Ethics Review Committee (Ethikkommission der Universität Ulm, Ulm, Germany) and registered at clinicaltrialsregister.eu (EudraCT Number: 2010-023409-37) and clinicaltrials.gov (NCT01237808, first posted 10/11/2010) and methods were performed in accordance with the relevant guidelines and regulations.
Cyto- and molecular genetics
Chromosome banding analysis was performed centrally in the two AMLSG Laboratories for Cytogenetics (Hannover, Ulm). Leukemia samples were analyzed for mutations in NPM1 and FLT3 (ITDs, and tyrosine kinase domain [TKD] mutations at codons D835/I836)2,3,26.
Study design
Patients were randomized (1:1) to receive low-dose chemotherapy with or without ATRA. In the first cycle patients received cytarabine 20 mg/day, s.c., bid, days 1–7, and etoposide or etoposidphosphate 50 mg/m2/day, continuously i.v., days 1–3; for cycles 2 to 6 the etoposide dose was increased to 100 mg/day, p.o. or i.v. (over 1 h), days 1–3. From May 2011 to March 2014, ATRA was given at a dose of 45 mg/m2/day p.o., days 8–28 with or shortly after meals distributed over 3 doses per day. After an interim safety analysis in March 2014, the dose of ATRA was reduced to 45 mg/m2 day 8–10, followed by 15 mg/m2 day 11–28 due to an increased frequency of toxicities, in particular infections, and deaths observed in the ATRA arm compared to the control arm.
Definition of response criteria, survival endpoints and hematologic recovery
In accordance with standard criteria, CR was defined as less than 5% bone marrow blasts, an absolute neutrophil count of > 1.0 G/L or higher, a platelet count of 100 G/L or higher, no blasts in the peripheral blood, and no extramedullary leukemia; CR with incomplete blood count recovery (CRi) was defined as CR except for residual neutropenia (neutrophils < 1.0 G/L) or thrombocytopenia (platelets < 100 G/L)27. Relapse was defined as more than 5% bone marrow blasts unrelated to recovery from the preceding course of chemotherapy or new extramedullary leukemia in patients with previously documented CR.
EFS and OS were defined as recommended27. Toxicities were defined and graded according to the National Cancer Institute (NCI) Common Toxicity Criteria, version 3.0.
Randomization, sample size calculation, and statistical analysis
Randomization was performed at the AMLSG Clinical Trials Office, two-arm in a ratio of 1:1 using the minimization approach of Pocock (Biometrics 1975) for the factors FLT3-ITD and HCT-CI score. After the randomization in the AMLSG Clinical Trials Office, the result of the randomization and the definite patient identification number (patient-ID) were marked on the registration confirmation form, which was returned by Fax to the registering physician and the local investigator.
The sample size calculation was based on data from the British MRC 14 trial23 leading to an expected survival probability after 2 years within the control arm of the proposed study of 10%. An improvement by 15% to a 2-year survival probability of 25% in the ATRA arm was defined as clinically relevant. Type I and II errors were fixed at 5% and 20%, respectively. This leaded to a sample size of 144 patients to be randomized. Trial duration was conservatively assumed with a 5-year accrual time and a minimum of 2 years of follow-up period. A drop-out rate of 5% in each arm was assumed for sample size calculation. Sample size estimation was done according to Lachin and Foulkes (1986) using PASS 2008 (NCSS Kaysville, USA).
Pairwise comparisons between patient subgroups were performed by the Mann–Whitney or Kruskal Wallis test for continuous variables and by Fisher’s exact test for categorical variables.
The analysis was performed on an ITT basis according to initial randomization. The primary endpoint of the study was OS; secondary endpoints were event-free survival (EFS), response to therapy (CR/CRi), cumulative incidences of relapse (CIR) and death in CR/CRi (CID) and therapy-related toxicity. The median duration of follow-up was calculated by the reverse Kaplan–Meier estimate28; the Kaplan–Meier method was used to estimate the distributions of OS and EFS. Survival distributions were compared using the log-rank test. Multivariable Cox regression models were used to evaluate prognostic variables and the following variables were evaluated: WBC (log10 transformed), age, randomization (CONTROL, ATRA), FLT3-ITD, HCT-CI score (≤ 2 versus > 2), ECOG performance status (0–1 versus > 1). A multivariable logistic regression model was applied to investigate the influence of covariates on response to therapy (same covariates as listed above).
CIR and CID were assessed using the time from achievement of CR/CRi until relapse or death in CR/CRi. CIR and CID and their standard errors were estimated according to the method of Aalen and Johansen29 and formal statistical comparison of the incidences was done using the test by Gray30. A cause-specific Cox model was used for the time to relapse with death in CR as competing event, including the same covariates as for OS and EFS. Fitting the analogous model for time to death in CR with relapse as a competing event was not possible due to low number of events.
Comparisons regarding safety endpoints were performed using Barnard’s test.
All statistical analyses were performed with the statistical software environment R, version 4.3.1, using the R packages survival, version 3.2-13, and cmprsk, version 2.2-1131.
Results
Patients and baseline characteristics
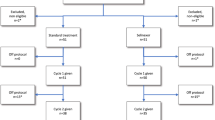
A total of 144 patients were randomized, 72 patients to ATRA and 72 to CONTROL. Recruitment started on May 11th 2011, last patient was enrolled on September 14th 2016 with no interruption of enrolment and a minimum follow-up after last patient in of 2 years. Patient demographics and presenting laboratory and genetic characteristics were balanced between the two treatment arms (Table 1). The trial flow is summarized in the diagram according to CONSORT statement in Fig. 1.
Applied Treatment and Response
After randomization all 144 patients received the first treatment cycle (n = 72 CONTROL, n = 72 ATRA). Overall, 254 treatment cycles were applied in CONTROL and 177 in ATRA (p < 0.001), the median number of cycles was 2.5 in CONTROL compared to 2 in ATRA. Only 38 patients (26%) received the intended 6 cycles of therapy, 26 (33%) in CONTROL and 12 (18%) in ATRA.
Best responses (CR/CRi) were achieved in 26 of 72 (36.1%) in CONTROL and in 24 of 72 (33.3%) patients in ATRA (p = 0.86) in median after 2.5 cycles (range, 1–6) in CONTROL and after 2.0 cycles (range, 1–5) in ATRA. A logistic regression model including treatment arm, ECOG status, HCT-CI risk score, age, WBC, and FLT3-ITD status did not reveal significant prognostic markers. During the first treatment cycle 17 patients died, n = 6 in CONTROL and n = 11 in ATRA (p = 0.30).
Survival analyses
Estimated median follow-up for survival was 29.8 months (95% CI, 25.4-Inf) without difference between the treatment arms (p = 0.58). Of 144 randomized patients 130 died.
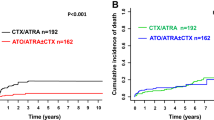
The log-rank test on an ITT basis for the primary endpoint OS revealed a significantly inferior survival for patients in the ATRA compared to patients in the CONTROL arm (p = 0.023, Fig. 2). Median OS times, 1 and 2-years OS rates were 9.2 and 5 months, 38% and 23%, 10% and 7% in CONTROL and ATRA, respectively. Multivariable analyses for OS revealed treatment with ATRA, HCT-CI Score > 2, higher WBC, and older age as significant unfavorable factors (Table 2). No significant difference in EFS was observed between the treatment arms. Of 50 patients achieving a CR/CRi, 45 patients relapsed and 2 patients in ATRA died in CR/CRi. There was no significant difference in CIR (p = 0.65) and CID (p = 0.14) between the treatment arms. Multivariable (cause-specific) Cox models did not reveal a significant effect of the addition of ATRA on EFS or CIR either. However, results for these endpoints do not provide further insight due to the low response rate.
Toxicity
Rates of early/hypoplastic death occurring during the first two treatment cycles did not differ significantly between arms but were with 18.1% in ATRA numerically higher compared to 8.5% in CONTROL (p = 0.09).
Infections analyzed on an as treated basis during the first treatment cycle occurred in 59% of patients with no difference between treatment arms (p = 0.73). In contrast, starting from treatment cycle 2 rates of infections were higher in ATRA compared to CONTROL (cycle 2, 59% vs. 32%, respectively). Whereas for cycle 2, this difference was statistically significant (p = 0.01), no significant difference was observed for treatment cycles after cycle 2. During the first treatment cycle no difference with regard to frequency of adverse event was identified between treatment arms although cardiac events were more frequent (p = 0.06) in ATRA (Table 3).
Discussion
We previously reported that ATRA given in combination with intensive chemotherapy improves survival in patients 61 years and older with newly diagnosed AML17 and in particularly in AML with mutated NPM118. The objective of the trial reported here was to perform a confirmatory study in an older patient population not fit for intensive chemotherapy exhibiting mutated NPM1. The chemotherapy backbone consisted of low-dose cytarabine23 in combination with etoposide32. Etoposide was added based on reports indicating a specific clinical efficacy of etoposide in myelomonocytic and monoblastic AML33,34 and the observation that patients exhibiting a normal karyotype benefit from induction therapy including etoposide in terms of CR rate35. Based on the early preclinical data, we decided to start ATRA at day 8, that is, after all cytotoxic drugs were administered10,11. The initial dose of ATRA (45 mg/m2, days 8–28) had to be reduced (45 mg/m2, days 8–10; 15 mg/m2, days 11–28) after 75 patients due to an increased toxicity, in particular infections.
Our results show that the addition of ATRA to low-dose cytarabine plus etoposide in an older, unfit patient population was not beneficial, but rather led to an inferior outcome. OS was significantly shorter in ATRA compared to CONTROL (median OS of 5 vs 9 months; p = 0.023). A possible explanation could be the cumulative effect of a higher early/hypoplastic rate, an increased rate of infectious complications by the addition of ATRA which was still present after dose reduction of ATRA in the second half of the trial (p = 0.90), and a higher rate of cardiac events in ATRA leading to the observed inferior outcome. Furthermore, the addition of etoposide to standard low-dose cytarabine in combination with ATRA might have increased susceptibility to infectious complications, particularly beyond cycle 1, by extensive mucosal toxicity in this very vulnerable older unfit patient population. Of note, extensive mucocutaneous changes leading to early termination of ATRA treatment was reported in a study of lung cancer patients receiving all-trans-retinoic acid plus cisplatin and etoposide36.
Two randomized studies have previously been conducted evaluating ATRA in combination with a standard of care backbone in older unfit patients, the MRC AML14 trial with low-dose cytarabine23, and the DECIDER trial with decitabine12. In the MRC AML 14 trial the addition of ATRA had no effect on all clinical endpoints, CR rate (p = 0.30), survival (p = 0.60), as well as analyzed toxicities23. In contrast, in the DECIDER trial the addition of ATRA to decitabine resulted in an in trend higher CR rate (p = 0.06) and a significantly improved survival (p = 0.006) with again no differences in toxicity12. Based on these results and the trial presented here one may speculate that there is an additive beneficial effect of ATRA with hypomethylating agents but not with conventional low dose cytotoxic drugs.
Although, the preclinical rationale for examining the addition of ATRA in AML was attractive we were not able to show consistently a beneficial effect across different patient populations6,17,18,19. However, the preclinical rationale as well as recently published clinical data underlines the effect of targeted BCL-2 inhibition in AML37 and at least based on data of a cohort study in AML with mutated NPM138. Furthermore, promising data support that NPM1-mutated AMLs are sensitive to chromatin complex inhibition via targeting menin39. In line, patient-derived xenograft models of NPM1-mutated AML showed promising results with the menin inhibitor VTP5046940 and first data of a subsequent phase I study demonstrated single agent activity also in patients with NPM1-mutated AML41. Furthermore, first clinical data of the oral spleen tyrosine kinase (SYK) inhibitor entospletinib given in combination with intensive chemotherapy in NPM1-mutated AML showed promising activity42 and triggered a double-blinded randomized phase-III evaluation (ClinicalTrials.gov Identifier: NCT05020665). In conclusion, ATRA in combination with non-intensive chemotherapy as used in our study led to increased toxicity and inferior OS in patients not fit for intensive chemotherapy with newly diagnosed NPM1-mutated AML.
Data availability
The datasets used and/or analyzed during the current study are available from the last author on reasonable request.
References
Falini, B. et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 352, 254–266 (2005).
Schlenk, R. F. et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N. Engl. J. Med. 358, 1909–1918 (2008).
Schlenk, R. F. et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia: Results from AMLSG Trial AML HD98B. Haematologica 94, 54–60 (2009).
Nagel, G. et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann. Hematol. 96, 1993–2003 (2017).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20), 2391–2405 (2016).
Schlenk, R. F. et al. Gemtuzumab ozogamicin in NPM1-mutated acute myeloid leukemia: Early results from the prospective randomized AMLSG 09–09 phase III study. J. Clin. Oncol. 38(6), 623–632 (2020).
Döhner, H., Wei, A. & Löwenberg, B. Towards precision medicine for AML. Nat. Rev. Clin. Oncol. 18(9), 577–590 (2021).
Lo-Coco, F. et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 369(2), 111–121 (2013).
Lo-Coco, F., Di Donato, L., Schlenk, R. F., German–Austrian Acute Myeloid Leukemia Study Group and Study Alliance Leukemia. Targeted therapy alone for acute promyelocytic leukemia. N. Engl. J. Med. 374(12), 1197–1198 (2016).
Hu, Z. B., Minden, M. D. & McCulloch, E. A. Direct evidence for the participation of bcl-2 in the regulation by retinoic acid of the Ara-C sensitivity of leukemic stem cells. Leukemia 9, 1667–1673 (1995).
Hu, Z. B., Minden, M. D. & McCulloch, E. A. Phosphorylation of BCL-2 after exposure of human leukemic cells to retinoic acid. Blood 92, 1768–1775 (1998).
Lübbert, M. et al. DECIDER Study Team. Valproate and retinoic acid in combination with decitabine in elderly nonfit patients with acute myeloid leukemia: Results of a multicenter, randomized, 2 × 2 Phase II Trial. J. Clin. Oncol. 38(3), 257–270 (2020).
Boutzen, H. et al. Isocitrate dehydrogenase 1 mutations prime the all-trans retinoic acid myeloid differentiation pathway in acute myeloid leukemia. J. Exp. Med. 213(4), 483–497 (2016).
Balusu, R. et al. Targeting levels or oligomerization of nucleophosmin 1 induces differentiation and loss of survival of human AML cells with mutant NPM1. Blood 118(11), 3096–3106 (2011).
Hajj, H. E. et al. Retinoic acid and arsenic trioxide trigger degradation of mutated NPM1 resulting in apoptosis of AML cells. Blood 125(22), 3447–3454 (2015).
Martelli, M. P. et al. Arsenic trioxide and all-trans-retinoic acid target NPM1 mutant oncoprotein levels and induce apoptosis in NPM1-mutated AML cells. Blood 125(22), 3455–3465 (2015).
Schlenk, R. F. et al. Phase III study of all-trans retinoic acid in previously untreated patients 61 years or older with acute myeloid leukemia. Leukemia 18, 1798–1803 (2004).
Schlenk, R. F. et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia: Results from AMLSG Trial AML HD98B. Haematologica 94, 54–60 (2009).
Schlenk, R. F. et al. All-trans retinoic acid as adjunct to intensive treatment in younger adult patients with acute myeloid leukemia: results of the randomized AMLSG 07–04 study. Ann Hematol. 95(12), 1931–1942 (2016).
Burnett, A. K. et al. The impact on outcome of the addition of all-trans retinoic acid to intensive chemotherapy in younger patients with nonacute promyelocytic acute myeloid leukemia: overall results and results in genotypic subgroups defined by mutations in NPM1, FLT3, and CEBPA. Blood 115, 948–956 (2010).
Estey, E. H. et al. Randomized phase II study of fludarabine+ cytosine arabinoside +idarubicin +/- all-trans retinoic acid +/- granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood 93, 2478–2484 (1999).
Burnett, A. K. et al. Does all-trans retinoic acid (ATRA) have a role in non-APL acute myeloid leukaemia? Results from 1666 patients in three MRC trials. Blood 104, 1794 (2004).
Burnett, A. K. et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 109, 1114–1124 (2007).
Milligan, D. W., Wheatley, K., Littlewood, T., Craig, J. I. O. & Burnett, A. K. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood 107(12), 4614–4622. https://doi.org/10.1182/blood-2005-10-4202 (2006).
Vardiman, J. W. et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114, 937–951 (2009).
Schlenk, R. F. et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood 133(8), 840–851 (2019).
Cheson, B. D. et al. Revised recommendations of the International Working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J. Clin. Oncol. 21(24), 4642–4649 (2003).
Schemper, M. & Smith, T. L. A note on quantifying follow-up in studies of failure time. Control Clin. Trials 17(4), 343–346 (1996).
Aalen, O. O. & Johansen, S. An empirical transition matrix for nonhomogeneous markov chains based on censored observations. Scand. J. Stat. 5, 141–150 (1978).
Gray, R. J. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 16(3), 1141–1154 (1988).
R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: 2020; Vienna, Austria.
Schlenk, R. F. et al. Intensive consolidation versus oral maintenance therapy in patients 61 years or older with acute myeloid leukemia in first remission: results of second randomization of the AML HD98-B treatment Trial. Leukemia 20(4), 748–750 (2006).
Hurd, D. D. et al. VP 16–213 and cyclophosphamide in the treatment of refractory acute nonlymphocytic leukemia with monocytic features. Med. Pediatr. Oncol. 9, 251–255 (1981).
Leoni, F. et al. Etoposide in remission induction of adult acute myeloid leukemia. Acta Haematol. 83, 82–85 (1990).
Farag, S. S. et al. Outcome of induction and postremission therapy in younger adults with acute myeloid leukemia with normal karyotype: A cancer and leukemia group B study. J. Clin. Oncol. 23, 482–493 (2005).
Kalemkerian, G. P. et al. A phase II study of all-trans-retinoic acid plus cisplatin and etoposide in patients with extensive stage small cell lung carcinoma: An eastern cooperative oncology group study. Cancer 83(6), 1102–1108 (1998).
DiNardo, C. D. et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 383(7), 617–629 (2020).
Lachowiez, C. A. et al. Outcomes of older patients with NPM1-mutated AML: Current treatments and the promise of venetoclax-based regimens. Blood Adv. 4(7), 1311–1320 (2020).
Kühn, M. W. et al. Targeting chromatin regulators inhibits leukemogenic gene expression in NPM1 mutant leukemia. Cancer Discov. 6, 1166–1181 (2016).
Borkin, D. et al. Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell 27(4), 589–602 (2015).
Stein, E. M. et al. Safety and efficacy of menin inhibition in patients (Pts) with MLL-rearranged and NPM1 mutant acute leukemia: A phase (Ph) 1, first-in-human study of SNDX-5613 (AUGMENT 101). Blood 138(Supplement 1), 699–699. https://doi.org/10.1182/blood-2021-146944 (2021).
Walker, A. R. et al. Entospletinib in combination with induction chemotherapy in previously untreated acute myeloid leukemia: Response and predictive significance of HOXA9 and MEIS1 expression. Clin. Cancer Res. 26, 5852–5859 (2020).
Acknowledgements
This work was supported by grant 01KG1004 [AMLSG 15-10: Randomized Phase III Study of Low-Dose Cytarabine and Etoposide with or without All Trans Retinoic Acid in Older Patients not Eligible for Intensive Chemotherapy with Acute Myeloid Leukemia and NPM1 Mutation] from the German Bundesministerium für Bildung und Forschung (BMBF). We are also grateful to all members of the German-Austrian AML Study Group (AMLSG) for providing leukemia specimens and clinical data.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conception and Design: R.F.S., H.D. Provision of study materials or patients: all authors. Collection and assembly of data: all authors. Data analysis and interpretation: all authors. Manuscript writing: all authors. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
Richard F. Schlenk: Consulting for or advisory board membership with Daiichi Sankyo, Pfizer, Astellas, and Novartis; research funding from PharmaMar, AstraZeneca, Pfizer, Roche, Boehringer Ingelheim, and Daiichi Sankyo; travel, accommodations, and expenses covered by Daiichi Sankyo. Hartmut Döhner: Consulting or advisory board: AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, BMS, Celgene, Daiichi Sankyo, Gilead, Janssen, Jazz, Novartis, Servier, Syndax; Clinical Research Funding to Institution: AbbVie, Agios, Amgen, Astellas, Bristol Myers Squibb, Celgene, Jazz Pharmaceuticals, Kronos Bio, Novartis, Pfizer. Helmut R. Salih: Consulting for or advisory board membership with BMS, Celgene, Novartis, Pfizer, Roche, Synimmune GmbH; research funding from ABL Bio, Celgene, Novartis, Synimmune GmbH. Arnold Ganser: Consultant or advisory board: Celgene, JAZZ Pharmaceuticals, Novartis. Elisabeth Koller: Consulting or advisory board: AbbVie, Astellas, BMS, Celgene, Daiichi Sankyo, Jazz, Novartis, Servier, Pfizer. Felicitas Thol: Advisory Board for BMS, Abbvie, Takeda, Novartis, Pfizer. All others have declared no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlenk, R.F., Weber, D., Krzykalla, J. et al. Randomized phase-III study of low-dose cytarabine and etoposide + /− all-trans retinoic acid in older unfit patients with NPM1-mutated acute myeloid leukemia. Sci Rep 13, 14809 (2023). https://doi.org/10.1038/s41598-023-41964-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41964-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.