Abstract
Endoprosthetic surgery can lead to relevant blood loss resulting in red blood cell (RBC) transfusions. This study aimed to identify risk factors for blood loss and RBC transfusion that enable the prediction of an individualized transfusion probability to guide preoperative RBC provision and blood saving programs. A retrospective analysis of patients who underwent primary hip or knee arthroplasty was performed. Risk factors for blood loss and transfusions were identified and transfusion probabilities computed. The number needed to treat (NNT) of a potential correction of preoperative anemia with iron substitution for the prevention of RBC transfusion was calculated. A total of 308 patients were included, of whom 12 (3.9%) received RBC transfusions. Factors influencing the maximum hemoglobin drop were the use of drain, tranexamic acid, duration of surgery, anticoagulation, BMI, ASA status and mechanical heart valves. In multivariate analysis, the use of a drain, low preoperative Hb and mechanical heart valves were predictors for RBC transfusions. The transfusion probability of patients with a hemoglobin of 9.0–10.0 g/dL, 10.0–11.0 g/dL, 11.0–12.0 g/dL and 12.0–13.0 g/dL was 100%, 33.3%, 10% and 5.6%, and the NNT 1.5, 4.3, 22.7 and 17.3, while it was 100%, 50%, 25% and 14.3% with a NNT of 2.0, 4.0, 9.3 and 7.0 in patients with a drain, respectively. Preoperative anemia and the insertion of drains are more predictive for RBC transfusions than the use of tranexamic acid. Based on this, a personalized transfusion probability can be computed, that may help to identify patients who could benefit from blood saving programs.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) belong to the most frequently performed orthopedic procedures worldwide. They can result in relevant blood loss and up to 46% of patients require red blood cell (RBC) transfusions during or following surgery1,2,3,4,5,6. Despite major improvements in blood transfusion safety in the last years, allogenic blood transfusion can still lead to several complications, including allergic reactions, infections7, circulatory overload and transfusion-associated lung injury2. In addition, blood transfusions can result in prolonged hospitalization, higher mortality and morbidity and may increase the risk for surgical site infections8,9. Preoperative anemia is a common condition in patients undergoing major surgery. A meta-analysis has shown an overall prevalence rate of preoperative anemia in major surgery of 39.1%10. Preoperative anemia was prevalent in approximately 25% of the patients admitted for elective orthopedic surgery8,11. Severe anemia is associated with increased mortality and higher incidence for RBC transfusions10. Iron deficiency anemia is the most common type of anemia in patients undergoing arthroplasty and it is associated with longer hospital stay, higher 90 days readmission rates and higher incidence of postoperative complications12,13.
Tranexamic acid (TXA) is a synthetic derivative of the amino acid lysine, which inhibits lysine binding to plasminogen and thus blocks its conversion to plasmin14. Recent literature demonstrated the effectiveness of intravenous tranexamic acid to reduce blood loss and transfusions rates in patients undergoing total hip and knee arthroplasty5,15,16,17. Patients receiving tranexamic acid had no increased risk for complications, such as thromboembolic events or acute kidney failure18,19.
In the present study we analyzed factors predicting blood loss and transfusion requirement after elective hip and knee arthroplasty with a special focus on the use of tranexamic acid (TXA), requirement of anticoagulation and preoperative conditions like anemia and impaired renal function.
Methods
Patient selection
All patients who underwent primary total hip arthroplasty or total knee arthroplasty at the University Hospital Leipzig between June 2018 and November 2019 were retrospectively analyzed. Starting from January 2019, all patients without contraindications received prophylactic infusion of TXA (1000 mg) 30 min prior to the beginning of the surgery as per institutional protocol. Contraindications for the administration of TXA were history of venous thromboembolism, severe renal dysfunction (GFR < 30 mL/min), known epilepsy, coronary artery disease, history of stroke or intake of anticoagulants. Surgery was performed by senior consultants specialized in arthroplasty using the same prosthesis designs. All patients without an indication for therapeutic anticoagulation received prophylactic low molecular weight heparin subcutaneously starting from 6 h after surgery, for 30 days (hip arthroplasty) or 14 days (knee arthroplasty) after surgery.
The following variables were collected for every patient: age, gender, body mass index, duration of surgery, American Society of Anesthesiologists (ASA) grade, Metabolic Equivalent of Task (MET) score and intraoperative insertion of suction drains. Hemoglobin levels were measured preoperatively and one and three to five days postoperatively. Blood transfusions were administered in patients with a Hb ≤ 6 g/dL or in patients with a Hb ≤ 8 g/dL presenting with symptoms of inadequate oxygen delivery, such as congestive heart failure, hypotension, tachycardia, chest pain or dyspnea. Based on our internal policy and recommendations for autologous hemotherapy20 prophylactic allocation of RBC was performed for procedures with a transfusion probability of ≥ 10% but no prophylactic collection of autologous RBCs was performed in any patient.
The mean blood loss was calculated with the Meunier’s21 and Lopez-Picard equations22. The blood volume was estimated according to the Nadler’s formula23 for the Meunier’s equation and with to the formula of the International Council for Standardization in Hematology (ICSH)24 for the Lopez-Picard equation.
Statistics
Data are given as median with interquartile range (IQR) in brackets. The distributions of all variables were tested with Kolmogorov–Smirnov-test. A t-test was used to compare means if normal distribution of variables could be assumed, otherwise the Mann–Whitney U test was performed. Multivariate analysis for the prediction of blood transfusion was made using a binary logistic regression model. The odds ratio (OR) and the 95% confidence interval was calculated. The level of significance was set at p < 0.05. Statistical analyses were performed using the software SPSS version 26 (SPSS Inc, Chicago, IL).
Ethical considerations
The study was approved by the Ethical committee of the University of Leipzig (reference 178/20-ek), and conducted according to the Declaration of Helsinki. The need for informed consent was waived by the Ethical Committee at the Medical Faculty of the Leipzig University, Germany, but according to the Ethical approval, patients had to be excluded who refused to have their data used for research purposes under the admission contract with the Leipzig University Hospital. During the observation period, no patient refused to have their data used for research purposes.
Results
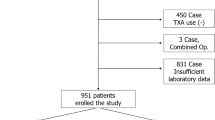
A total of 308 patients were included into the analysis. Median age was 66 (IQR 58–76) years, and 53.6% were female. Total hip arthroplasty was carried out in 202 patients (69.5%), while 79 patients (25.6%) underwent a total knee arthroplasty. A minimal invasive anterolateral approach was used in 75 (37.1%) patients receiving a total hip arthroplasty. A fully cemented knee arthroplasty was performed in 58 (73.4%), a hybrid operation (uncemented femoral and cemented tibial component) in 18 (22.8%) and a cementless arthroplasty in 3 (3.8%) patients. Twenty-five patients received unicondylar knee arthroplasty and two patients underwent an isolated replacement of the femoropatellar joint. A total of 87 (28.2%) patients were under anticoagulation, predominantly platelet aggregation inhibitors (n = 49, 15.9%). The median duration of surgery was 73 (61–90) minutes, without a significant difference between knee and hip arthroplasty.
Patient characteristics are given in Table 1.
Patients undergoing knee arthroplasty had a higher median body weight (90 vs. 78 kg, p < 0.001) and a higher BMI (31.7 vs. 27.8 kg/m2, p < 0.001) than patients with hip arthroplasty, resulting in a higher blood volume according to Nadler’s formula: 5091 vs. 4695 ml (p = 0.001) and the ICSH formula: 4919 vs. 4653 mL (p = 0.02).
Perioperative hemoglobin values
Overall, the median preoperative Hb was 13.9 (12.7–14.7) g/dL. Significantly lower Hb was found in women than in men (13.0 g/dL vs. 14.3 g/dL, p < 0.001), as well as in patients > 66 years old than in patients ≤ 66 years (13.2 g/dL vs.14.0 g/dL, p < 0.001). Patients with an ASA of > 2 also had lower Hb than those with an ASA ≤ 2 (13.0 vs. ASA: 13.9 g/dL, p = 0.008). The Hb level was also lower in patients with a MET score of < 5 compared to those with a score ≥ 5 (13.1 vs. 14.0, p = 0.001). There was a trend towards a lower Hb in patients receiving therapeutic anticoagulation than in those without this therapy (13.8 g/dL vs.13.5 g/dL, p = 0.063). The preoperative Hb did not correlate with BMI (BMI < 28 kg/m2: 13.7 g/dL vs. BMI > 28 kg/m2 13.7 g/dL). Patients with a preoperative Hb < 12 g/dL had a lower GFR (64.0 [47.5–91.0] vs. 84.0 [69–94] mL/min, p = 0.004), a lower mean corpuscular hemoglobin (MCH: 1.83 [1.77–1.88] vs. 1.88 [1.81–1.94] fmol, p = 0.009) and a lower mean corpuscular hemoglobin concentration (MCHC: 21.2 [20.7–21.4] vs. 21.5 [21.1–22.0] mmol/L, p = 0.001) than those with a Hb of > 12 g/dl. In addition, the proportion of patients with a GFR, MCH and MCHC below the reference range was higher in patients with low Hb (Supplementary Table 1). There were no patients with a mean corpuscular volume (MCV) below the reference range in our cohort.
The median drop in Hb between the pre-operative value and the first postoperative day was 2.6 (IQR 1.8–3.7) g/dL and the maximum drop in Hb postoperatively was 3.9 (IQR 2.6–4.8) g/dL. The maximum drop in Hb was not influenced by the type of operation (knee vs. hip arthroplasty: 4.2 (IQR 3.2–5.3) vs. 4.0 (IQR 3.1–4.8) g/dL, p = 0.223), age and MET score. Patients receiving anticoagulants had a higher maximum drop in Hb but there was no difference between different types of anticoagulants. Factors influencing the maximum drop in Hb are shown in Table 2.
Calculation of perioperative blood loss
The mean blood loss calculated with the Meunier’s formula was higher compared to the Lopez-Picard formula (1949 mL vs. 1496 mL), but there was a high correlation between the results for each patient (Spearman r = 0.902, p < 0.001). The perioperative blood loss was lower in patients receiving hip compared to knee arthroplasty, in patients without a suction drain, in patients receiving tranexamic acid, in patients with a lower Hb, in non-anticoagulated patients and in patients with low ASA status.
The role of anticoagulation
Patients on anticoagulation (either platelet inhibitor, direct oral anticoagulant or vitamin K antagonist) were older (median 75 vs. 64 years, p < 0.001), had a higher maximum drop in Hb (median 4.4 vs. 3.5 g/dL, p = 0.004) and were longer hospitalized postoperatively (median 7 vs. 6 days, p = 0.014) than those without any anticoagulant treatment. Patients receiving VKA had a higher transfusion rate compared to patients on DOAC (p = 0.032) and to patients with platelet inhibitors (p = 0.012). There were three patients with mechanical heart valve replacement (aortic valve, n = 2 and mitral valve, n = 1) receiving VKA. Two of these patients required RBC transfusions. The preoperative Hb was 10.9 and 14.8 g/dL in the two patients receiving RBC transfusions and 15.5 g/dL in the patient without RBC transfusion.
Transfusion requirement
A total of 12 (3.9%) patients received RBC transfusions. In one patient, a single RBC unit was transfused, eight patients received two units, two patients received four RBCs and six RBCs were administered in one patient. The median postoperative hospitalization was 7 days in patients receiving transfusions vs. 6 days in patients without transfusions, without reaching statistical significance (p = 0.12). Factors influencing RBC transfusions are displayed in Table 3.
Age ≥ 75 years, BMI < 30 kg/m2, ASA status > 2, the use of drains, the avoidance of tranexamic acid, a low preoperative Hb and an MCHC below the lower limit of normal (LLN) were associated with a significantly higher transfusion frequency. The transfusion frequency in different cohorts of patients and predictors for transfusions are shown in Table 4.
Multivariate analysis
A multivariate analysis including the univariate risk factors: use of drain, use of tranexamic acid, contraindications for the use of tranexamic acid, lower preoperative hemoglobin and preoperative hemoglobin < 13 g/dL, older age and age ≥ 75 years, lower BMI and BMI < 30 kg/m2, ASA status > 2, duration of the operation > 73 min, the use of anticoagulants, the use of VKA and existence of mechanical heart valve was performed. In multivariate analysis, only the use of drain (OR: 0.053 [95% 0.006–0.489], p = 0.01), lower preoperative hemoglobin level (OR: 0.205 [95% CI 0.090–0.465], p < 0.001) and the existence of a mechanical heart valve (OR: 0.002 [95% CI 0.000–0.080], p = 0.001) were significant risk factors for transfusions. After exclusion of patients with mechanical heart valves, only the use of drain (OR: 0.054 [95% 0.006–0.496], p = 0.01) and lower preoperative hemoglobin level (OR: 0.214 [95% CI 0.096–0.477], p < 0.001) persisted as multivariate risk factors.
Patient blood management
The transfusion frequency in different patient cohorts with different preoperative Hb ranges was calculated. The threshold for prophylactic allocation of RBC of ≥ 10% is reached at a hemoglobin level of 13.2 g/dL in the total cohort, at 14.0 g/dL in patients not receiving tranexamic acid and at 14.8 g/dL in patients in whom a suction drain is used. Based on this threshold and preoperative hemoglobin values, prophylactic allocation of RBC would not be necessary in 189 patients (61.4%). Taking into account a planned use of tranexamic acid and the preoperative hemoglobin levels, 10 of 118 (8.5%) patients receiving tranexamic acid, but 108 of 190 (56.8%) patients not receiving tranexamic acid have a transfusion probability of ≥ 10%. As the transfusion frequency in patients not receiving a drain was only 0.6%, prophylactic provision is not necessary in these patients, while 101 of 136 (74.3%) patients with a drain had a transfusion frequency > 10%.
The number needed to treat (NNT) for the reduction of transfusion requirement was determined based on the fact that with the infusion of 1000 mg iron isomaltoside, a median increase in hemoglobin level of 1.0 g/dL within four weeks can be achieved25. The transfusion frequency among patients with a preoperative Hb < 11 g/dL was 67%. The theoretical NNTs for the whole cohort of patients with a hemoglobin level of 9.0–10.0 g/dL, 10.0–11.0 g/dL and 11.0–12.0 g/dL was 1.5, 4.3 and 22.7, respectively. The transfusion frequency and NNTs for all patients and different subgroups of high-risk patients are given in Table 5. Patients with mechanical heart valves were excluded from the calculation due to their high transfusion frequency irrespective of the preoperative Hb.
Discussion
The aim of this study was to investigate factors affecting perioperative hemoglobin loss and transfusion frequency in patients following hip and knee arthroplasty. We identified the use of drain a lower preoperative Hb and the existence of a mechanical heart valve as independent predictors for RBC transfusions. According to our institutional protocol and recent guidelines26, patients with mechanical heart valves receive a perioperative anticoagulation with continuous infusion of unfractionated heparin at a therapeutic dose starting early after the operation when hemostasis is achieved. It is well known that heparin bridging of anticoagulation with VKA leads to an increased risk of perioperative bleeding events27,28 and in patients after implantation of mechanical heart valves29,30. Therefore, the higher transfusion frequency in patients with mechanical heart valves and in patients receiving anticoagulation is well explained by the bridging therapy.
Predictors for blood loss and transfusion
The insertion of drains after knee or hip arthroplasty is still a matter of controversy31,32,33. The insertion of a suction drain has no scientifically proven benefits and drains are not used routinely anymore. Using enhanced recovery protocols in the arthroplasty leads to a changing role for drains, particularly with the use of tranexamic acid. Most literature is from the pre-tranexamic era and there are only few studies on the use of drains in combination with tranexamic acid. In our cohort, the use of suction drains was associated with a greater Hb drop and was an independent risk factor for higher transfusion rates (8.1% vs. 0.6%). Similar results were published by Concina et al. who have also found a decrease in Hb and a lower transfusion frequency in total knee arthroplasty34. Although Chen et al. determined a higher Hb drop in patients after knee arthroplasty with a drain but no higher transfusion frequency35. Others found no correlation between the use of drains and Hb drop31,36, but the number of patients included in these last two studies was much lower than in the cohort reported by Chen and colleagues. In our study, none of the 80 patients in whom tranexamic acid was used and suction drains were avoided received a blood transfusion. In contrast, the transfusion rate was 10.2% in patients not receiving tranexamic acid but a suction drain.
Tranexamic acid has been proved to be effective in preventing blood transfusions as well as reducing perioperative blood loss. Recent meta-analyses reported on reduced blood loss and lower transfusion rates for patients receiving tranexamic acid, without an increased risk for deep vein thrombosis, pulmonary embolism or other complications37,38. These findings are consistent with our results. However, our data indicate that the avoidance of suction drains might be as important as the use of tranexamic acid for the reduction of perioperative blood loss.
Preoperative anemia is common in orthopedic surgery and is prevalent in approximately 25% of patients8,11. Low preoperative Hb has been identified as an independent risk factor for postoperative transfusions39,40,41. Ryan et al. reported a Hb of 12.5 g/dL as an optimal cutoff for predicting postoperative transfusion with a specificity 76.4% and a sensitivity of 84.8%42. We found a transfusion probability of > 10% at a hemoglobin level of 13.2 g/dL in the total cohort, at 14.0 g/dL in patients not receiving tranexamic acid and at 14.8 g/dL in patients in whom a suction drain was used. Based on our internal policy and recommendations for autologous hemotherapy43, prophylactic allocation of RBC is recommended for procedures with a transfusion probability of ≥ 10%. Taking into account a planned use of tranexamic acid and the pre-operative hemoglobin level, only 10 of 118 (8.5%) patients receiving tranexamic acid but 108 of 190 (56.8%) patients not receiving tranexamic acid have a transfusion probability of ≥ 10% and therefore require prophylactic provision of packed RBC according to our policy. As the transfusion frequency in patients not receiving a suction drain was only 0.6%, prophylactic provision of RBC units is not necessary in these patients; but 74.3% of patients with a drainage had a transfusion probability > 10% based on their pre-operative hemoglobin level. Based on these data a personalized transfusion probability can be calculated, resulting in a further reduction of prophylactic RBC provision.
Patient blood management
Perioperative blood optimization programs have been developed in the last years to reduce transfusion frequency and to increase patient safety. These programs include the pre-operative optimization of the hemoglobin level and the optimal hemostasis obtained during surgery. The comparably low transfusion rate of 3.9% observed in our cohort might be in part explained with the use of minimal invasive surgery in total hip arthroplasty. Various studies have shown a significant lower blood loss by using the minimal invasive anterolateral compared to the standard lateral approach due to smaller skin and muscle incisions44,45. In our cohort, patients who received a minimal invasive approach had a transfusion rate of only 2.7%. In addition, most patients undergoing knee arthroplasty received a cemented prosthesis which causes lower blood loss compared to a cementless arthroplasty46. Optimal hemostasis during surgery was maintained by avoiding hypothermia and optimization of pH level. A meta-analysis reported that even mild hypothermia significantly increases blood loss and the risk for transfusion by 22%47. In addition, RBC transfusions were administered restrictively only in patients with Hb ≤ 6 g/dL or in symptomatic patients with Hb ≤ 8 g/dL.
Kopanidis et al. achieved a lower blood transfusion rate and higher postoperative hemoglobin values after implementing a patient blood management program48. Pinilla-Gracia et al. also showed lower transfusion rates, shorter length of stay and a corrected preoperative anemia in 79% of cases49. Prevention of postoperative anemia is a fundamental issue to avoid blood transfusions and potential complications and also to save costs. Fenelon reported on a 46% reduction of cross-matched blood and an annual cost saving of €54,375 after introduction of an enhanced recovery program50. Apart from cost saving, supplementation with iron isomaltoside is very well tolerated with a number needed to harm for side effects of 15–25 and for serious side effects > 50051 resulting in a positive risk–benefit ratio especially in patients with additional risk factors and a low NNT. In addition, the co-administration of erythropoietin has shown to further reduce blood transfusions in patients undergoing major orthopedic surgery52. Several studies have reported that blood transfusions are associated with an increased perioperative mortality, more complications, especially surgical site infections and prolonged hospitalization3,10,53,54,55. Therefore, the inclusion of these patients in preoperative blood saving programs and pre-operative adjustment of iron deficiency anemia is highly warranted. According to our data, particularly patients with a low preoperative hemoglobin level who are not receiving tranexamic acid and a suction drain is used, are at high risk and should be included in such programs.
In addition, we were able to show that a personalized estimation of the transfusion probability can be calculated. This formula will be implemented in the clinical decision support system AMPEL (Analysis and Monitoring System for Patient Safety by Enhanced Laboratory Decision Support), which is used at the University Hospital Leipzig56 to allow for an automatized identification of patients with increased risk for postoperative RBC transfusions.
Limitations
The present study has several limitations, mainly due to its retrospective design. Secondly, patients have been operated by different surgeons, which might have influenced bleeding. Thirdly, the criteria for insertion of suction drains were not standardized. Thus, surgeons’ expertise and patient risk factors may have influenced the decision on the use of suction drains. Finally, tranexamic acid was not given in patients on therapeutic anticoagulation, resulting in a selection bias. However, as the use of anticoagulants, apart from patients with mechanical heart valves, was not predictive for transfusions in the multivariate analysis, this bias seems to be of limited relevance.
Conclusion
Preoperative anemia and the insertion of drains are the strongest predictors for RBC transfusion requirement in our analysis and turned out to be even more predictive than the well-established use of tranexamic acid. Therefore, the insertion of drains should be avoided and patients should be screened for preoperative anemia. In addition, the calculation of a personalized transfusion probability is feasible and may enable automatized clinical decision systems with regard to preoperative packed red cells allocation and other blood patient management options such as iron substitution. This could contribute to save resources, reduce costs and enhance patient safety.
References
Ponnusamy, K. E., Kim, T. J. & Khanuja, H. S. Perioperative blood transfusions in orthopaedic surgery. J. Bone Joint Surg. Am. 96, 1836–1844 (2014).
Bierbaum, B. E. et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J. Bone Joint Surg. Am. 81, 2–10 (1999).
Song, K., Pan, P., Yao, Y., Jiang, T. & Jiang, Q. The incidence and risk factors for allogenic blood transfusion in total knee and hip arthroplasty. J. Orthop. Surg. Res. 14, 273 (2019).
Menendez, M. E. et al. Variation in use of blood transfusion in primary total hip and knee arthroplasties. J. Arthropl. 31, 2757–2763.e2 (2016).
Carling, M. S., Jeppsson, A., Eriksson, B. I. & Brisby, H. Transfusions and blood loss in total hip and knee arthroplasty: A prospective observational study. J. Orthop. Surg. Res. 10, 48 (2015).
Saleh, A. et al. Allogenic blood transfusion following total hip arthroplasty: Results from the nationwide inpatient sample, 2000–2009. J. Bone Joint Surg. Am. 96, e155 (2014).
Taylor, R. W. et al. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit. Care Med. 30, 2249–2254 (2002).
Spahn, D. R. Anemia and patient blood management in hip and knee surgery: A systematic review of the literature. Anesthesiology 113, 482–495 (2010).
Jans, Ø., Jørgensen, C., Kehlet, H. & Johansson, P. I. Role of preoperative anemia for risk of transfusion and postoperative morbidity in fast-track hip and knee arthroplasty. Transfusion 54, 717–726 (2014).
Fowler, A. J. et al. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 102, 1314–1324 (2015).
Muñoz, M. et al. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 72, 826–834 (2017).
Mathew, K. K. et al. Is iron deficiency anemia a risk factor for poorer outcomes in primary total knee arthroplasty?. J. Arthropl. 35, 1252–1256 (2020).
Viola, J., Gomez, M. M., Restrepo, C., Maltenfort, M. G. & Parvizi, J. Preoperative anemia increases postoperative complications and mortality following total joint arthroplasty. J. Arthropl. 30, 846–848 (2015).
Okamoto, S., Sato, S., Takada, Y. & Okamoto, U. An active stereo-isomer (trans-form) of amcha and its antifibrinolytic (antiplasminic) action in vitro and in vivo. Keio J. Med. 13, 177–185 (1964).
Benoni, G., Fredin, H., Knebel, R. & Nilsson, P. Blood conservation with tranexamic acid in total hip arthroplasty: A randomized, double-blind study in 40 primary operations. Acta Orthop. Scand. 72, 442–448 (2001).
Husted, H. et al. Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: A prospective randomized double-blind study in 40 patients. Acta Orthop. Scand. 74, 665–669 (2003).
Rosencher, N. et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: Blood management in elective knee and hip arthroplasty in Europe. Transfusion 43, 459–469 (2003).
Poeran, J. et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: Retrospective analysis of effectiveness and safety. BMJ (Clin. Res. Ed.) 349, g4829 (2014).
Ho, K. M. & Ismail, H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: A meta-analysis. Anaesth. Intensive Care 31, 529–537 (2003).
Müller, M. M., Geisen, C., Zacharowski, K., Tonn, T. & Seifried, E. Transfusion of packed red cells: Indications, triggers and adverse events. Deutsches Arzteblatt Int. 112, 507–517 (2015) (quiz 518).
Meunier, A., Petersson, A., Good, L. & Berlin, G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 95, 120–124 (2008).
Lopez-Picado, A., Albinarrate, A. & Barrachina, B. Determination of perioperative blood loss: Accuracy or approximation?. Anesth. Analg. 125, 280–286 (2017).
Nadler, S. B., Hidalgo, J. H. & Bloch, T. Prediction of blood volume in normal human adults. Surgery 51, 224–232 (1962).
Pearson, T. C. et al. Interpretation of measured red cell mass and plasma volume in adults: Expert Panel on Radionuclides of the International Council for Standardization in Haematology. Br. J. Haematol. 89, 748–756 (1995).
Sinclair, R. C., Duffield, K. E. & de Pennington, J. H. Improving preoperative haemoglobin using a quality improvement approach to treat iron deficiency anaemia. BMJ Open Qual. 9, e000776. https://doi.org/10.1136/bmjoq-2019-000776 (2020).
Baumgartner, H. et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 38, 2739–2791 (2017).
Douketis, J. D. et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N. Engl. J. Med. 373, 823–833 (2015).
Ayoub, K. et al. Perioperative heparin bridging in atrial fibrillation patients requiring temporary interruption of anticoagulation. Evidence from meta-analysis. J. Stroke Cerebrovasc. Diseases Off. J. Natl. Stroke Assoc. 25, 2215–2221 (2016).
Passaglia, L. G., de Barros, G. M. & de Sousa, M. R. Early postoperative bridging anticoagulation after mechanical heart valve replacement. A systematic review and meta-analysis. J. Thromb. Haemost. JTH 13, 1557–1567 (2015).
Li, B.-X. et al. Comparison of different bridging anticoagulation therapies used after mechanical heart valve replacement in Chinese patients—A prospective cohort study. J. Cardiothorac. Surg. 15, 40 (2020).
Maniar, R. N. et al. Role of suction drain after knee arthroplasty in the tranexamic acid era: A randomized controlled study. Clin. Orthop. Surg. 11, 73–81 (2019).
Sharma, G. M., Palekar, G. & Tanna, D. D. Use of closed suction drain after primary total knee arthroplasty—An overrated practice. SICOT-J. 2, 39 (2016).
Chen, Z.-Y., Gao, Y., Chen, W., Li, X. & Zhang, Y.-Z. Is wound drainage necessary in hip arthroplasty? A meta-analysis of randomized controlled trials. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 24, 939–946 (2014).
Concina, C., Crucil, M., Fabbro, S. & Gherlinzoni, F. Do tourniquet and drainage influence fast track in total knee arthroplasty? Our results on 151 cases. Acta Bio-Medica Atenei Parmensis 90, 123–129 (2019).
Chen, J. Y. et al. Drain use in total knee arthroplasty is neither associated with a greater transfusion rate nor a longer hospital stay. Int. Orthop. 40, 2505–2509 (2016).
Wang, D. et al. Closed suction drainage is not associated with faster recovery after total knee arthroplasty: A prospective randomized controlled study of 80 patients. Orthop. Surg. 8, 226–233 (2016).
Zhang, H., Chen, J., Chen, F. & Que, W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 20, 1742–1752 (2012).
Gandhi, R., Evans, H. M. K., Mahomed, S. R. & Mahomed, N. N. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: A meta-analysis. BMC Res. Notes 6, 184 (2013).
Noticewala, M. S., Nyce, J. D., Wang, W., Geller, J. A. & Macaulay, W. Predicting need for allogeneic transfusion after total knee arthroplasty. J. Arthropl. 27, 961–967 (2012).
Guerin, S., Collins, C., Kapoor, H., McClean, I. & Collins, D. Blood transfusion requirement prediction in patients undergoing primary total hip and knee arthroplasty. Transfus. Med. 17, 37–43 (2007).
Ogbemudia, A. E., Yee, S. Y., MacPherson, G. J., Manson, L. M. & Breusch, S. J. Preoperative predictors for allogenic blood transfusion in hip and knee arthroplasty for rheumatoid arthritis. Arch. Orthop. Trauma Surg. 133, 1315–1320 (2013).
Ryan, S. P. et al. Preoperative hemoglobin predicts postoperative transfusion despite antifibrinolytics during total knee arthroplasty. Orthopedics 42, 103–109 (2019).
Frank, S. M. et al. Optimizing preoperative blood ordering with data acquired from an anesthesia information management system. Anesthesiology 118, 1286–1297 (2013).
Martin, R., Clayson, P. E., Troussel, S., Fraser, B. P. & Docquier, P.-L. Anterolateral minimally invasive total hip arthroplasty: A prospective randomized controlled study with a follow-up of 1 year. J. Arthropl. 26, 1362–1372 (2011).
Speranza, A., Iorio, R., Ferretti, M., D’Arrigo, C. & Ferretti, A. A lateral minimal-incision technique in total hip replacement: A prospective, randomizes, controlled trial. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 17, 4–8 (2007).
Hood, M., Dilley, J. E., Ziemba-Davis, M. & Meneghini, R. M. Greater blood loss in contemporary cementless total knee arthroplasty than cemented total knee arthroplasty despite tranexamic acid use: A match-controlled retrospective study. J. Knee Surg. https://doi.org/10.1055/s-0039-1695796 (2019).
Rajagopalan, S., Mascha, E., Na, J. & Sessler, D. I. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 108, 71–77 (2008).
Kopanidis, P. et al. Perioperative blood management programme reduces the use of allogenic blood transfusion in patients undergoing total hip and knee arthroplasty. J. Orthop. Surg. Res. 11, 28 (2016).
Pinilla-Gracia, C., Mateo-Agudo, J., Herrera, A. & Muñoz, M. On the relevance of preoperative haemoglobin optimisation within a Patient Blood Management programme for elective hip arthroplasty surgery. Blood Transfusion Trasfusione del Sangue 18, 182–190 (2020).
Fenelon, C. et al. Saving blood and reducing costs: Updating blood transfusion practice in lower limb arthroplasty. Ir. Med. J. 111, 730 (2018).
Moore, R. A., Gaskell, H., Rose, P. & Allan, J. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord. 11, 4 (2011).
Muñoz, M., Gómez-Ramírez, S. & Auerbach, M. Stimulating erythropoiesis before hip fracture repair for reducing blood transfusion: Should we change the hemoglobin cutoff level for defining anemia in females?. Transfusion 56, 2160–2163 (2016).
Kim, Y.-H., Pandey, K., Park, J.-W. & Kim, J.-S. Comparative efficacy of intravenous with intra-articular versus intravenous only administration of tranexamic acid to reduce blood loss in knee arthroplasty. Orthopedics 41, e827–e830 (2018).
Friedman, R., Homering, M., Holberg, G. & Berkowitz, S. D. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J. Bone Joint Surg. Am. 96, 272–278 (2014).
Roque-Castellano, C. et al. Perioperative blood transfusion is associated with an increased mortality in older surgical patients. World J. Surg. 40, 1795–1801 (2016).
Eckelt, F. et al. Verbesserte Patientensicherheit durch “clinical decision support systems” in der Labormedizin. Der Internist 61, 452–459 (2020).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
C.Pe. was responsible for the design of the study, data collection, interpretation of data and writing the manuscript. R.W., P.P., S.P., R.H. was responsible for the interpretation of data and revising the manuscript. M.F. was responsible for the laboratory analysis and revising the manuscript. A.R. was responsible for the design of the study, interpretation of data and revising the manuscript. C.Pf. was responsible for the design of the study, data collection, statistical analysis, interpretation of data and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pempe, C., Werdehausen, R., Pieroh, P. et al. Predictors for blood loss and transfusion frequency to guide blood saving programs in primary knee- and hip-arthroplasty. Sci Rep 11, 4386 (2021). https://doi.org/10.1038/s41598-021-82779-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82779-z
This article is cited by
-
A neck-sparing short stem shows significantly lower blood loss in total hip arthroplasty compared to a neck-resecting short stem
Scientific Reports (2023)
-
Artificial neural networks for the prediction of transfusion rates in primary total hip arthroplasty
Archives of Orthopaedic and Trauma Surgery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.