Abstract
People with osteoarthritis often experience pain and depression. These meta-analyses examined and compared nonpharmacological randomized controlled trials (RCTs) for pain and symptoms of depression in people living with osteoarthritis. RCTs published up until April 2022 were sourced by searching electronic databases EMBASE, PUBMED & MEDLINE, Web of Science, CINAHL and PEDro. Random-effects meta-analyses were performed to calculate pooled effect sizes (ES) and 95% confidence intervals (CI) for pain and depression. Subgroup analyses examined intervention subtypes. For pain, 29 interventions (n = 4382; 65 ± 6.9 years; 70% female), revealed a significant effect on reducing pain (ES = 0.43, 95% CI [0.25, 0.61], p < 0.001). Effect sizes were significant (p < 0.001) for movement meditation (ES = 0.52; 95% CI [0.35, 0.69]), multimodal approaches (ES = 0.37; 95% CI [0.22, 0.51]), and psychological therapy (ES = 0.21; 95% CI [0.11, 0.31]), and significant (p = 0.046) for resistance exercise (ES = 0.43, 95% CI [− 0.07, 0.94]. Aerobic exercise alone did not improve pain. For depression, 28 interventions (n = 3377; 63 ± 7.0 years; 69% female), revealed a significant effect on reducing depressive symptoms (ES = 0.29, 95% CI [0.08, 0.49], p < 0.001). Effect sizes were significant for movement meditation (ES = 0.30; 95% CI [0.06, 0.55], p = 0.008) and multimodal interventions (ES = 0.12; 95% CI [0.07, 0.18], p < 0.001). Resistance/aerobic exercise or therapy alone did not improve depressive symptoms. Mind–body approaches were more effective than aerobic/resistance exercise or therapy alone for reducing pain and depression in people with osteoarthritis.
Systematic review registration: PROSPERO CRD42022338051.
Similar content being viewed by others
Introduction
Arthritis and psychological conditions (i.e. mental health conditions, for example, depression and anxiety) were in the top three most prevalent chronic conditions experienced in Australia during 2020–20211. Osteoarthritis (OA) is a degenerative joint condition where cartilage that cushions the ends of the bones deteriorates. It is the most common form of arthritis and most commonly affects hands, knees, hips and lower back and neck. Depression and anxiety are both common psychological co-morbidities that occur alongside OA2,3 in addition to increased pain and other mental health issues4. Chronic pain is experienced by one in five Australians aged 45 and over5.
OA has also been associated with increased dementia risk6,7, higher prevalence rates of dementia8, and is considered an early risk factor for cognitive decline7. Depression is another risk factor for dementia9 and is a common psychological symptom associated with dementia10. People living with dementia often experience other behavioural changes and psychological symptoms (e.g. agitation) that may be associated with underlying pain that is not always identified by care staff due to communication difficulties11. In Australia, dementia is the leading cause of death in women and second leading cause of death following heart disease in all Australians1,12. High rates of OA and dementia are also observed globally13.
There are no medications that can cure OA, though analgesics, non-steroidal anti-inflammatory drugs (NSAIDs) and topical therapies are often administered to relieve pain and inflammation. However, all medicines have side effects, for example, NSAIDS can increase risk of stomach ulcers and bleeding14, paracetamol can have adverse side effects when taken long-term15, and the use of opioids is controversial, with increasing evidence showing an increase in addiction, morbidity, and mortality16. Therefore, effective nonpharmacological interventions are needed to address pain and depression in OA, which are all well-established precursors to dementia. No previous systematic reviews have sought to determine the most effective approaches for reducing pain levels and symptoms of depression in OA. Reducing these co-morbidities will reduce dementia risk.
Physical and psychological health conditions rarely occur in isolation, and it is more likely people will go onto experience multiple comorbidities. For example, co-morbidities including psychological conditions are more likely in those with pain than those without5. The ABS reported that in 2020, 19% of Australians with long-term health conditions had two or more chronic conditions1. The interactions between physical and psychological conditions and associated symptoms are complex, and conditions may present independently or be highly intertwined. Common brain-related conditions (e.g. depression, anxiety, and dementia) can be exacerbated by physical conditions (e.g. OA, cardiovascular disease (CVD)) and vice-versa.
There are several models/theories that describe proposed mechanisms involved in these interactions. The biopsychosocial model which recognizes the contribution of all biological, psychological, social, and behavioural factors that dynamically interact with one another to generate the experience of pain17. Inflammation models describe how localized peripheral inflammation can trigger the development of neuroinflammation and subsequently the induction of dementia pathology18. The pain-sensitisation model recognises highly variable experiences of pain between individuals and the role of neuroplastic changes occurring in the peripheral and central nervous system, resulting in pain sensitisation, and impacting the person’s experience of pain19. The fear-avoidance model of musculoskeletal pain describes a psychological process where concern or fear about pain can lead to fear-avoidance behaviour which paradoxically can exacerbate pain and function20. The role of the gut microbiome has also been implicated with pain in OA21, depression22,23 and neurological conditions24.
There are clear associations between physical, psychological, and cognitive health outcomes (i.e. OA, anxiety, depression, and cognitive decline). However, it is not yet known what the most effective approaches are for improving psychological outcomes as well as physical outcomes (e.g. pain) in people living with OA. Physical activity interventions delivered by Accredited Exercise Physiologists (AEPs) have been shown to be highly effective for people living with 26 chronic conditions25, including OA26,27, anxiety and depression28,29, cognitive decline/dementia30,31, diabetes32 and cardiovascular disease33. Examples of effective exercise strategies include resistance training/strengthening exercises34, yoga35, and aquatic exercise36. Interventions that include lifestyle education and information on managing chronic conditions (e.g. information specific to OA, diabetes, depression, etc.) also provide additive beneficial effects37. Indeed, exercise and education are key components of clinical guidelines for OA. It is important that clients understand how to self-manage their pain and remain engaged with exercise to improve outcomes.
Research shows that therapeutic approaches such as cognitive behavioural therapy (CBT) are highly effective in treating mild-moderate depression and anxiety38. Furthermore, evidence suggests that the combination of exercise and behavioural therapies provides greater reduction in depressive symptomology than therapy alone39,40. Behavioural therapy approaches have been adapted or investigated in specific populations such as diabetes41, sleep disorders42, eating disorders43, and chronic pain44. Multimodal approaches that include a combination of exercise, therapeutic approaches and/or lifestyle education may be more effective than single mode approaches due to the focus on both psychological and physical health outcomes. However, there is limited evidence around the effect of multimodal treatment on multimorbidity.
Therefore, the aims of this systematic review and meta-analysis are to determine the most effective nonpharmacological approaches for reducing pain and symptoms of depression in people living with OA. We aimed to:
-
1.
Identify effective approaches for improving pain and reducing symptoms of depression in people with OA.
-
2.
Identify whether single or multi-modal approaches are more effective.
-
3.
Present evidence-based recommendations for treatments for people with OA and associated depression.
In better understanding how to reduce OA-related symptoms and co-morbidities in populations who are at higher-risk of experiencing cognitive decline (i.e. people living with OA6), we may also reduce dementia prevalence rates, as well as improve psychological outcomes for people living with OA.
Methods
The review was preregistered with the international prospective register of systematic reviews (PROSPERO), in accordance with PRISMA guidelines (PROSPERO CRD42022338051)45.
Search strategy
Relevant studies published from electronic database inception until April 2022 were sourced by searching: EMBASE, PUBMED & MEDLINE, Web of Science, CINAHL and PEDro. Studies that included outcome measures relevant to physical outcomes (e.g. pain and physical function) and psychological outcomes (i.e. depression and anxiety symptomatology) were included in the initial search and this review. Search terms were Osteoarthritis OR OA; Depression OR anxiety OR mood OR affective OR psychological; Exercise OR physical activity OR education OR therapy OR CBT OR cognitive behavioural OR cognitive-behavioural OR lifestyle intervention OR mindfulness; RCT OR Randomised controlled trial OR Randomized controlled trial OR randomised controlled trial OR Randomized controlled trial (see Supplementary Material S1 for full list of search terms).
Eligibility criteria and study selection
Only randomized controlled trials (RCTs) were included in this review. Studies were included from all settings such as community clinics, hospitals, and in people’s homes via online platforms, if pain or symptoms of depression in people with OA were assessed. Studies were only included where two or more conditions were compared (control versus intervention). Exclusion criteria were studies having an active control group (e.g. aerobic activity versus resistance activity), studies where data was not presented separately for people with OA if they included people with other pain-related conditions (e.g. lower back pain), studies not available in English, and studies with insufficient information to conduct a meta-analysis. When means and standard deviations were not provided, authors were contacted and requested to share this data.
Only post-intervention scores were compared across conditions (i.e. post-intervention, control versus intervention group scores in pain and symptoms of depression). Cochrane methods and those developed by statisticians were used to convert data46,47,48. Pooled, weighted effect sizes were calculated for all approaches, followed by subgroups determined by intervention types (e.g. multimodal, aerobic activity only, resistance activity only, therapeutic only, movement meditation).
Interventions
Only nonpharmacological interventions were included that involved either exercise, lifestyle education, therapeutic and/or multimodal approaches. These were categorized into groups as they emerged and refined during the screening process. The final groups were ‘multimodal approaches’, ‘movement mediation’ (including yoga, tai chi, and chi gong), ‘resistance activity only’, ‘aerobic activity only’, and ‘therapeutic approaches only’. Studies were included if they had one or more active interventions and an inactive control group (e.g. treatment as usual). Only data from the combined intervention and control group were used for the meta-analyses.
Outcome measures
Studies were included that used several different measurement tools to determine pain and depression in people living with OA. For pain these included the Western Ontario and McMaster Universities Arthritis Index (WOMAC)49, Knee Injury and Osteoarthritis Outcome Score (KOOS)50, Hip Disability and Osteoarthritis Outcome Score (HOOS)51, 36-Item Short Form Survey (SF-36) (pain component)52, (PISF), Harris Hip Score (HHS) (pain component)53, and arthritis self-efficacy (ASE) (pain component)54. For depression these included the Centre for Epidemiological Studies Depression (CESD) scale55, Hospital Anxiety and Depression Scale (HADS)56, Geriatric Depression Scale (GDS)57, Beck Depression Inventory (BDI), (AIMS) (psychological component)34, Emotional Distress and Depression Short-Form (EDD-SF)58, Depression Anxiety Stress Scales (DASS21)59, and the Patient Health Questionnaire (PHQ-9)60. Studies were included with a range of intervention durations and some included follow-up periods (e.g. 6 months, 1-year) which were considered when rating of the quality of the research article was completed. Articles were only included if they were in English.
Quality rating
All studies were quality rated based on the study design, participant characteristics, outcome measures and statistical analyses using a Quality Rating Tool10 (see Supplementary Material S2) developed by adapting and combining several different previously used tools10,61,62,,62. The tool ensured quality ratings reflected ability to determine potential statistical and clinical significance. Points were given based on study design and statistical analysis. Total scores on the quality rating tool ranged from 0 to 16 (i.e. higher scores indicated higher quality research).
Statistical analysis and clinical interpretation
The statistical analysis was conducted in Meta-Essentials developed by Suurmond and colleagues, Netherlands63. A random effects meta-analysis was completed with restricted maximum likelihood estimation (REML), assuming that the true effects differ across studies, as these studies vary in their study design and methodology (e.g. intervention type, duration, and outcome measure). Sensitivity analyses were performed to determine potential studies driving heterogeneity. Subgroup analyses were performed to investigate different types of interventions on clinical outcomes (pain and depression) and associated significance and effect sizes. The effect size of interventions was determined using Hedges’ g64 (page 110). Bias corrected standardized mean difference calculations (Hedges’ g) are recommended for studies with small sample sizes. According to Cohen, effect sizes less than 0.2 are trivial, 0.2 are small, 0.8 are medium, and 0.8 or higher are large65. Evidence for minimally clinically important differences (MCID) and moderate improvement estimates of change in OA-related pain had been published66. For the KOOS and HOOS short form pain measures, evidence has been published for MCID and moderate improvement estimates (2.2 and 15 respectively). Though reliability was much lower in knee compared to hip OA66.
A 95% confidence interval was used to determine the efficacy of combined interventions versus control on pain and symptoms of depression. Heterogeneity of the effects was assessed by calculating the I2 index and prediction intervals. The I2 index value reflects the proportion of true heterogeneity in the observed variance 69, 47], where 0% indicates no observed heterogeneity and increased heterogeneity is indicated by larger values where 25%, 50% and 75% reflect low, medium, and high heterogeneity respectively67. Prediction intervals are also presented throughout to ensure the predicted range of effects is unambiguous68. To understand the observed heterogeneity, we performed subgroup analyses to test intervention type. Publication bias was assessed graphically with contour-enhanced funnel plots and Egger’s regression test69. Funnel plots show if studies were missing only from areas of low statistical significance indicating that any asymmetry is likely to be caused by publication bias. The Egger regression gives “the degree of funnel plot asymmetry as measured by the intercept from regression of standard normal deviates against precision”69. If Egger’s test results in a p value less than 0.05, this implicates publication bias.
Results
Included studies
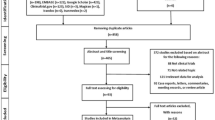
In total 1984 studies were found through the database search of studies published up until April 2022 (Fig. 1). Following removal of duplicates, article screening and removal of studies that did not meet eligibility criteria, 61 studies remained that included measures of pain and/or symptoms of depression in people with OA (Fig. 1). Thirty-eight studies were then excluded for not having sufficient data for meta-analysis, an active control condition, pain or depression outcomes not being reported post-intervention, and articles that did not meet methodology requirements (i.e. not RCTs, protocol papers). The mean quality rating of the 23 remaining studies (see Table 1) used in the final meta-analyses was high (mean: 13; SD = 1.6; total possible score: 16; range: 10–16). Participant characteristics are outlined in Table 2.
Meta-analyses
Pain
For pain, 29 interventions from 21 published articles were included in the meta-analysis (n = 4,382; 65 ± 6.9 years; 70% female; people with knee/hip OA at varying severity). There was a significant overall medium effect on reducing pain (ES = 0.43, 95% CI [0.25, 0.61], p < 0.001). Heterogeneity was significant and substantial (I2 = 71.5%, pq < 0.001) (Fig. 2a).
Main meta-analysis (a) and sub-group meta-analysis (b) for pain. Subgroup meta-analysis results showing effect sizes (i.e. SMDs), CIs (black lines), PIs (red lines) and I2 values for interventions. Movement meditation included yoga, tai chi, and qigong. Resistance exercise included hydrotherapy. Multimodal approaches included a combination of exercise and therapeutic/education-based approaches. CI confidence interval, I2 percentage of variation across studies that is due to heterogeneity, PI prediction interval, SMD standardized mean difference, w weight.
Sensitivity analyses were performed to examine potential outliers. A study was excluded with a very large effect size that was in the intervention subtype defined as ‘resistance exercise only’36. This analysis included 28 interventions from 20 published articles (n = 4322). The overall effect was ES = 0.33, 95% CI [0.24, 0.42], p < 0.001, I2 = 32.33%, pq = 0.0052. According to Cohen, it is a small to medium effect. Heterogeneity was at an acceptable level.
Subgroup analysis was then performed using random effects models for type of intervention and were defined as aerobic exercise only, resistance exercise only, therapeutic only, multimodal approaches and movement meditation. Effect sizes for reducing pain were significant for movement meditation (7 interventions: ES = 0.52, 95% CI [0.35, 0.69], p < 0.001; I2 = 0.0%), multimodal approaches (9 interventions: ES = 0.37, 95% CI [0.22, 0.51], p < 0.001; I2 = 41.2%, pq = 0.0092), resistance exercise (2 interventions: ES = 0.43, 95% CI [− 0.07,0.94], p = 0.046; I2 = 74.4%, pq = 0.0048), and therapeutic approaches (7 interventions: ES = 0.21, 95% CI [0.11, 0.31], p < 0.001; I2 = 3.1%, pq = 0.402). Aerobic exercise alone (including 3 interventions) did not significantly improve pain (Fig. 2b). Analysis of variance revealed significant differences between subgroup effect sizes for pain between movement meditation and aerobic exercise (p < 0.05) and movement meditation and therapeutic approaches (p < 0.01). Heterogeneity was at an acceptable level for all subgroups except resistance exercise though there are too few studies in this group to do further subgroup analysis.
Depression
For depression, 28 interventions from 20 published articles were included in the meta-analysis (n = 3377; 63 ± 7.0 years; 69% female; people with knee/hip OA at varying severity). There was a significant overall small effect on reducing symptoms of depression (ES = 0.29, 95% CI [0.08, 0.49], p < 0.001). Heterogeneity was significant and substantial (I2 = 73.3%, pq < 0.001) (Fig. 3a).
Main meta-analysis (a) and sub-group meta-analysis (b) for symptoms of depression. Subgroup meta-analysis results showing effect sizes (i.e. SMDs), CIs (black lines), PIs (red lines) and I2 values for interventions. Movement meditation included yoga, tai chi, and qigong. Resistance exercise included hydrotherapy. Multimodal approaches included a combination of exercise and therapeutic/education-based approaches. CI confidence interval, I2 percentage of variation across studies that is due to heterogeneity, PI prediction interval, SMD standardized mean difference, w weight.
For the sensitivity analysis two studies were excluded with very large effect sizes. They included a resistance intervention36 and a movement meditation intervention74. This analysis included 26 interventions from 18 published articles (n = 3274). The overall effect is small and significant ES = 0.16, 95% CI [0.08, 0.24], p < 0.001, I2 = 11.78%, pq = 0.293. Heterogeneity was at an acceptable level.
Subgroup analysis was then performed using random effects models for type of intervention and were defined as aerobic exercise only, resistance exercise only, therapeutic only, multimodal approaches and movement meditation. Effect sizes for reducing symptoms of depression were significant for movement meditation (6 interventions: ES = 0.30, 95% CI [0.06, 0.55], p = 0.008, I2 = 18.9%, pq = 0.291), and multimodal interventions (8 interventions: ES = 0.12, 95% CI [0.07, 0.18], p < 0.001, I2 = 0%, pq = 0.940). Resistance exercise, aerobic exercise, or therapeutic approaches alone (2, 4 and 6 interventions respectively) did not improve symptoms of depression (Fig. 3b). Analysis of variance revealed no significant differences between subgroup effect sizes for depression. Heterogeneity was at an acceptable level for movement meditation, multimodal approaches and aerobic exercise though was high for the remaining subgroups.
Publication bias
A funnel plot and the Egger test of asymmetry were used to examine publication bias (Fig. 4). The funnel plot was asymmetric. The Egger test was significant for pain (p < 0.001) suggesting potential publication bias and/or small-study effects91, though was not significant for depression (p = 0.216).
Discussion
Summary of main findings
Overall, meta-analyses from 23 high quality rated studies showed that nonpharmacological approaches significantly reduced pain (ES = 0.43, 95% CI [0.25, 0.61], 29 interventions) and symptoms of depression (ES = 0.29, 95% CI [0.08, 0.49], 28 interventions) in people with OA. Heterogeneity was lowered after removing outliers. For pain, subgroup analysis showed significance for movement meditation, multimodal approaches, therapeutic approaches, and resistance exercise only. However, heterogeneity was considerably high for resistance exercise. Movement meditation and multimodal approaches were also significant for symptoms of depression.
Based on these findings, approaches that are either multimodal (e.g. education/therapeutic approaches alongside exercise) or include focus on both the body and mind (e.g. focused breathing and mindfulness techniques used alongside movement, seen in yoga, qigong, and tai chi) are effective for reducing pain and symptoms of depression in people with knee/hip OA and warrant further investigation.
How these findings compare to others
Our findings support and expand on others regarding beneficial effects of exercise for reducing pain in people living with OA23,87. There are also differences between our findings and those of other studies. Fransen et al.’s26 Cochrane review based on 44 trials indicated that exercise significantly reduced pain and improved physical function26. Uthman et al.’s92 review based on 60 trials compared the effectiveness of different exercise interventions92.
Our analysis included only two resistance and three aerobic interventions. Both Cochrane reviews included more articles than our review. This was likely due to the main outcome of interest being pain and did not include depression, and because we only included studies with an inactive control group. Our findings align with other studies regarding reducing pain26,92, though extend our current understanding by investigating exercise type. In Fransen et al.’s review, no subgroup analysis was performed to examine potential different effects of exercise type, despite the heterogeneity and marked variability that was present regarding the exercise interventions assessed and other aspects of methodology26.
A more recent network meta-analysis investigated the relative efficacy of different types of exercise (aerobic, mind–body, strengthening, flexibility/skill or mixed) and found that aerobic had an equivalent beneficial effect (SMD = 1.11) to mind–body approaches in reducing pain93. Additionally, reporting that mixed exercise was the least effective. However, these approaches did not include education/therapeutic components as we did here, nor did they examine depression symptomatology. In another network analysis that examined different approaches on mental health measures in people with OA, the authors concluded that strengthening exercise was most beneficial for overall mental health, or mixed exercise for symptoms of depression94, though pain was not examined.
Strengths and limitations
Strengths of this study include that we were able to perform a meta-analysis including 23 studies where one or more intervention groups were compared with a control group. We also achieved high agreement levels between independent reviewers on ratings of study quality. We showed that similar approaches were effective for reducing both pain and symptoms of depression in OA, suggesting that these benefits will likely transfer to additional improvements in quality of life92,90. Further, identifying effective therapeutic options can support informed choice for people living with OA, and thus support shared decision-making processes in collaboration with healthcare professionals and family members to tailor treatments that best suit their individual needs and preferences95.
However, there are several limitations to consider when interpreting these results. Although the overall effect obtained for the meta-analyses for both pain and depression were significant, not all studies reported beneficial effects (e.g. pain72,85, symptoms of depression79,89). Possible explanations for observed non-significant effects in individual studies include low intervention dose, baseline depression scores and/or lack of formal depression diagnoses, and that participants differed between studies in the diagnostic site of OA, e.g. knee or hip. In our study, this information is included in Tables 1 and 2 where available.
Publication bias should also be considered, and some studies had very small sample sizes which may increase the likelihood of Type II errors. The funnel plots and Egger’s regression indicated publication bias was likely for pain level data though not for symptoms of depression, possibly due to pain more frequently being recorded as a primary outcome measure and depression a secondary outcome measure in these studies. Therefore, the overall effect size for pain may be an overestimation. This can be caused when small sample studies have not been published because significant effects were not obtained.
We included RCTs that measured pain and symptoms of depression in OA. Studies not measuring depression though were effective in reducing pain in OA may not have been captured in this review, for example therapeutic exercise and pain education96. Safety is a key consideration in intervention studies additional to effectiveness and adverse effects have been captured in other reviews26,97. However, we did not extract data for this outcome. Exercise is usually safe and well-tolerated for people with OA and depression, but this is based on limited data as studies seldom collect this as an outcome. Future trials would benefit from more focus and rigorous reporting on safety.
Clinical implications
In this systematic review we provide evidence for several approaches for reducing pain and symptoms of depression in OA, a condition with a substantial health burden in which co-morbidities are common. We highlight the importance of considering psychological health, i.e. symptoms of depression, in addition to physical health, i.e. pain, when providing quality healthcare to people living with OA. Interventions may be more effective when they are tailored to the individual and involve healthcare professionals working collaboratively across disciplines, e.g. nutrition, exercise, psychology, as well as with patients/clients. Further investigation is warranted on combining psychological and physical health focused interventions as well as multidisciplinary expertise to optimize patient outcomes.
Pain is experienced by at least half of people living with dementia and is most often attributed to OA98. Given the strong associations between OA and dementia6,7,8, 98, and that forty percent of dementia cases could be prevented or delayed by targeting risk factors including physical inactivity and associated chronic conditions31,99, targeting associated chronic conditions including OA will likely lead to reductions in dementia prevalence rates.
Conclusion
In this study, mind–body approaches were more effective than aerobic/resistance exercise or psychological therapy alone for reducing pain and depression in people with OA. Future research is required to determine the optimal approach for OA based on diagnostic site and symptom severity of comorbid conditions such as depression. Further, given the high prevalence rates of chronic conditions and people who experience multimorbidity, a better understanding of how the underlying mechanisms of co-occurring chronic conditions interact is also required. Incorporating qualitative studies such as focus groups and interviews to examine the experience, perspectives and preferences of people living with OA and depression will also add considerable value to this area.
Data availability
The data will be made available on request to the corresponding author.
References
Australian Bureau of Statistics (ABS). Causes of Death, Australia, 2020. https://www.abs.gov.au/statistics/health/causes-death/causes-death-australia/latest-release#australia-s-leading-causes-of-death-2020 (Accessed 18 January 2023) (2022).
Sharma, A., Kudesia, P., Shi, Q. & Gandhi, R. Anxiety and depression in patients with osteoarthritis: Impact and management challenges. Open Access Rheumatol. 8, 103–113 (2016).
Axford, J. et al. Prevalence of anxiety and depression in osteoarthritis: use of the Hospital Anxiety and Depression Scale as a screening tool. Clin. Rheumatol. 29(11), 1277–1283 (2010).
Australian Institute of Health and Welfare (AIHW) Osteoarthritis. https://www.aihw.gov.au/reports/chronic-musculoskeletal-conditions/osteoarthritis/contents/impact-of-osteoarthritis (Accessed 18 January 2023) (2020).
Australian Institute of Health and Welfare (AIHW). Chronic pain in Australia. https://www.aihw.gov.au/reports/chronic-disease/chronic-pain-in-australia/summary (Accessed 18 January 2023) (2022).
Guo, R. et al. The Association Between Osteoarthritis with Risk of Dementia and Cognitive Impairment: A Meta-Analysis and Systematic Review. Journal of Alzheimer's disease 89(4), 1159–1172. https://doi.org/10.3233/JAD-220568 (2022).
Weber, A. et al.Association between osteoarthritis and increased risk of dementia: A systemic review and meta-analysis. Medicine, 98(10), e14355. https://doi.org/10.1097/MD.0000000000014355 (2019).
Huang, SW., Wang, WT., Chou, LC. et al. Osteoarthritis Increases the Risk of Dementia: A Nationwide Cohort Study in Taiwan. Sci Rep 5, 10145. https://doi.org/10.1038/srep10145(2015).
Kuring, J., Mathias, J. & Ward, L. Risk of dementia in persons who have previously experienced clinically-significant depression, anxiety, or PTSD: A systematic review and meta-analysis. J. Affect. Disord. 274, 247–261 (2020).
Burley, C. V., Burns, K., Lam, B. C. & Brodaty, H. Nonpharmacological approaches reduce symptoms of depression in dementia: A systematic review and meta-analysis. Ageing Res. Rev. 79, 101669 (2022).
May, K. & Scammell, J. Nurses’ experiences of pain management in end-of-life dementia care: A literature review. Int. J. Palliat. Nurs. 26(3), 110–118 (2020).
Dementia Australia, 2022 Dementia statistics https://www.dementia.org.au/statistics (Accessed 18 January 2023).
WHO. Ageing and health. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (Accessed 18 January 2023) (2022).
Wallace, J. L. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself?. Physiol. Rev. 88(4), 1547–1565 (2008).
McCrae, J., Morrison, E., MacIntyre, I., Dear, J. & Webb, D. Long-term adverse effects of paracetamol–A review. Br. J. Clin. Pharmacol. 84(10), 2218–2230 (2018).
Yip, K. & Oettinger, J. Why are we still using opioids for osteoarthritis?. Int. J. Clin. Pract. 74(1), e13416 (2020).
Bartley, E. J., Palit, S. & Staud, R. Predictors of osteoarthritis pain: The importance of resilience. Curr. Rheumatol. Rep. 19(9), 1–9 (2017).
Kyrkanides, S. et al. Osteoarthritis accelerates and exacerbates Alzheimer’s disease pathology in mice. J. Neuroinflamm. 8(1), 1–8 (2011).
Arendt-Nielsen, L. Pain sensitisation in osteoarthritis. Clin. Exp. Rheumatol. 107(5), 68–74 (2017).
Bhatt, N. G., Sheth, M. S. & Vyas, N. J. Correlation of fear avoidance beliefs with pain and physical function in subjects with osteoarthritis of knee. Int. J. Ther. Rehabil. Res. 4, 117–121 (2015).
Sánchez Romero, E. A. et al. Relationship between the gut microbiome and osteoarthritis pain: Review of the literature. Nutrients 13(3), 716 (2021).
Clapp, M. et al. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 7(4), 987 (2017).
Liu, L. et al. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 90, 104527 (2023).
Sorboni, S. G., Moghaddam, H. S., Jafarzadeh-Esfehani, R. & Soleimanpour, S. A Comprehensive review on the role of the gut microbiome in human neurological disorders. Clin. Microbiol. Rev. 35(1), e0033820 (2022).
Pedersen, B. K. & Saltin, B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 25, 1–72 (2015).
Fransen, M. et al. Exercise for osteoarthritis of the knee: A Cochrane systematic review. Br. J. Sports Med. 49(24), 1554–1557 (2015).
Fransen, M., Nairn, L., Winstanley, J., Lam, P. & Edmonds, J. Physical activity for osteoarthritis management: A randomized controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthritis Care Res. 57(3), 407–414 (2007).
Rosenbaum, S., Tiedemann, A., Sherrington, C., Curtis, J. & Ward, P. B. Physical activity interventions for people with mental illness: A systematic review and meta-analysis. J. Clin. Psychiatry 75(9), 14465 (2014).
McDowell, C. P., Dishman, R. K., Gordon, B. R. & Herring, M. P. Physical activity and anxiety: A systematic review and meta-analysis of prospective cohort studies. Am. J. Prev. Med. 57(4), 545–556 (2019).
Jia, R., Liang, J., Xu, Y. & Wang, Y. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 19(1), 1–14 (2019).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396(10248), 413–446 (2020).
Quirk, H., Blake, H., Tennyson, R., Randell, T. & Glazebrook, C. Physical activity interventions in children and young people with type 1 diabetes mellitus: A systematic review with meta-analysis. Diabet. Med. 31(10), 1163–1173 (2014).
Polese, J. C., Ada, L., Dean, C. M., Nascimento, L. R. & Teixeira-Salmela, L. F. Treadmill training is effective for ambulatory adults with stroke: A systematic review. J. Physiother. 59(2), 73–80 (2013).
Kuntz, A. B. et al. Efficacy of a biomechanically-based yoga exercise program in knee osteoarthritis: A randomized controlled trial. PLoS One 13(4), e0195653 (2018).
Moonaz, S. H., Bingham, C. O., Wissow, L. & Bartlett, S. J. Yoga in sedentary adults with arthritis: Effects of a randomized controlled pragmatic trial. J. Rheumatol. 42(7), 1194–1202 (2015).
Taglietti, M. et al. Effectiveness of aquatic exercises compared to patient-education on health status in individuals with knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 32(6), 766–776 (2018).
Gay, C., Chabaud, A., Guilley, E. & Coudeyre, E. Educating patients about the benefits of physical activity and exercise for their hip and knee osteoarthritis. Systematic literature review. Ann. Phys. Rehabilit. Med. 59(3), 174–183 (2016).
Beck AT, Steer RA, Brown G. Beck depression inventory–II. Psychol Assess (1996).
Gourgouvelis, J., Yielder, P., Clarke, S. T., Behbahani, H. & Murphy, B. A. Exercise leads to better clinical outcomes in those receiving medication plus cognitive behavioral therapy for major depressive disorder. Front. Psychiatry 9, 37 (2018).
Bourbeau, K., Moriarty, T., Ayanniyi, A. & Zuhl, M. The combined effect of exercise and behavioral therapy for depression and anxiety: Systematic review and meta-analysis. Behav. Sci. 10(7), 116 (2020).
Li, C. et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for patients with diabetes and depression. J. Psychosom. Res. 95, 44–54 (2017).
Rossman, J. Cognitive-behavioral therapy for insomnia: An effective and underutilized treatment for insomnia. Am. J. Lifestyle Med. 13(6), 544–547 (2019).
Atwood, M. E. & Friedman, A. A systematic review of enhanced cognitive behavioral therapy (CBT-E) for eating disorders. Int. J. Eat. Disord. 53(3), 311–330 (2020).
Morley, S., Eccleston, C. & Williams, A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 80(1–2), 1–13 (1999).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 10(1), 1–11 (2021).
Cochrane. Chapter 10: Analysing data and undertaking meta-analyses. https://training.cochrane.org/handbook/current/chapter-10 (Accessed 18 January 2023) (2022).
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14(1), 1–13 (2014).
Chinn, S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat. Med. 19(22), 3127–3131 (2000).
Bellamy, N., Buchanan, W. W., Goldsmith, C. H., Campbell, J. & Stitt, L. W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 15, 1833–1840 (1988).
Bekkers, J., de Windt, T. S., Raijmakers, N., Dhert, W. & Saris, D. Validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the treatment of focal cartilage lesions. Osteoarthr. Cartil. 17(11), 1434–1439 (2009).
Nilsdotter, A. K., Lohmander, L. S., Klässbo, M. & Roos, E. M. Hip disability and osteoarthritis outcome score (HOOS)–validity and responsiveness in total hip replacement. BMC Musculoskelet. Disord. 4(1), 1–8 (2003).
McHorney, C. A., Ware Johne, J. & Anastasiae, R. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 31(3), 247–263 (1993).
Söderman, P. & Malchau, H. Is the Harris hip score system useful to study the outcome of total hip replacement?. Clin. Orthop. Relat. Res. 1976–2007 384, 189–197 (2001).
Barlow, J., Williams, B. & Wright, C. The reliability and validity of the arthritis self-efficacy scale in a UK context. Psychol. Health Med. 2(1), 3–17 (1997).
Devins, G. M. et al. Measuring depressive symptoms in illness populations: Psychometric properties of the center for epidemiologic studies depression (CES-D) scale. Psychol. Health 2(2), 139–156 (1988).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67(6), 361–370 (1983).
Montorio, I. & Izal, M. The geriatric depression scale: A review of its development and utility. Int. Psychogeriatr. 8(1), 103–112 (1996).
Liu, H. et al. Representativeness of the patient-reported outcomes measurement information system internet panel. J. Clin. Epidemiol. 63(11), 1169–1178 (2010).
Akin, A. & Çetin, B. The depression anxiety and stress scale (DASS): The study of validity and reliability. Kuram ve Uygulamada Egitim Bilimleri 7(1), 260 (2007).
Spitzer, R. L., Kroenke, K., Williams, J. B., Patient Health Questionnaire Primary Care Study Group, Patient Health Questionnaire Primary Care Study Group. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA 282(18), 1737–1744 (1999).
Hopewell, S. et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 5(1), e20 (2008).
Verhagen, A. P. et al. The art of quality assessment of RCTs included in systematic reviews. J. Clin. Epidemiol. 54(7), 651–654 (2001).
Suurmond, R., van Rhee, H. & Hak, T. Introduction, comparison, and validation of meta-essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 8(4), 537–553 (2017).
Hedges, L. V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 6(2), 107–128 (1981).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences, no. 1 (Lwrence Earlbaum Associates Publishers, 1988).
Singh, J. A., Luo, R., Landon, G. C. & Suarez-Almazor, M. Reliability and clinically important improvement thresholds for osteoarthritis pain and function scales: A multicenter study. J. Rheumatol. 41(3), 509–515 (2014).
Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. Introduction to Meta-analysis (Wiley, 2021).
Borenstein, M., Higgins, J. P., Hedges, L. V. & Rothstein, H. R. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res. Synth. Methods 8(1), 5–18 (2017).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Cheung, C. et al. Managing knee osteoarthritis with yoga or aerobic/strengthening exercise programs in older adults: A pilot randomized controlled trial. Rheumatol. Int. 37(3), 389–398 (2017).
French, H. P. et al. Exercise and manual physiotherapy arthritis research trial (EMPART) for osteoarthritis of the hip: A multicenter randomized controlled trial. Arch. Phys. Med. Rehabil. 94(2), 302–314 (2013).
Lin, Y., Lee, W. & Hsieh, R. Active video games for knee osteoarthritis improve mobility but not WOMAC score: A randomized controlled trial. Ann. Phys. Rehabil. Med. 63(6), 458–465 (2020).
Park, J. et al. The effect of chair yoga on biopsychosocial changes in English-and Spanish-speaking community-dwelling older adults with lower-extremity osteoarthritis. J. Gerontol. Soc. Work 59(7–8), 604–626 (2016).
Zhang, S. et al. The effectiveness of traditional Chinese Yijinjing Qigong exercise for the patients with knee osteoarthritis on the pain, dysfunction, and mood disorder: A pilot randomized controlled trial. Front. Med. https://doi.org/10.3389/fmed.2021.792436 (2022).
Ahn, Y. H. & Ham, O. K. Evaluation of the interaction model of client health Behavior-based multifaceted intervention on patient activation and osteoarthritis symptoms. Jpn. J. Nurs. Sci. 17(2), e12306 (2020).
Barlow, J. H., Turner, A. P. & Wright, C. C. A randomized controlled study of the Arthritis Self-Management Programme in the UK. Health Educ. Res. 15(6), 665–680 (2000).
Hurley, M. et al. Clinical effectiveness of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain: A cluster randomized trial. Arthritis Care Res. 57(7), 1211–1219 (2007).
Somers, T. J. et al. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: A randomized controlled study. Pain 153(6), 1199–1209 (2012).
Tak, E., Staats, P., Van Hespen, A. & Hopman-Rock, M. The effects of an exercise program for older adults with osteoarthritis of the hip. J. Rheumatol. 32(6), 1106–1113 (2005).
Walsh, N. et al. Facilitating activity and self-management for people with arthritic knee, hip or lower back pain (FASA): A cluster randomised controlled trial. Musculoskelet. Sci. Pract. 50, 102271 (2020).
Yip, Y., Sit, J. W., Wong, D. Y., Chong, S. Y. & Chung, L. A 1-year follow-up of an experimental study of a self-management arthritis programme with an added exercise component of clients with osteoarthritis of the knee. Psychol. Health Med. 13(4), 402–414 (2008).
Lorig, K. & Fries, J. The Arthritis Helpbook, 4th edn. Addison-Wesley, Reading, MA (1995).
Yip, Y.B. et al. Impact of a self-management arthritis programme with an added exercise component for osteoarthritic knee sufferers on improving pain, functional outcomes, and use of health care services: An experimental study. Patient Educ. Couns. 65, 113–121 (2007).
Bossen, D. et al. Effectiveness of a web-based physical activity intervention in patients with knee and/or hip osteoarthritis: Randomized controlled trial. J. Med. Internet Res. 15(11), e2662 (2013).
Allen, K. D. et al. Pain coping skills training for African Americans with osteoarthritis: Results of a randomized controlled trial. Pain 160(6), 1297–1307 (2019).
Broderick, J. E. et al. Nurse practitioners can effectively deliver pain coping skills training to osteoarthritis patients with chronic pain: A randomized, controlled trial. Pain 155(9), 1743–1754 (2014).
Hausmann, L. R. et al. Testing a positive psychological intervention for osteoarthritis. Pain Med. 18(10), 1908–1920 (2017).
Helminen, E., Sinikallio, S. H., Valjakka, A. L., Väisänen-Rouvali, R. H. & Arokoski, J. P. Effectiveness of a cognitive–behavioural group intervention for knee osteoarthritis pain: A randomized controlled trial. Clin. Rehabil. 29(9), 868–881 (2015).
Lin, E. H. et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: A randomized controlled trial. JAMA 290(18), 2428–2429 (2003).
O’Moore, K. A. et al. Internet cognitive–behavioral therapy for depression in older adults with knee osteoarthritis: A randomized controlled trial. Arthritis Care Res. 70(1), 61–70 (2018).
Debray, T. P., Moons, K. G. & Riley, R. D. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: A comparison of new and existing tests. Res. Synth. Methods 9(1), 41–50 (2018).
Uthman, O. A. et al. Exercise for lower limb osteoarthritis: Systematic review incorporating trial sequential analysis and network meta-analysis. BMJ 347, f5555 (2013).
Goh, S. et al. Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: Systematic review and network meta-analysis. Sports Med. 49(5), 743–761 (2019).
Hall, M. et al. Comparative effectiveness of exercise programs for psychological well-being in knee osteoarthritis: A systematic review and network meta-analysis. Semin. Arthritis Rheum. 51(5), 1023–1032 (2021).
Johnson, C. B. A personalized shared decision-making tool for osteoarthritis management of the knee. Orthop. Nurs. 40(2), 64–70 (2021).
Siddall, B. et al. Short-term impact of combining pain neuroscience education with exercise for chronic musculoskeletal pain: A systematic review and meta-analysis. Pain 163(1), e20–e30. https://doi.org/10.1097/j.pain.0000000000002308 (2022).
Geneen, L. J. et al. Physical activity and exercise for chronic pain in adults: An overview of cochrane reviews. Cochrane Database Syst. Rev. 1(1), CD011279 (2017).
Lin, P., Li, C., Chou, P., Chen, Y. & Lin, L. Prevalence of pain-related diagnoses in patients with dementia: A nationwide study. J. Pain Res. 11, 1589–1598 (2018).
Siette, J. et al. Advancing Australian public health initiatives targeting dementia risk reduction. Australas. J. Ageing 41(2), e190–e195 (2022).
Acknowledgements
This research was supported by philanthropic funding provided by Faye Williams.
Author information
Authors and Affiliations
Contributions
C.B. designed the study, collected data, carried out quality ratings, analysed the data, and drafted the article. A.-N. Casey collected data, carried out quality ratings, analysed data and assisted with writing the article. B.P. supervised data collection and analysis and assisted with writing the article. M.J. and K.W. assisted with clinical interpretation of results and writing the article. All authors reviewed and authorized the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burley, C.V., Casey, AN., Jones, M.D. et al. Nonpharmacological approaches for pain and symptoms of depression in people with osteoarthritis: systematic review and meta-analyses. Sci Rep 13, 15449 (2023). https://doi.org/10.1038/s41598-023-41709-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41709-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.