Abstract
Microbial pathogens are known for causing great environmental stress, owing to which emerging challenges like lack of eco-friendly remediation measures, development of drug-resistant and mutational microbial strains, etc., warrants novel and green routes as a stepping stone to serve such concerns sustainably. In the present study, palladium (Pd) doped manganese (II, III) oxide (Mn3O4) nanoparticles (NPs) were synthesized using an aqueous Syzygium aromaticum bud (ASAB) extract. Preliminary phytochemical analysis of ASAB extract indicates the presence of polyphenolics such as phenols, alkaloids, and flavonoids that can act as potential capping agents in NPs synthesis, which was later confirmed in FTIR analysis of pure and Pd-doped Mn3O4 NPs. XRD, Raman, and XPS analyses confirmed the Pd doping in Mn3O4 NPs. FESEM and HRTEM study reveals the mixed morphologies dominated by nanocorns appearance. Zeta potential investigation reveals high stability of the synthesized NPs in colloidal solutions. The developed Pd-doped Mn3O4 NPs were tested against two fungal phytopathogens, i.e., Sclerotinia sclerotiorum and Colletotrichum gloeosporioides, known for causing great economic losses in yield and quality of different plant species. The antifungal activity of synthesized Pd‐doped Mn3O4 NPs displayed a dose‐dependent response with a maximum of ~92%, and ~72% inhibition was recorded against S. sclerotiorum and C. gloeosporioides, respectively, at 1000 ppm concentration. However, C. gloeosporioides demonstrated higher sensitivity to Pd‐doped Mn3O4 NPs upto 500 ppm) treatment than S. sclerotiorum. The prepared NPs also showed significant antibacterial activity against Enterococcus faecalis. The Pd-doped Mn3O4 NPs were effective even at low treatment doses, i.e., 50–100 ppm, with the highest Zone of inhibition obtained at 1000 ppm concentration. Our findings provide a novel, eco-benign, and cost-effective approach for formulating a nanomaterial composition offering multifaceted utilities as an effective antimicrobial agent.
Similar content being viewed by others
Introduction
Nanobiotechnology integrates biotechnology and nanotechnology, dealing with applications of nanomaterials in biological sciences1,2,3,4. With the advent of nanobiotechnology, nanostructures' biological and physiochemical properties are tuned to serve the most relevant areas of human welfare, like medicine and agriculture5. Among different types, metallic nanoparticles (MNPs) are one of the widely exploited antimicrobial nanomaterials against phyto- and animal pathogens6,7,8,9,10,11,12. Researchers have recently witnessed a growing interest in synthesizing biocompatible metal-based NPs, utilizing green chemistry and bioinspired fabrication routes13,14,15,16,17,18,19,20,21.
Biological synthesis offers an eco-friendly and cost-effective method for the fabrication of NPs22 and is preferred over conventional methods23. Different biogenic sources for the synthesis of MNPs, like bacteria, plants, algae, fungi, yeasts, and actinomycetes, cause considerable modifications in the properties of their corresponding metals24. Among such sources, plant-based bioinspired fabrication of NPs is one of the preferred approaches24,25. The bioactive compounds in plant extracts can act as potential reducing and capping agents in synthesizing MNPs24,25. Green synthesis of MNPs like silver, copper, gold, iron, titanium, zinc, platinum, palladium, etc., has been extensively investigated26,27,28. However, limited investigations for green synthesis of manganese (Mn) NPs have been reported so far24, despite various applications in catalysis, biomedicine, electronics, electrochemistry, energy, optics, biosensors, food, pharmaceutical, cosmetics, textile industries, etc.24,29.
Metal oxides such as ZnO, CuO, TiO2 and MnO etc. have also a great potential to have excellent antimicrobial activity19. Chamaecostus cuspidatus extract is used to green synthesis CeO2 and ZnO nanoparticles (NPs) and effective antibacterial activities. The anticancer effects of CeO2 and ZnO nanoparticles were investigated in human breast cancer cell lines21. Similarly, Green synthesis is used to prepare Cerium oxide nanoparticles (CeO2 NPs) from Artabotrys hexapetalus leaf extracts. The prepared NPs exhibit excellent antibacterial activity against a variety of bacterial species. The anticancer potential of the compound was studied against the (MCF-7) human breast cancer cell line22. In addition to that, Zinc oxide nanoparticle (ZnO NPs) was prepared utilizing starch in a single step green synthesis and had highly porous, novel hollow multi-sphere in morphology. Because of their morphology and porosity, the synthesized ZnO NPs can be employed in a variety of drug delivery applications19. Mn has been reported as the transition element with the third highest abundance on earth followed by iron and titanium30. Among various 3d transition metal-oxides, Mn-oxides (MnO, MnO2, Mn2O3, Mn3O4, and Mn5O8) have obtained key attention owing to their compositional and structural diversity24,31. Mn-oxides NPs also possess structural adaptability with varying physicochemical qualities32. Mn-oxide NPs have excessive potential for sustainable-nanotechnology research and innovation24,30. Mn-oxides can have applications in optoelectronics, magnetic storage devices, imaging contrast agents, magnetic materials, drug delivery, catalysis, wastewater treatment, solar cells, etc.24.
Sclerotinia sclerotiorum is a necrotrophic phytopathogen that harbors a broad host range and causes stem rot disease in different crops including soybean, oilseed rape, sunflower, tomato, etc., resulting huge losses of agricultural produce33. On the other hand, C. gloeosporioides follows the hemibiotrophic infection mode and is known for causing anthracnose in fruits like papaya, mango, avocado, apple, guava, banana, papaya, cashews, grapes, pitaya, etc., resulting in serious postharvest losses34,35. Although chemical fungicides have been utilized for their control, their indiscriminate utilization has serious environmental consequences that necessitate the search for novel and eco-friendly alternatives36. E. faecalis is known to colonize the human intestine, and its occurrence in aquatic bodies implies fecal contamination37. These bacteria have been reported as multidrug-resistance microbial pathogens associated with hospital-acquired infections37,38.
To combat such underlying issues, present study focused on developing nanoformulation comprised of Pd-doped Mn3O4 NPs through the green chemistry route as a potential tool to offer a broad spectrum of antimicrobial activity.
In the current study, green synthesis of Mn3O4 NPs was completed by using Syzygium aromaticum bud extract as a potential reducing and capping agent. Adding novelty to this work, attempts were made to modify the structural attributes of Mn3O4 NPs through Pd doping to prioritize their multifaced applications in sectors such as agriculture, environment and medicine. To the best of our knowledge and available literature, this is the first report on the bioinspired fabrication of nanocorn-like Pd-doped Mn3O4 NPs using ASAB extract.
This present work investigated the antimicrobial potential of bioinspired Pd-doped Mn3O4 NPs against S. sclerotiorum, C. gloeosporioides, and E. faecalis.
Experimental
Materials and methods
All the chemicals used in the present study (such as manganese chloride tetrahydrate, palladium chloride, sodium hydroxide, dextrose, agar, hydrochloric acid, potassium hydroxide, sodium chloride, yeast extract, beef extract, and peptone) were of analytical grade and utilized without any further purifications. The solutions and reagents were prepared in double-distilled water (DDW).
Collection of plant material
The evenly looking dried flower buds of S. aromaticum were obtained from the nearby local market of Meerut (Uttar Pradesh, India). The taxonomical evaluation was performed by Prof. Vijai Malik, Head, Department of Botany, CCS University, Meerut (UP) (letter reference no. Bot/PB/380). Plant material was procured as per applicable Institutional, International, and National guidelines. The specimens were deposited in the University Herbarium at the Department of Botany (Accession no. Bot. 26 V2L4).
Preparation of S. aromaticum bud extract
The procured flower buds were thoroughly washed with distilled water to remove dirt particles and dried at 40 °C for 48 h. The dried flower buds were homogenized into a fine powdered form and stored in an air-tight container until used. To prepare the extract, powdered flower buds were macerated in DDW (1:10, w/v) at 60 °C for 2 h. After cooling at room temperature, the aqueous solution of phytoextract was filtered using Whatman filter paper number 1 at stored at 4 °C temperature until use.
Green synthesis of pure and Pd-doped Mn3O4 nanoparticles
The NPs were synthesized using a sol–gel method39, assisted by the addition of phytoextract as a potential source of capping agent (Fig. 1). Briefly, 2% (w/v) PdCl2 was added to the aqueous solution of MnCl2.4H2O (49 mM) followed by drop-wise addition of 10 ml aqueous Syzygium aromaticum bud extract under continuous stirring at 650 rpm at 90 °C for one hour. After getting the light brown color of the solution, 25 mM aqueous solution of NaOH was added drop-wise to adjust the pH. The alkali-mediated synthesis provides rapid precipitation leading to the mixture of manganese dihydroxide (Mn (OH)2) and manganese trihydroxide (Mn (OH)3)40,41. The appearance of a dark brownish-black precipitate indicated the formation of Pd-doped Mn3O4 NPs. The precipitate was collected using centrifugation at 5000 rpm for 10 min. The recovered precipitate gave multiple washings with ethanol and DDW to remove impurities, then dried at 150 °C for 3.5 h in a hot air oven. The finally dried residue was transformed into fine powder through mechanical grinding. The obtained Mn3O4 NPs were stored in an airtight bottle for further characterization. Except for the dopant addition, all other synthesis steps for pure Mn3O4 NPs were the same as those mentioned for Pd-doped Mn3O4 NPs. The key reaction steps in synthesizing Mn3O4 NPs are mentioned below40.
Characterization of synthesized nanoparticles
XRD analysis was performed to study the crystallinity and phases of prepared NPs (Bruker AXS, D8 Advance). The phase transitions and chemical composition of synthesized NPs were examined by Raman spectroscopy. The morphology and elemental composition were studied by FESEM (FEI, Quanta 200F), and EDAX, respectively. FTIR analysis conducted on ASAB extract and synthesized NPs to investigate the role of bioactive compounds in the development of NPs. The zeta potential and structural properties were determined using Zetasizer (Malvern Nano ZS) and HRTEM/SAED, respectively. XPS analysis was conducted to study the composition, and oxidation states of Pd-doped Mn3O4 NPs. The optical properties of prepared NPs were determined by UV–Visible spectroscopy.
Antimicrobial activity of nanoparticles
The antifungal activity of NPs was tested against S. sclerotiorum and C. gloeosporioides using the poisoned food technique. The fungal cultures were procured from Indian Type Culture Collection (ITCC), Division of Plant Pathology, Indian Agriculture Research Institute, New Delhi, India. The potato dextrose agar (PDA) media was prepared for fungal growth with following composition: dextrose (2% w/v), potato starch (0.4% w/v), and agar (1.5% w/v). The pH of 5.6 ± 0.2 was adjusted using 0.1N KOH/0.1N HCl. The synthesized NPs were dispersed in PDA media to get the desired concentrations (up to 1000 ppm). The media containing NPs was poured into a Petri plate. At the next step, a ~ 8 mm piece of actively growing mycelia from 5 to 8 days old pure cultures of S. sclerotiorum and C. gloeosporioides were placed in the middle of each plate and incubated for five days at ~ 25 ± 2 °C and ~ 28 ± 2 °C temperature, respectively. The media plates without NPs treatment served as the negative control, and plates with 2 mg/ml of carbendazim + mancozeb were designated as the positive control. The % of growth inhibition was calculated using the below formula:
The antibacterial activity of NPs was determined using agar disc diffusion assay (ADDA). The pure culture of E. faecalis was inoculated to freshly prepared nutrient broth media (NBM) and maintained at 37 °C for ~ 18 h. The composition of NBM was Yeast extract (0.2% w/v), Beef extract (0.1% w/v), Peptone (0.5% w/v), NaCl (0.5% w/v), and pH ~ 7.4 ± 0.2. 2% (w/v) agar was added to the nutrient broth for preparing solid media plates (SPM). The pure culture of E. faecalis at the active log phase was uniformly spread on SPM. The sterile filter paper discs of about 6 mm diameter, each dipped in various concentrations of NPs (0 to 1000 ppm), were placed on SPM. The DDW-dipped discs were served as a negative control. The Petri plates were placed in an incubator overnight, and the ZOI was measured in millimetre. All the experiments on antimicrobial activity were performed in triplicates under aseptic conditions in a laminar airflow chamber. The nutrient media, glassware, and other utilities were autoclaved at 121 °C for 15 min at 15 psi pressure before use to maintain aseptic conditions throughout the assay.
Statistical analysis
All of the experiments were completed in triplicates and recorded data presented as mean ± standard deviation using Microsoft Excel®.
Results and discussion
Green synthesis of nanoparticles and FTIR analysis
Pure and Pd-doped Mn3O4 NPs were synthesized using an aqueous Syzygium aromaticum bud (ASAB) extract. Preliminary phytochemical analysis indicated the presence of phenols (ferric chloride test)42, flavonoids (lead acetate test)43, alkaloids (Wagner test), carbohydrates (fehling's test), and tannins (ferric chloride test)44. The results were in concordance with the findings of Jimoh et al.43, which established the suitability of the tested plant as a potential substrate for developing Phyto inspired nanoparticles45,46,47. Through FTIR analysis, Rajesh et al.45 predicted the role of metabolites present in S. aromaticum bud extract, such as flavonoids, tannins, alkaloids, and carotenoids, in the green synthesis of CuNPs. We have compared the FTIR spectra of ASAB extract, pure and Pd-doped Mn3O4 NPs to validate the capping and stabilizing potential of bioactive compounds present in phytoextract (Fig. 2).
FTIR spectrum of ASAB extract revealed a broad band at ~ 3431.62 cm−1, which corresponds to the OH group45, alkyl CH stretching (sp3), and C–O ester group was observed at ~ 2931.95 and ~ 1711.92 cm−1, respectively48. A sharp peak at ~ 1611.99 cm−1 belongs to –C = C aromatic stretching vibrations and C = O stretching vibrations of proteins denoting amide linkages 45. The aromatic groups were indicated at ~ 1511.36 cm−148, while two separate peaks at ~ 1366.60 cm−145 and ~ 1051.25 cm−148 denoted the C–O group. These characteristic FTIR spectral peaks suggest for eugenol presence in ASAB extract48. The peak at ~ 640 cm-1 are attributed to the Mn–O in synthesized nanoparticles49. The peaks common to ASAB extract, pure and Pd-doped Mn3O4 NPs (~ 3450–3400, ~ 1750–1700, ~ 1650–1600, ~ 1400–1350, and ~ 1050 cm-1) depicted the capping potential of bioactive compounds in phytoextract.
Structural analysis
The XRD confirms the crystalline structure of the prepared manganese oxide NPs with two distinct phases, as shown in Fig. 3. The diffraction peaks of Mn3O4 samples at 2θ values of 31.04, 32.36, 36.13, 44.47, 53.83, 58.57, 59.90, and 64.69 matched well with the (200), (103), (211), (220), (312), (321), (224), and (400) crystal planes of Mn3O4 phase respectively50. The above (hkl) planes correspond to the Hausmannite phase of the Mn3O4 crystal structure (JCPDS 24–0734)51. The intensity of XRD peaks was decreased in the case of Pd doping, indicating peak shifting towards lower diffraction angles and crystalline lattice expansion52, suggesting the successful incorporation of Pd in Mn3O4. There is no shifting of XRD peaks in the case of Mn3O4 NPs, indicating that as-synthesized NPs are comprised of the tetragonal Hausmannite phase53. The sharp peaks confirmed the highly crystalline nature of NPs54. There were no other peaks in the XRD pattern that demonstrated the phase purity of the produced NPs55.
The crystallite size of synthesized NPs were calculated by Scherrer’s formula at a extremely intense peak (Eq. 5), and the values were ~ 32 and ~ 28 nm for pure and Pd-doped Mn3O4 NPs, respectively56,57. The Pd-doped Mn3O4 NPs were smaller than the pure Mn3O4 NPs, which could be owing to the fact that ionic radii of Pd (0.137 nm) are much larger than that of Mn (0.082 nm)58.
where D is crystallite size, λ is the wavelength, θ is Bragg’s angle, and β is FWHM.
Surface morphology and elemental analysis
The surface morphology of the pure Mn3O4 NPs in Fig. 4a, b appeared to be rod-like nanostructures. In contrast, Fig. 4d, e showed the surface of Pd doped-Mn3O4 NPs with the likely appearance of nanocorn-like structures. The morphological changes from rod (Mn3O4 NPs) to nanocorn-like nanocorn (Pd-doped Mn3O4 NPs) could be owing to the decoration of Mn3O4 with Pd2+. EDAX analysis revealed Pd, Mn, and O in their suitable stoichiometric proportion, as given in Fig. 4c, f 59.
Optical studies
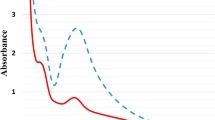
The absorption spectra of pure and Pd-doped Mn3O4 NPs were recorded at room temperature using a UV–visible spectrophotometer. In absorption spectra, the optical absorption edge was shifted to a higher wavelength region with Pd doping; consequently, the red shift was noticed in Pd-doped Mn3O4 NPs. The energy band gap of developed NPs was calculated by Tauc’s relation (Eq. 6)60.
where α, hν, Eg, and B are the absorption coefficient, photon energy, the band gap energy, and constant, respectively. The value of index ‘n’ calculated from Tauc’s Plot was 2 (Fig. 5). The estimated band gap was ~ 3.79 and ~ 3.75 eV for pure and Pd-doped Mn3O4 NPs, respectively, which is in agreement with the previous reports for Mn3O4 NPs61. It was noticed that the band gap decreased in Pd-doped Mn3O4 NPs, compared to pure Mn3O4 NPs. A red shift was observed in the band gap of Pd-doped Mn3O4 NPs. This may be due to intermediate levels forming between the CB and VB of the host Mn3O4 matrix62,63. Pd atoms act as an accepter to decrease the band gap of Mn3O4 NPs64,65. Therefore, variation in the energy band gap of Mn3O4 NPs by Pd doping may have applications in photocatalytic activity66.
Electronic states and elemental composition analysis
The oxidation states of Pd-doped Mn3O4 NPs were determined by XPS analysis67. The survey spectrum (Fig. 6a) revealed the presence of Pd, Mn, and O, confirming their existence in the product, i.e., Pd-doped Mn3O4 NPs. Further analysis of the Pd 3d spectrum showed a doublet feature68, providing evidence of Pd species' presence in the material. The peaks observed at 332.17 eV and 335.56 eV in the Pd 3d5/2 region, along with 340.57 eV and 344.58 eV in the Pd 3d3/2 region, corresponded to Pd (II) and Pd (IV) states68. Moving on to the Mn 2p core-level spectrum, two distinct peaks were observed at binding energies of 654.94 eV, 653.56 eV and 643.53 eV, 642.13 eV for pure and Pd-doped Mn3O4 nanocorns, respectively. These peaks were associated with Mn 2p1/2 and Mn 2p3/2 in Mn3O4 NPs and indicating a spin-orbital splitting of 11.4 eV (Fig. 6b)69,70. Additionally, the O1s spectrum peaked at 529.68 eV for pure Mn3O4 NPs and 532.89 eV for Pd-doped Mn3O4 NPs (Fig. 6d)70. This peak confirmed the presence of oxygen in both materials. The XPS analysis provided conclusive evidence that the prepared manganese oxide material was indeed Pd-doped Mn3O4 NPs, with the oxidation states of Pd (II) and Pd (IV) and specific Mn 2p states characteristic of Mn3O4.
Transmission electron microscopy analysis
The TEM micrograph of the green synthesized Mn3O4 NPs in Fig. 7a shows that the Mn3O4 NPs were composed of nearly uniform types of particles. The SAED patterns of Mn3O4 NPs in Fig. 7b displayed bright rings with some bright spots, suggesting the high crystallinity of the materials71. Figure 7c represents the high-resolution TEM images of the Pd-doped Mn3O4 NPs, and the magnified calibrated lattice fringes of Mn3O4 NPs for the crystal plane of (103) and (211) revealed an interplanar spacing (d-spacing) of 2.7 and 2.4 Å in Fig. 7d. These planes were also observed in the XRD analysis, and the reduction of the d-spacing of these planes is in good agreement with the shifting of the XRD peaks. Therefore, these findings indicate the successful formation of Pd-doped Mn3O4 NPs, which was also consistent with the XRD patterns72,73.
Raman Spectroscopy analysis
Figure 8 presents the Raman spectra of pure and Pd-doped Mn3O4 NPs. Two characteristic peaks at ~ 629 and ~ 630 cm−1 were observed, corresponding to the skeletal vibrations for pure and Pd-doped samples, respectively. The strongest peaks at ~ 629 and ~ 630 cm−1 are consistent with the reported data74 for both materials. These sharp peaks can be assigned to the A1g mode, representing the Mn–O breathing vibration of divalent manganese ions in tetrahedral coordination. This mode is a characteristic feature of Hausmannite75,76. The comparison of the Raman spectra between pure and Pd-doped Mn3O4 NPs reveals similarities in the characteristic peaks, indicating that the introduction of Pd did not significantly alter the skeletal vibrations and Mn–O breathing vibrations in the tetrahedral coordination. The Raman spectra analysis of pure and Pd-doped Mn3O4 NPs confirmed the presence of specific vibrational modes and provides key insights into the structural properties of these materials. The similarities in the Raman spectra between pure and Pd-doped samples suggested that the Pd-doping did not cause significant changes in the observed vibrational features.
Zeta potential studies
A particle's surface potential substantially impacts its dispersion stability77, which may also influence its bactericidal potential78. Without proper surfactants or capping agents, nanoparticles tend to agglomerate, and their surface area-to-volume ratio decreases due to their increased size79. The zeta potential studies allow us to investigate nanoparticles' surface charge and stability in colloidal solutions80 (Fig. 9). The surface charge of NPs can be influenced by the charged dopants77, which is also observed in the present study. We have obtained highly stable NPs (ZP > 30 mV)81, with recorded values of −33.2 ± 0.404 and −36.6 ± 1.74 mV for pure and Pd-doped Mn3O4, respectively. The NPs with greater ZP values (negative or positive) prevent agglomeration via electrostatic repulsion, hence conferring stability80.
Antifungal activity
The developed NPs showed mycelium growth inhibition in a dose-dependent manner. In the case of Mn3O4, NPs, we have observed maximum antifungal activity at 1000-ppm concentration with over 50% inhibitions in the growth of S. sclerotiorum and C. gloeosporioides was recorded at 500 ppm dose (Fig. 10). Overall, S. sclerotiorum exhibited higher sensitivity to the Mn3O4 NPs treatment than C. gloeosporioides. The inhibition of mycelial growth in the case of Pd-doped Mn3O4 NPs was higher than pure Mn3O4 NPs against both fungal strains (Fig. 11). This could be due to the significant modification in structural properties of Mn3O4 NPs as a result of Pd doping, such as the reduction in crystallite size and nanocorn-like morphology. At 1000 ppm concentration, Pd-doped Mn3O4 NPs caused ~ 92% and ~ 72% growth inhibition of S. sclerotiorum and C. gloeosporioides, respectively. Interestingly, C. gloeosporioides was more sensitive to Pd-doped Mn3O4 NPs treatment, specifically at lower doses, and showed ~ 65%, ~ 23%, and ~ 10% higher inhibition compared to pure Mn3O4 NPs at 500, 100, and 10 ppm concentration, respectively. The Pd-doped Mn3O4 NPs at 1000 ppm showed antifungal activity comparable to those of positive control (2 mg/ml of carbendazim + mancozeb; commercial chemical grade fungicide formulation). Hence, doping Mn3O4 NPs with Pd favoured their antifungal potential66, which is highly explicitly recommended in the case of C. gloeosporioides to decrease the effective dose. Overall, the bioinspired fabrication of nanocorn-like Pd doped Mn3O4 NPs can be used as an effective antifungal nano-pesticide against different necrotrophic and hemibiotrophic phytopathogens, known for causing enormous loss to agricultural food crops globally.
Antibacterial activity
The antibacterial activity of pure and Pd-doped Mn3O4 NPs was also investigated against E. faecalis to establish their broad spectrum of antimicrobial potential, in terms of pathogen diversity, i.e., phytopathogens and animal pathogens. As stated earlier, E. faecalis is a well-known human pathogen known for hospital acquired infections. This bacterium colonizes the intestine of animals including humans, and its presence in waterbodies is an indicative of fecal contamination. The resistance of E. faecalis against various antibiotics necessitated the search of novel materials possessing significant antibacterial potential against such nosocomial pathogens. In the present work, both pure and Pd-doped Mn3O4 NPs showed dose-dependent increment in ZOI (Fig. 12). The ZOI values showed effect of Pd doping on improving antibacterial activity of Mn3O4 NPs. When compared to pure Mn3O4 NPs, Pd doping showed ~ 14%, ~ 17%, and ~ 16% higher ZOI values at 50, 100, and 200 ppm doses of Mn3O4 NPs respectively.
Mechanism of antimicrobial activity of Pd-doped Mn3O4 NPs
The plausible routes of inducing antimicrobial activity by Pd-doped Mn3O4 NPs are illustrated in Fig. 1336,66,82. The NPs in fungal cells usually gain entry via diffusion and endocytosis83 and may cause growth inhibition through multiple actions such as DNA damage, protein denaturation, breakdown of the cell wall and cell membrane, ROS-mediated lipid peroxidation, ribosome disassembly, denaturation of enzymes, perforations in the cell wall and cell membrane, mitochondrial damage, release of cytochrome-c from mitochondria to cytosol, and increase levels of metacaspase and promotes cell death36. Similar effects have been proposed in the case of bacterial cells where NPs can cause protein and enzyme denaturation, damage to chromosomal and plasmid DNA, ribosomal depolymerization, interference in ETC, the release of cellular contents, disruption of the cell membrane, etc.84,85.
Schematic representation highligting plausible mechanisms involved in antimicrobial action of Pd-doped Mn3O4 NPs (1. breakdown of cell wall and cell membrane; 2. perforations in cell wall and cell membrane; 3. mitochondrial damage; 4. release of cytochrome-c from mitochondria to cytosol, increase levels of metacaspase and promotes cell death; 5. chromosomal DNA damage; 6. ribosome disassembly; 7. Protein damage; 8. release of cellular contents; 9. denaturation of enzymes; 10. plasmid DNA damage; 11. disruption of cell membrane; 12. perforation in cell membrane; 13. interference in ETC and damage to protein-efflux pump; 14. destruction of membranous proteins; CB = conduction band, VB = valance band; ROS: reactive oxygen species).
In general, NPs have direct and indirect effects on microbial cells. The direct damage occurs via the electrostatic interaction of NPs with cell membrane resulting in membrane depolarization and loss of membrane integrity leading to the disruption in ETC and cell lysis39,86. The indirect damage to microbial cells is reported via ROS generation (Eqs. 7, 8, 9, 10, 11, 12)39,87. The doping in pure nanomaterial leads to lattice defects (alters band gap), causing overlapping of Fermi levels, variation in cellular redox potential, promotes ROS generation (Fig. 14) and can impart enhanced antimicrobial properties39, which was observed in the present study as well, where Pd-doped Mn3O4 NPs showed higher antifungal and antibacterial activity compared to pure NPs. In addition, doping can improve the binding capacity and cellular internalization ability of NPs. NPs generate ROS outside the cellular environment or can produce it inside the cell due to interference in ETC39. The oxygen molecules that are not reduced in the water get oxidized into free radicals (such as superoxide anion, singlet oxygen, or hydroxyl radicals) in mitochondria (Eqs. 7, 8, 9, 10, 11, 12)39,88. ROS causes alteration in protein structures, oxidative stress, lipid peroxidation, and DNA damage88.
Effect of doping on Fermi levels leading to ROS generation (VL = vacuum level, CB = conduction band, VB = valance band) (conceptualized and redrawn from39).
Conclusion
The present investigation demonstrates a successful green chemistry approach to synthesis pure and Pd-doped Mn3O4 NPs via utilizing an aqueous extract of S. aromaticum buds. Adding Pd in Mn3O4 resulted in significant changes to their structural attributes, including morphology, crystallite size, and lattice defects. The Pd-doped Mn3O4 NPs exhibited antimicrobial activity in a dose-dependent manner and provides higher inhibitory effects than pure Mn3O4 NPs against S. sclerotiorum, C. gloeosporioides, and E. faecalis. The outcome of this study provides a novel, cost-effective method to develop Pd-doped Mn3O4 based nanomaterials for highly effective antimicrobial applications against tested microbial pathogens. This breakthrough opens up new possibilities in the area of green nanotechnology to develop sustainable and multifaceted antimicrobial agents.
Data availability
All data generated or analyzed during this study are included within the article.
Abbreviations
- ADDA:
-
Agar disc diffusion assay
- ASAB:
-
Aqueous Syzygium aromaticum bud
- CB:
-
Conduction band
- DDW:
-
Double-distilled water
- DNA:
-
Deoxy ribose nucleic acid
- EDAX:
-
Energy dispersive X-ray analysis
- ETC:
-
Electron transport chain
- eV:
-
Electron volt
- FESEM:
-
Field emission scanning electron microscopy
- FTIR:
-
Fourier-transform infrared spectroscopy
- HCl:
-
Hydrochloric acid
- JCPDS:
-
Joint Committee on Powder Diffraction Standards
- KOH:
-
Potassium hydroxide
- mm:
-
Milli meter
- mM:
-
Milli molar
- Mn:
-
Manganese
- Mn3O4 :
-
Manganese (II, III) oxide
- MnCl2·4H2O:
-
Manganese chloride tetrahydrate
- MNPs:
-
Metallic nanoparticles
- NaCl:
-
Sodium chloride
- NaOH:
-
Sodium hydroxide
- NBM:
-
Nutrient broth media
- nm:
-
Nanometer
- Pd:
-
Palladium
- PDA:
-
Potato dextrose agar
- PdCl2 :
-
Palladium chloride
- ppm:
-
Parts per million
- ROS:
-
Reactive oxygen species
- SAED:
-
Selected area electron diffraction
- SPM:
-
Solid media plates
- TEM:
-
Transmission electron microscopy
- VB:
-
Valence band
- w/v:
-
Weight by volume
- XPS:
-
X-ray Photoelectron Spectroscopy
- XRD:
-
X-Ray diffraction
- ZOI:
-
Zone of Inhibition
- ZP:
-
Zeta potential
References
Alshora, D. H., Ibrahim, M. A., Alanazi, F. K. (2016). Nanotechnology from particle size reduction to enhancing aqueous solubility. In Surface Chemistry of Nanobiomaterials. Elsevier, pp. 163–191. https://doi.org/10.1016/B978-0-323-42861-3.00006-6.
Al-Zahrani, F. A. M. et al. Green synthesis and antibacterial activity of Ag/Fe2O3 nanocomposite using buddleja lindleyana extract. Bioengineering 9, 452. https://doi.org/10.3390/bioengineering9090452 (2022).
Salem, S. S. A mini review on green nanotechnology and its development in biological effects. Arch Microbiol 205, 128. https://doi.org/10.1007/s00203-023-03467-2 (2023).
Salem, S. S. & Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol Trace Elem Res 199, 344–370. https://doi.org/10.1007/s12011-020-02138-3 (2021).
Asghari F, Jahanshiri Z, Imani M, et al (2016) Antifungal nanomaterials. In Nanobiomaterials in Antimicrobial Therapy. Elsevier, pp. 343–383. https://doi.org/10.1016/B978-0-323-42864-4.00010-5.
Ali, Md. A. et al. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 10, 1146. https://doi.org/10.3390/nano10061146 (2020).
Zhang, S. et al. Antimicrobial properties of metal nanoparticles and their oxide materials and their applications in oral biology. J. Nanomater. 2022, 1–18. https://doi.org/10.1155/2022/2063265 (2022).
Abdelghany, T. M. et al. Phytofabrication of zinc oxide nanoparticles with advanced characterization and its antioxidant, anticancer, and antimicrobial activity against pathogenic microorganisms. Biomass Conv Bioref 13, 417–430. https://doi.org/10.1007/s13399-022-03412-1 (2023).
Salem, S. S. Bio-fabrication of selenium nanoparticles using Baker’s yeast extract and its antimicrobial efficacy on food borne pathogens. Appl Biochem Biotechnol 194, 1898–1910. https://doi.org/10.1007/s12010-022-03809-8 (2022).
Shehabeldine, A. M. et al. Potential antimicrobial and antibiofilm properties of copper oxide nanoparticles: Time-kill kinetic essay and ultrastructure of pathogenic bacterial cells. Appl Biochem Biotechnol 195, 467–485. https://doi.org/10.1007/s12010-022-04120-2 (2023).
Soliman, M. K. Y., Salem, S. S., Abu-Elghait, M. & Azab, M. S. Biosynthesis of silver and gold nanoparticles and their efficacy towards antibacterial, antibiofilm, cytotoxicity, and antioxidant activities. Appl Biochem Biotechnol 195, 1158–1183. https://doi.org/10.1007/s12010-022-04199-7 (2023).
Vikal, S., Gautam, Y., Kumar, A., Kumar, A., Singh, N., Singh, H., Singh, B. (2022). Effect of silver (Ag) doping on structural, optical and antimicrobial properties of copper oxide (CuO) nanostructures. Nano Express 4(2023), 025004. https://doi.org/10.1088/2632-959X/acdc41.
Alqarni, L. S., Alghamdi, M. D., Alshahrani, A. A. & Nassar, A. M. Green nanotechnology: Recent research on bioresource-based nanoparticle synthesis and applications. J. Chem. 2022, 1–31. https://doi.org/10.1155/2022/4030999 (2022).
Abdelmoneim, H. E. M. et al. Multiple applications of CdS/TiO2 nanocomposites synthesized via microwave-assisted sol–gel. J Clust Sci 33, 1119–1128. https://doi.org/10.1007/s10876-021-02041-4 (2022).
Mohamed, A. A., Abu-Elghait, M., Ahmed, N. E. & Salem, S. S. Eco-friendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm and antifungal applications. Biol Trace Elem Res 199, 2800–2801. https://doi.org/10.1007/s12011-020-02391-6 (2021).
Said, A., Abu-Elghait, M., Atta, H. M. & Salem, S. S. Antibacterial activity of green synthesized silver nanoparticles using lawsonia inermis against common pathogens from urinary tract infection. Appl Biochem Biotechnol https://doi.org/10.1007/s12010-023-04482-1 (2023).
Salem, S. S. Baker’s yeast-mediated silver nanoparticles: characterisation and antimicrobial biogenic tool for suppressing pathogenic microbes. BioNanoSci 12, 1220–1229. https://doi.org/10.1007/s12668-022-01026-5 (2022).
Soliman, M. K. Y., Abu-Elghait, M., Salem, S. S. & Azab, M. S. Multifunctional properties of silver and gold nanoparticles synthesis by Fusarium pseudonygamai. Biomass Conv Bioref https://doi.org/10.1007/s13399-022-03507-9 (2022).
Jeyabharathi, S. et al. Self-assembled hollow ZnO nano and micro donut shape by starch and its antimicrobial potentials. Mater Lett. 275, 128128. https://doi.org/10.1016/j.matlet.2020.128128 (2020).
Kalam, A. et al. Antimicrobial properties of silver nanoparticles prepared by Acacia etbaica (Schweinf.) valve extract. Mater. Lett. 300, 130233. https://doi.org/10.1016/j.matlet.2021.130233 (2021).
Parvathy, S., Subramanian, P., Arun Karthick, S. & Subbaiya, R. The structural, optical, antimicrobial and anticancer properties of biocompatible astaxanthin coated ZnO and CeO2 nanoparticles. Mater. Lett. 312, 131669. https://doi.org/10.1016/j.matlet.2022.131669 (2022).
Parvathy, S., Manjula, G., Balachandar, R. & Subbaiya, R. Green synthesis and characterization of cerium oxide nanoparticles from Artabotrys hexapetalus leaf extract and its antibacterial and anticancer properties. Materials Letters 314, 131811. https://doi.org/10.1016/j.matlet.2022.131811 (2022).
Bhardwaj et al. (2020). https://doi.org/10.1016/10.34172/apb.2020.067.
Hoseinpour, V. & Ghaemi, N. Green synthesis of manganese nanoparticles: Applications and future perspective—a review. J. Photochem. Photobiol. B 189, 234–243. https://doi.org/10.1016/j.jphotobiol.2018.10.022 (2018).
Suresh, K. C. & Balamurugan, A. Evaluation of structural, optical, and morphological properties of nickel oxide nanoparticles for multi-functional applications. Inorganic Nano-Metal Chem. 51, 296–301. https://doi.org/10.1080/24701556.2020.1770793 (2021).
Alavi, M., Karimi, N. & Valadbeigi, T. Antibacterial, antibiofilm, antiquorum sensing, antimotility, and antioxidant activities of green fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis muralis lichen aqueous extract against multi-drug-resistant bacteria. ACS Biomater Sci Eng 5, 4228–4243. https://doi.org/10.1021/acsbiomaterials.9b00274 (2019).
Bao, Y. et al. Plant-extract-mediated synthesis of metal nanoparticles. J. Chem. 2021, 1–14. https://doi.org/10.1155/2021/6562687 (2021).
Kumar, B. Green synthesis of gold, silver, and iron nanoparticles for the degradation of organic pollutants in wastewater. J Compos Sci 5, 219. https://doi.org/10.3390/jcs5080219 (2021).
Nicolas, J. et al. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev 42, 1147–1235. https://doi.org/10.1039/C2CS35265F (2013).
Veeramani, H. et al. Low-temperature green synthesis of multivalent manganese oxide nanowires. ACS Sustain. Chem. Eng. 1, 1070–1074. https://doi.org/10.1021/sc400129n (2013).
Prasad, A. S. Green synthesis of nanocrystalline manganese (II, III) oxide. Mater. Sci. Semicond. Process. 71, 342–347. https://doi.org/10.1016/j.mssp.2017.08.020 (2017).
Fei, J. B. et al. Controlled preparation of MnO2 hierarchical hollow nanostructures and their application in water treatment. Adv Mater 20, 452–456. https://doi.org/10.1002/adma.200701231 (2008).
Gong, Y. et al. Sclerotinia sclerotiorum SsCut1 modulates virulence and cutinase activity. JoF 8, 526. https://doi.org/10.3390/jof8050526 (2022).
Sharma, M. & Kulshrestha, S. Colletotrichum gloeosporioides: An anthracnose causing pathogen of fruits and vegetables. Biosci. Biotechnol. Res. Asia 12, 1233–1246. https://doi.org/10.13005/bbra/1776 (2015).
Siddiqui Y, Ali A (2014) Colletotrichum gloeosporioides (Anthracnose). In Postharvest Decay. Elsevier, pp. 337–371. https://doi.org/10.1016/B978-0-12-411552-1.00011-9.
Dubin, K., & Pamer, E. G. (2017) Enterococci and their interactions with the intestinal microbiome. Microbiol. Spectr. 5:5.6.01. https://doi.org/10.1128/microbiolspec.BAD-0014-2016.
Olawale, K., Fadiora, S., Taiwo, S. (2011). Prevalence of hospital acquired enterococci infections in two primary-care hospitals in Osogbo, Southwestern Nigeria. Afr. J. Infect. Dis. 5. https://doi.org/10.4314/ajid.v5i2.66513.
Gnana Sundara Raj, B., Asiri, A. M., Wu, J. J. & Anandan, S. Synthesis of Mn3O4 nanoparticles via chemical precipitation approach for supercapacitor application. J. Alloy. Compd. 636, 234–240. https://doi.org/10.1016/j.jallcom.2015.02.164 (2015).
Patra, T. et al. Effect of calcination temperature on morphology and phase transformation of MnO2 nanoparticles: A step towards green synthesis for reactive dye adsorption. Chemosphere 288, 132472. https://doi.org/10.1016/j.chemosphere.2021.132472 (2022).
Revathy, J. S., Priya, N. S. C., Sandhya, K. & Rajendran, D. N. Structural and optical studies of cerium doped gadolinium oxide phosphor. Bull Mater Sci 44, 13. https://doi.org/10.1007/s12034-020-02299-w (2021).
Amer, A. A., Reda, S. M., Mousa, M. A. & Mohamed, M. M. Mn3O4/graphene nanocomposites: Outstanding performances as highly efficient photocatalysts and microwave absorbers. RSC Adv. 7, 826–839. https://doi.org/10.1039/C6RA24815B (2017).
Arniati, A., Pratama, M., Baits, M., & Tahir, M. (2022). Determination of total phenolic levels of ethyl acetate fraction of clove leaves (Syzygium aromaticum (L.) Merr). Pharmaceut. Rep., 1(2).
Jimoh, S. O., Arowolo, L. A. & Alabi, K. A. Phytochemical screening and antimicrobial evaluation of Syzygium aromaticum extract and essential oil. Int. J. Curr. Microbiol. Appl. Sci. 6, 4557–4567. https://doi.org/10.20546/ijcmas.2017.607.476 (2017).
Lone, Z. A. & Jain, N. K. Phytochemical analysis of clove (Syzygium aromaticum) dried flower buds extract and its therapeutic importance. J. Drug Deliv. Therap. 12, 87–92. https://doi.org/10.22270/jddt.v12i4-S.5628 (2022).
Rajesh, K. M. et al. Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical, optical and antimicrobial properties. Optik 154, 593–600. https://doi.org/10.1016/j.ijleo.2017.10.074 (2018).
Jardón-Romero, E. A. et al. Antimicrobial activity of biogenic silver nanoparticles from Syzygium aromaticum against the five most common microorganisms in the oral cavity. Antibiotics 11, 834. https://doi.org/10.3390/antibiotics11070834 (2022).
Lakshmeesha, T. R. et al. Biofabrication of zinc oxide nanoparticles with Syzygium aromaticum flower buds extract and finding its novel application in controlling the growth and mycotoxins of Fusarium graminearum. Front Microbiol 10, 1244. https://doi.org/10.3389/fmicb.2019.01244 (2019).
Parthipan, P., AlSalhi, M. S., Devanesan, S. & Rajasekar, A. Evaluation of Syzygium aromaticum aqueous extract as an eco-friendly inhibitor for microbiologically influenced corrosion of carbon steel in oil reservoir environment. Bioprocess Biosyst Eng 44, 1441–1452. https://doi.org/10.1007/s00449-021-02524-8 (2021).
Atique Ullah, A. K. M. et al. Oxidative degradation of methylene blue using Mn3O4 nanoparticles. Water Conserv Sci Eng 1, 249–256. https://doi.org/10.1007/s41101-017-0017-3 (2017).
Li, Q. et al. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci Rep 7, 9894. https://doi.org/10.1038/s41598-017-09897-5 (2017).
Kadam, A. et al. Template free synthesis of ZnO/Ag2O nanocomposites as a highly efficient visible active photocatalyst for detoxification of methyl orange. J. Photochem. Photobiol. B 154, 24–33. https://doi.org/10.1016/j.jphotobiol.2015.11.007 (2016).
Muñoz-Fernandez, L., Sierra-Fernandez, A., Milošević, O. & Rabanal, M. E. Solvothermal synthesis of Ag/ZnO and Pt/ZnO nanocomposites and comparison of their photocatalytic behaviors on dyes degradation. Adv. Powder Technol. 27, 983–993. https://doi.org/10.1016/j.apt.2016.03.021 (2016).
Liu, H. et al. Microwave-assisted one-pot synthesis of Ag decorated flower-like ZnO composites photocatalysts for dye degradation and NO removal. Ceram. Int. 45, 20133–20140. https://doi.org/10.1016/j.ceramint.2019.06.279 (2019).
Zeferino, R. S., Flores, M. B. & Pal, U. Photoluminescence and Raman Scattering in Ag-doped ZnO Nanoparticles. Journal of Applied Physics 109, 014308. https://doi.org/10.1063/1.3530631 (2011).
Nie, M. et al. Photocatalytic property of silver enhanced Ag/ZnO composite catalyst. Chem. Phys. Lett. 768, 138394. https://doi.org/10.1016/j.cplett.2021.138394 (2021).
AL-Jawad, S. M. H., Sabeeh, S. H., Taha, A. A., Jassim, H. A. (2018). Studying structural, morphological and optical properties of nanocrystalline ZnO:Ag films prepared by sol–gel method for antimicrobial activity. J. Sol-Gel Sci. Technol. 87:362–371. https://doi.org/10.1007/s10971-018-4724-9.
Hosny, N. M. & Dahshan, A. Facile synthesis and optical band gap calculation of Mn3O4 nanoparticles. Mater. Chem. Phys. 137, 637–643. https://doi.org/10.1016/j.matchemphys.2012.09.068 (2012).
Mallesham, B. et al. Crystal chemistry, band-gap red shift, and electrocatalytic activity of iron-doped gallium oxide ceramics. ACS Omega 5, 104–112. https://doi.org/10.1021/acsomega.9b01604 (2020).
Kösemen, A. et al. Electrochemical growth of Pd doped ZnO nanorods. J Electrochem Soc 162, D142–D146. https://doi.org/10.1149/2.0341504jes (2015).
Kumar, S., Singh, V. & Tanwar, A. Structural, morphological, optical and photocatalytic properties of Ag-doped ZnO nanoparticles. J Mater Sci: Mater Electron 27, 2166–2173. https://doi.org/10.1007/s10854-015-4227-1 (2016).
Sagadevan, S., Pal, K., Chowdhury, Z. Z. & Hoque, M. E. Structural, dielectric and optical investigation of chemically synthesized Ag-doped ZnO nanoparticles composites. J Sol–Gel Sci Technol 83, 394–404. https://doi.org/10.1007/s10971-017-4418-8 (2017).
Slavin, Y. N. & Bach, H. Mechanisms of antifungal properties of metal nanoparticles. Nanomaterials 12, 4470. https://doi.org/10.3390/nano12244470 (2022).
Jamil, S. et al. Synthesis of saucer shaped manganese oxide nanoparticles by co-precipitation method and the application as fuel additive. J. Clust. Sci. 29, 1099–1106. https://doi.org/10.1007/s10876-018-1428-9 (2018).
Oba, M., Oaki, Y. & Imai, H. A microbial-mineralization-inspired approach for synthesis of manganese oxide nanostructures with controlled oxidation states and morphologies. Adv. Funct. Mater. 20, 4279–4286. https://doi.org/10.1002/adfm.201000361 (2010).
Gurunathan, S., Lee, A. R. & Kim, J. H. Antifungal effect of nanoparticles against COVID-19 linked black fungus: A perspective on biomedical applications. IJMS 23, 12526. https://doi.org/10.3390/ijms232012526 (2022).
Mubeen, B. et al. Nanotechnology as a novel approach in combating microbes providing an alternative to antibiotics. Antibiotics 10, 1473. https://doi.org/10.3390/antibiotics10121473 (2021).
Gnana Sundara Raj, B., Angulakshmi, R., Baskaran, N., Wu, J. J., Anandan, S., Ashok Kumar, M. Pseudocapacitive performance of Mn3O4–SnO2 hybrid nanoparticles synthesized via ultrasonication approach. J. Appl. Electrochem. https://doi.org/10.1007/s10800-020-01421-4.
Wang, P. et al. PdO/SnO2 heterostructure for low-temperature detection of CO with fast response and recovery. RSC Adv. 9, 22875. https://doi.org/10.1039/c9ra03171e (2019).
Ren, L. et al. Magnetic properties of Mn3O4 film with a coexistence of two preferential orientations. J Appl Phys 116, 023906 (2014).
Jin, R., Liu, H., Guan, Y., Zhou, J. & Li, G. Molten salt synthesis of fuorine-doped Mn3O4 nanobelts as anode materials for Li-ion batteries. Cryst Eng Commun 17, 7717–7722 (2015).
Acharyya, S. S., Ghosh, S., Sharma, S. K. & Bal, R. Cetyl alcohol mediated fabrication of forest of Ag/Mn3O4 nanowhiskers catalyst for the selective oxidation of styrene with molecular oxygen. RSC Adv. 5, 89879–89887 (2015).
Chen, X. et al. Performance enhancement of asymmetric supercapacitors with bud-like Cu-doped Mn3O4 hollow and porous structures on nickel foam as positive electrodes. RSC Adv. 8, 35878–35887 (2018).
Sukhdev, A., Challa, M., Narayani, L., Manjunatha, A. S., Deepthi, P. R., Angadi, J. V., Kumar, P. M., Pasha, M. Synthesis, phase transformation, and morphology of hausmannite Mn3O4 nanoparticles: Photocatalytic and antibacterial investigations. Heliyon 6, e03245 (2020).
Bussamara, R., Melo, W. W. M., Scholten, J. D., Migowski, P., Marin, G., Zapata, M. J. M., Machado, G., Teixeira, S. R., Novak, M., Dupont, J. Dalton Trans. 42, 14473 (2013).
Buciuman, F., Patcas, F., Craciun, R., Zahn, D. R. T. Phys. Chem. Chem. Phys. 1, 185 (1999).
Moulder, J. F., Stickle, W. F., Sobol, P. E., Bomben, K. D. Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA (1979).
Pinton. A. P., Bulhões, L. O. D. Synthesis, characterization, and photostability of manganese-doped titanium dioxide nanoparticles and the effect of manganese content. Mater. Res. Express 6, 125015. https://doi.org/10.1088/2053-1591/ab533b (2019).
Ichimaru, H. et al. Gold coating of silver nanoplates for enhanced dispersion stability and efficient antimicrobial activity against intracellular bacteria. Langmuir 34, 10413–10418. https://doi.org/10.1021/acs.langmuir.8b00540 (2018).
Ullah, M., Bee, S. & Hamid, A. Surfactant-assisted ball milling: a novel route to novel materials with controlled nanostructure: A review. Rev. Adv. Mater. Sci. 37(2014), 1–14 (2014).
İlhan M, Gültekin HE, Rençber S, et al (2022) Aquasomes: A novel platform for drug delivery. In Systems of Nanovesicular Drug Delivery. Elsevier, pp. 191–206.
Das, P. & Das, M. K. Chapter 4: Production and physicochemical characterization of nanocosmeceuticals. In Nanocosmeceuticals (ed. Das, M. K.) 95–138 (Academic Press, 2022).
Abebe, B., Zereffa, E. A., Tadesse, A. & Murthy, H. C. A. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res Lett 15, 190. https://doi.org/10.1186/s11671-020-03418-6 (2020).
Naik, M. M. et al. Multifunctional properties of microwave-assisted bioengineered nickel doped cobalt ferrite nanoparticles. J Sol-Gel Sci Technol 91, 578–595. https://doi.org/10.1007/s10971-019-05048-6 (2019).
Singh, A. et al. Zinc oxide nanoparticles: a review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J Mater Sci 53, 185–201. https://doi.org/10.1007/s10853-017-1544-1 (2018).
Bhattacharya, P. & Neogi, S. Antibacterial properties of doped nanoparticles. Rev. Chem. Eng. 35, 861–876. https://doi.org/10.1515/revce-2017-0116 (2019).
Jin, S.-E. & Jin, H.-E. Antimicrobial activity of zinc oxide nano/microparticles and their combinations against pathogenic microorganisms for biomedical applications: From physicochemical characteristics to pharmacological aspects. Nanomaterials 11, 263. https://doi.org/10.3390/nano11020263 (2021).
Navarro-López, D. E., Garcia-Varela, R., Ceballos-Sanchez, O., et al. (2021). Effective antimicrobial activity of ZnO and Yb-doped ZnO nanoparticles against Staphylococcus aureus and Escherichia coli. Mater. Sci. Eng.: C 123:112004. https://doi.org/10.1016/j.msec.2021.112004.
Khalid, A., Ahmad, P., Alharthi, A. I., et al. (2021). Synergistic effects of Cu-doped ZnO nanoantibiotic against Gram-positive bacterial strains. PLoS ONE 16, e0251082. https://doi.org/10.1371/journal.pone.0251082
Acknowledgements
Authors are thankful to DST, Govt. of India, for providing a FIST grant for SEM facility. Prof. Ramesh Chandra for providing XRD, FESEM, and XPS facilities at Institute Instrumentation Center (IIC), IIT Roorkee, India. Dr. Nazia Tarannum, Department of Chemistry, CCS University Meerut, UP (India) for providing FTIR facility. Dr. Amit K. Chawla, Department of Physics, UPES, Dehradun, India for providing Raman facility, and Central Instrumentation facility, Jamia Milia Islamia, New Delhi, India for Zetasizer analysis in this research work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the research concept and study design. The conceptualization and supervision was done by Y.K.G., As.K. and Aj.K. Methodology was done by S.V., J.S., D.P., N.S. Data curation and data analysis was done by Y.K.G., S. V., A.K., D.P. Editing was done by Y.K.G., B.P., As.K. Visualization was done by D.P.,N.S. The original draft preparation was done by S.V. and Aj.K. The final manuscript was read and approved by all authors prior to submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vikal, S., Gautam, Y.K., Kumar, A. et al. Bioinspired palladium-doped manganese oxide nanocorns: a remarkable antimicrobial agent targeting phyto/animal pathogens. Sci Rep 13, 14039 (2023). https://doi.org/10.1038/s41598-023-40822-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40822-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.