Abstract
Infants make spontaneous movements from the prenatal period. Several studies indicate that an atypical pattern of body motion during infancy could be utilized as an early biomarker of autism spectrum disorders (ASD). However, to date, little is known about whether the body motion pattern in neonates is associated with ASD risk. The present study sought to clarify this point by examining, in a longitudinal design, the link between features of spontaneous movement at about two days after birth and ASD risk evaluated using the Modified Checklist for Autism in Toddlers by their caregivers at 18 months old. The body movement features were quantified by a recently developed markerless system of infant body motion analysis. Logistic regression analysis revealed that ASD risk at 18 months old is associated with the pattern of spontaneous movement at the neonatal stage. Further, logistic regression based on body movement features during sleep shows better performance in classifying high- and low-risk infants than during the awake state. These findings raise the possibility that early signs of ASD risk may emerge at a developmental stage far earlier than previously thought.
Similar content being viewed by others
Introduction
Infants spontaneously make body movements without external stimulation from 9–10 weeks after gestation1,2. Subcortical centers of motor control such as central pattern generator in brainstem and spinal cord supposedly innervate multiple muscles in concert to generate discernible body motions3, and recent studies further indicated that cortical as well as subcortical structures contribute to the emergence of spontaneous movements4. Many repertoires of body movement are being observable as early as during the foetal period2. However, neural regions responsible for spontaneous movement are still immature and body movement pattern shows prominent changes both in quality and quantity during the first year of life3,4.
Among early motor repertoire5,6, researchers have paid special attention to a constellation of whole body movements called general movement (GM)3,4,7,8. From foetal period onward, infant starts to show “writhing movement”, temporally-coordinated and complex pattern of entire body movement that sometimes creates an impression of “fluency and elegance”4,7. Around after three to five months of life, writhing movement gradually disappears, and gets replaced by “fidgety movement” characterized by tiny movements of the neck and limbs in all directions with varying intensity4.
Abnormality in neural development can be detected non-invasively by GM assessment (GMA). As an illustrative success, Prechtl and colleagues have reported in a series of studies that large proportion of preterm infants with cerebral palsy show abnormal patterns of GM, such as monotonous and rigid movements and lack of movement varieties4,7. GM during infancy is reported to be associated with long-term development of cognitive functions into the school year9,10. Several studies have made an attempt to see linkage between GM pattern and psychiatric conditions11,12, but the number of such studies is limited so far.
According to DSM-5, autism spectrum disorder (ASD) is a neurodevelopmental condition whose diagnostic criteria includes persistent deficits in social communication and social interaction, and restricted, repetitive patterns of behavior, and interests13. Currently, a formal diagnosis of ASD is being made at around three years after birth. However, diagnosis can be delayed due to multiple factors such as comorbidities, and large-scale studies report that the median age of ASD diagnosis is around 4.5 to 5.5 years old14,15.
Diagnosis can be made as early as two years of age16 when a clinical diagnosis is assisted by well-tested instruments such as Pre-linguistic Autism Diagnostic Observation Schedule (ADOS)17,18. In addition, a growing number of studies show that infants later diagnosed as ASD exhibit early signs well before they reach three years old19,20,21,22. A pioneering study by Osterling et al. that analyzed 1-year old birthday videos of ASD children revealed a lower frequency of fixating on other’s faces in ASD children compared to typically developing ones23. At this point, there is no definitive treatment for ASD, but studies have pointed out that early intervention to children at risk of ASD leads to better prognosis and social adaptation24,25,26,27. Thus, the development of methods to assess early signs of ASD risk is of primary importance in improving the well-beings of children with ASD and their caregivers.
A number of studies have revealed an atypical pattern of motoric control in ASD children. These atypicalities include lateral asymmetry in posture and movement28,29, hypotonia19, and spastic limb movement24. In prospective studies, Landa and her colleagues30,31 reported that children with ASD show atypical motor control in both gross and fine movements before 24 months old. Teitelbaum et al. proposed a framework for the early diagnosis of ASD by qualitative assessment of body movement at around five months old32. Other studies have also suggested the utility of an atypical pattern of body movement as potential biomarker for early screening of ASD risk33,34,35,36,37. However, these studies focused on infants after four months old, and it remains unclear whether an assessment of body movement pattern at the neonatal stage is useful in early screening of children at risk for ASD.
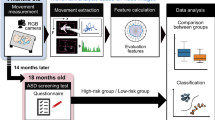
The present study sought to examine whether spontaneous movement in neonates contains information predictive of later emergence of ASD risk. To achieve this end, we quantified characteristics of spontaneous movements in neonates by our markerless system of GM analysis38. In a longitudinal design, ASD risk of these infants was evaluated by their caregivers at 18 months old. The main focus of the analysis was to see if ASD risk at 18 months old is associated with spontaneous movement characteristics at the neonatal stage. The overall framework of the present study is schematically described in Fig. 1. Recently, an increasing number of studies have reported the successful application of a markerless system to the analysis of GM (see Silva et al.39, for a recent review). Many of these have utilized automatic pose estimation and classification of motion patterns by neural networks40,41,42,43. Instead, we opted to use feature extraction by computer vision37,38 and logistic regression classifier in the present study to develop a classification model with high explainability.
In the present study, patterns of spontaneous movement linked to the later emergence of ASD risk were searched for during sleep and awake state separately. Of particular relevance to the present study, Denisova and Zhao revealed the potential of head movement during sleep as biomarker of ASD risk that emerges during the 1–2 months of life44. Furthermore, previous studies on the spontaneous movement of newborns revealed that the pattern of motor control differs depending on arousal state in human and model animals45,46.
Schematic depiction of the design of present study. The movement of a newborn infant is measured with a camera in the first few days after birth, and evaluation features are calculated through video analysis. At this time, the state of the newborn is distinguished into sleep state and awake state according to the level of arousal. In the video analysis, changes in the image center of gravity (iCOG) are calculated by background subtraction processing, and body movements are calculated by interframe difference processing, obtaining a total of 26 features from them. The relationship between the obtained features and the ASD screening test at 18 months of age is analyzed.
Methods
Participants
75 mother-infant pairs participated in the present study. The cohort included 48 male and 27 female infants. Mothers and fathers were 32.6 ± 4.7 and 34.1 ± 5.8 years old, respectively. Birth weight was 3056.1 ± 323.8 g and gestational age was 39.2 ± 1.2 weeks. Apgar scores were 8.77 ± 0.42 (ranging from 8 to 9) at 1 min and 9.74 ± 0.47 (ranging from 8 to 10) at 5 min. Fifty-five infants were delivered normally, six by vacuum extraction, and nine by cesarean section (information was missing for the remaining five infants). No congenital neurological defects were identified in all infants. Background information such as age and socioeconomic status was collected by a self-administered questionnaire at the time of first enrollment.
The study had been carried out from February 2014 to October 2016 in accordance with the declaration of Helsinki and its protocol had been approved by the institutional ethical committee of Hiroshima University (Approval No. E-1150-2) and Chiba University (Approval No. 451). Written informed consent was obtained from all participants. In the case of infants, informed consent was obtained from a parent and/or legal guardian.
Procedure
This study was carried out as a part of a long-term longitudinal study47. The mothers enrolled in the study during pregnancy. The video-recording of spontaneous body movement took place at a gynecological clinic in north-western area of Chiba prefecture, Japan, during 1–4 days after the delivery. The averaged days between delivery and video-recording was \(2.13 \pm 0.66\).
Newborn was taken out from the newborn nursery, and laid in a supine position wearing a diaper on a bed in an adjacent room. Caregiver was absent from the room in which the video recording took place. A video camera was fixed by a special equipment on a transparent ceiling of the bed so that the camera was directed downward towards the neonate. The camera’s location was adjusted to capture the newborn’s entire body and facial expressions. Care was taken to remove any unintended influences on the infant’s state, such as lighting condition or attention-grabbing objects (e.g., moving toys) within the visual field of the newborns48. The frame rate was 30 fps, and the resolution was set to \(W \times H = 720 \times 480\) pixels. Neonate was left alone under this setting and his/her body movement was recorded for about 18–22 mins. Nurses were instructed to make a minimum level of interventions to and observations of the neonate and refrain from making loud noises nearby the bed.
When the infant reached 18 months, a set of questionnaires was sent out to the mothers, and they were asked to answer the Japanese translation of M-CHAT49,50,51. The Japanese version of M-CHAT is reported to show good test-retest reliability of \(r = 0.99\) and external validity50. We did not make a follow-up telephone interview that is recommended in ASD risk screening by M-CHAT49,50,51. Mothers submitted the questionnaires after answering all the items.
Analysis
Extraction of body movement features
Quantitative features characterizing the pattern of spontaneous movement were extracted from the video-recordings. From the original video-recordings, temporal segments were deleted during which the infant cried/fussed or apparently slept, or nurses made external stimulation to the infant. A video-segment with deep sleep was defined as a segment during which the eyes were closed, and the infants showed no discernible movement. The entire video-recordings were visually inspected by R.U. and A.F., and final decisions were made by R.U. regarding which video-segments should be submitted for further analysis. The features extracted from the video-recordings are tabulated in Table 1. The exact definition and extraction procedure of each feature are described in more details in our previous studies37,38 and are briefly summarized in Supplementary Materials. Because the video recording took place in a hospital room, there was ambient background noise, such as voices and operational sounds, throughout the recording session. The infants seemed to have habituated to such continuous noise. We decided to retain these time-segments because discarding them results in too small number of time-segments available for feature extraction. Abrupt onset of high-level noise, such as noises accompanying door opening or closing, might influence the pattern of an infant’s body movement48. We deleted video-segments with high-level noise with abrupt onset.
Grouping of infants and group comparison
Based on the scores of M-CHAT administered at 18 months old, infants were classified into high and low ASD risk groups. High risk group included infants who either scored (i) three or more out of 23 items, or, (ii) one or more in the ten critical items50,51. These criteria were adopted from the cut-off values used in a large-scale study using the Japanese version of M-CHAT (first stage screening; Kamio et al.52). The remaining infants were classified into low risk group. Data from infants with small body movement, \({}^{(A_9)}I_1 \le 5\%\) (proportion of segments with above-threshold movement frequency is equal to or below 5%), were discarded, resulting in 57 low risk and 16 high risk infants. We set the criteria of \({}^{(A_9)}I_1 \le 5\%\) based on the results of visual inspection of the videos; Infants with \({}^{(A_9)}I_1 \le 5\%\) showed virtually no discernible body movements. Supplementary Figure S2 shows the histogram of \(^{(A_9)}I_1\) for all infants.
High risk group included 12 male and 4 female infants, while low risk group included 35 males and 22 females. There was no significant difference in the distribution of sex between high and low risk group (\(p = 0.387\); Fisher’s exact test). No significant group difference was found between low and high risk infants by Brunner-Munzel test in gestational age, weight at birth, days after birth at video-recording, and weight at the point of video recording, as summarized in Table 2. Weight data at the point of recording was missing for one infant. Thus, group comparison of this variable was conducted for the remaining 56 infants.
ASD risk group classification by logistic regression
We developed a classifier to predict whether an infant belongs to high or low ASD risk group based on the 26 body movement features. These features were selected because our previous study37 succeeded in predicting ASD risk classification in 4-month-olds by entering these features into machine learning. Since several of the previous studies found a differential pattern of motor control depending on arousal state44,45,46, we created two separate logistic regression classifiers based on body movement features during time-segments of sleep and awake states. Arousal state was determined by eye closure as the criterion. Specifically, we defined periods during which the eyes were opened as “awake state”, and the remaining periods as “sleep state”. Arousal state during the video segment was determined in a frame-by-frame manner by R.U. Dataset of sleep included recordings from 40 infants (33 low risk infants with a male:female ratio of 22:11 and 7 high risk infants with a male:female ratio of 6:1) whereas the dataset of awake state from 55 infants (41 low risk infants with a male:female ratio of 25:16 and 14 high risk infants with a male:female ratio of 11:3).
Logistic regression was conducted to search for body movement features that differentiate high and low risk infants. The probability q with which an infant belongs to high-risk group according to our criteria was modeled by the generalized linear model described below. Body movement features were standardized before being entered into the model. Sex was also included in the model as a binary dummy variable.
Intercept, \(\beta _0\) and regression coefficients, \(\beta _1\)–\(\beta _{27}\), were estimated by maximum likelihood estimation so as to maximize joint probability of all the observations in the dataset. Among the 26 features extracted from video recording, a set of body movement features was searched for by a forward-stepwise variable selection procedure to reduce redundancy and retain only informative features. Features to be retained were determined based on the Akaike information criterion (AIC)53. More specifically, a set of features that gives the smallest AIC was retained in the model. The classification performance of the resultant model was evaluated by the area under the curve (AUC) in the receiver operation characteristic (ROC) analysis. Classification threshold was defined so that the F-measure is maximized. Sensitivity and specificity were calculated at this threshold.
Results
The model with the smallest AIC is described below for sleep and awake state, respectively. AIC of the resultant model was 31.642 for sleep and 55.629 for awake state. Statistical results for the final model in each state are summarized in Table 3.
As can be seen in the table, higher ASD risk was linked to a larger standard deviation of rhythm of the motor alteration in the upper body, \(^{(A_5)} I_8\), and smaller rhythms of the motor alteration in the lower limb, \(^{(A_6)} I_7\) during sleep. At the same time, higher ASD risk was associated with a larger ratio of movement frequency in the upper body and in the lower body, \(^{(A_{5,6})}I_4\), and a higher standard deviation of rhythm of the image center of gravity fluctuation in the vertical axis, \(^{(A_9)} I_{12_x}\) during awake state. Boxplots of retained body movement features in high- and low-risk groups are shown for sleep and awake state in Fig. 2. In any models, sex was not retained as a significant predictor.
Boxplots of selected evaluation index. (a) Sleep state. (b) Awake state. \({}^{(A_5)}I_8\) : Standard deviation of rhythm of the motor alteration in upper body, \({}^{(A_6)}I_7\): Rhythm of the motor alteration in lower body, \({}^{(A_{5,6})}I_4\) : Ratio of movement frequency in upper body and in lower body, \({}^{(A_9)}I_{12x}\) : Standard deviation of rhythm of the image center of gravity fluctuation in vertical axis.
The ROCs and AUCs of the resultant models are shown in Fig. 3. The AUCs were compared between sleep and awake state, but the Delong's test failed to reveal a significant difference at a significance threshold of 0.05.
Performance indicators, i.e. sensitivity, specificity and F-measure, and confusion matrices at the cut-off value are summarized in Fig. 4. AUC and F-measure indicate that classification based on body movement features during sleep show numerically superior performance as evaluated by F-measure. This is largely due to higher specificity in sleep than awake state whereas sensitivity was higher in awake state. Classification performance based on the data during sleep was even superior to classification based on all the data combining datasets from both sleep and awake states.
Discussion
The present study investigated whether the pattern of spontaneous movement in neonates contains information predictive of ASD risk at 18 months old. To achieve this goal, the body movement pattern of neonates was quantified by our markerless movement analysis system37,38, and the association was examined between body motion characteristics at the neonatal stage and ASD risk evaluated by the Japanese version of M-CHAT at 18-months old.
During awake state, neonates with high ASD risk showed relatively larger motion frequency in the upper body compared to the lower body, and a larger standard deviation in center of gravity movement along the vertical axis. The former observation is consonant with our previous finding that four-month-olds with high ASD risk show reduced strength of lower limb motions37. These observations raise the possibility that ASD risk might be screened by focusing on muscle tone and motion in the lower limb during the first four months after delivery.
Large standard deviation around central frequency in neonates with high ASD risk was observed along the vertical axis in the present study, whereas a similar observation was obtained in 4-month-olds along the medial-lateral axis37. The cause for this difference remains elusive at this point. Three-to-four months after delivery is a transitory period during which the dominant pattern of spontaneous motion changes in qualitative as well as quantitative terms, as illustrated in the replacement of writhing by fidgety GM4. The body axis along which a lack of rhythmicity in high ASD risk infants is observed differs between neonates and four 4-month-olds probably due to such timeline in motor development.
Performance of logistic regression classifiers was numerically higher in sleep state than awake state. This observation resonates with the findings by Denisova and Zhao that a more frequent occurrence of noisy head movement in 1–2 month-olds with high than low ASD risk infants was prominent during sleep compared to awake state44. Classification performance in sleep was even higher than that of the logistic regression classifier trained by all the data combining sleep and awake state, which indicates the importance of considering arousal state in ASD risk prediction based on body movement features. One reason for the numerical difference in classification performance between sleep and awake state is the unbalanced size of the training dataset. At the same time, it is equally possible that body movement makes a differential contribution to neural development depending on arousal state, which claim gains some support from the observation that a different set of features was retained in the logistic regression model in sleep from awake state.
During sleep state, neonates with high ASD risk exhibited larger standard deviation around the central frequency in the upper body and lower central frequency in the lower body. As stated above, larger standard deviation in the distribution of frequency power indicates a lack of a prominent rhythmic component in body motion. This feature can be used as a marker of ASD risk irrespective of the infant’s arousal state. People with ASD are less adept at making rhythmic motion in sync with external stimulation (for a review, see Tordjman et al.54) which this ability is of primary importance in socio-emotional and sensori-motor development during early infancy55,56,57. It is conceivable that a lack of rhythmicity in body movement at the neonatal stage is an early sign of atypical rhythmic movement generation in ASD people.
Smaller central frequency in lower limb motion during sleep was associated with a higher ASD risk. Recent studies have suggested that the occurrence of a twitch, a sporadic and jerky movement, of faces and limbs plays primary roles in the fine-tuning of the sensorimotor map in rodents and humans alike45,46. Interestingly, a twitch is observed predominantly during sleep at the neonatal stage45,46. Based on these, one potential explanation for our observation is that neonates with high ASD risk make a smaller number of high-frequency twitches during sleep.
Neural function underlying the generation of body motion differs between sleep and awake states. For example, afferent input is inhibited during awake state and functional coupling between motor command and proprioceptive information is enhanced during sleep45,46. The widely-practiced procedure of GMA deals with the qualitative evaluation of spontaneous movement during wakefulness. Taking these into consideration, it is important to note that our findings alone do not tell whether there is an inherent linkage between body movement during sleep and the development of higher-order cognitive functions. Previous studies on motor function during sleep reported a potential contribution of body motion during sleep to the fine-tuning of somatotopic representation56,57,58,59. However, it remains to be seen whether the development of other domains of neural functions, such as socio-emotional functions, are causally linked to motor-related neural activity during sleep in infants.
There are several limitations that qualify the interpretation of the present findings. First and foremost, several previous studies question the reliability of M-CHAT as a screening tool for ASD risk60,61,62; Children with a score of 3 to 7 are generally categorized as a group with medium risk for ASD. Worse, we did not carry out a follow-up telephone interview49, which must have inflated the false positive rate that infants were classified into high risk group in the present study. Inada et al.50 recommended criteria slightly more lenient compared to the original one63. For the purpose of lowering the false positive rate, we chose to adopt a more stringent criteria than Inada et al.50 following the procedure of the first-stage screening in Kamio et al52. However, the proportion of high-risk infant was 17.5% (7/40) in sleep state and 25.4% (14/55) in awake state dataset. Both of these values are far larger than the ASD prevalence rate of 1 in 50 reported in previous studies (for example, Xu et al.64). Relatedly, the present study included a relatively small number of infants, and thus, high risk infants in the present study probably do not meet the diagnostic criteria of ASD during their toddlerhood, especially because we did not specifically recruit children with a familial risk of ASD. Thus, to validate our main claim that signs of ASD risk emerge at the neonatal stage, it is required in the future study to include infants with high ASD risk who have a genetic liability for ASD, and check whether the infant receives a diagnosis of ASD in a longitudinal design. It is also indispensable to evaluate a participant’s symptoms using an established and more reliable assessment tool such as ADOS.
Second, it is unclear what physiological condition the “sleep state” in the present study corresponds to. In the present study, we classified the neonate’s arousal state based on whether their eyes are opened or not65. But with such qualitative criteria, we cannot say with confidence whether sleep as per definition in our study corresponds to sleep or incidental closing of the eyes during awake state. Lastly, the recording environment was not strictly controlled. Since video-recording took place in a hospital, we could not entirely exclude background noises and casual interventions to the infants by hospital staffs, such as looking into the baby bed, during recording. Though video-segments with external stimulation were carefully discarded from the analysis, it is possible that overlooked stimulation from the environment influenced neonates’ arousal state and movement pattern. To address the last two points, it is desirable to record an infant’s body movement under a controlled laboratory environment simultaneously with scalp EEG data for arousal state classification.
Fourth, we deleted data from infants with small or no body movement, which leaves open the possibility that discarded data included infants with the lowest frequency of limb motion. We decided to discard these data because reliable quantification of body movement features was not feasible for these cases. Given the discussion above that reduced frequency of lower limb motion might be an early biomarker of ASD, it is conceivable that we failed to include the most severe case of ASD-related motor atypicality.
Fifth, the sample size of the present study was relatively small. This small sample size might explain the lack of significant contribution of sex in the prediction of ASD risk. Many previous studies found a sex difference in symptoms and behavioral phenotypes of ASD66,67,68. Relatedly, a structural brain imaging study69 found notable sex differences in motor-related neural regions. Considering these, it is possible that we could detect the effect of sex in a study with a larger sample size.
Conclusion
The present study has shown that characteristics of spontaneous movement at the neonatal stage reflects ASD risk evaluated by M-CHAT at 18 months old, which raises the possibility that early signs of ASD emerge at the neonatal stage, far earlier than previously thought. Predictability of ASD risk was higher when classification was made based on body movement features during sleep than awake state. At the same time, the present study suffers from many limitations. Most importantly, the proportion of high-risk infant was much higher than the reported prevalence rate of ASD, which indicates that high-risk group in the present study included many false positives. Thus, the present findings alone do not tell us whether features extracted from spontaneous movement could be utilized as an early biomarker of ASD.
Investigation into the association between early motor development and ASD is still at a nascent stage. Several studies indicate an association between GM and a later diagnosis of ASD (see Einspieler et al.36 for a review). However, definitive conclusions cannot be drawn from these studies, as the sample size in many of them is quite small. The introduction of an automated system greatly reduces the cost of GMA. Although preliminary, the present findings support the prospect of large-scale research on the association between early motor development and ASD, assisted by semi-automatic assessment of an infant’s spontaneous movement.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Einspieler, C., Prayer, D. & Marschik, P. B. Fetal movements: The origin of human behaviour. Dev. Med. Child Neurol. 63, 1142–1148 (2021).
Lüchinger, A. B., Hadders-Algra, M., Van Kan, C. M. & De Vries, J. I. Fetal onset of general movements. Pediatr. Res. 63, 191–195 (2008).
Hadders-Algra, M. Putative neural substrate of normal and abnormal general movements. Neurosci. Biobehav. Rev. 31, 1181–1190 (2007).
Einspieler, C., Bos, A. F., Libertus, M. E. & Marschik, P. B. The general movement assessment helps us to identify preterm infants at risk for cognitive dysfunction. Front. Psychol. 7, 406 (2016).
Salavati, S. et al. A comparison of the early motor repertoire of very preterm infants and term infants. Eur. J. Paediatr. Neurol. 32, 73–79 (2021).
Bruggink, J. L. et al. Quantitative aspects of the early motor repertoire in preterm infants: Do they predict minor neurological dysfunction at school age?. Early Hum. Dev. 85, 25–36 (2009).
Prechtl, H. F. Qualitative changes of spontaneous movements in fetus and preterm infant are a marker of neurological dysfunction. Early Hum. Dev. 23, 151–158 (1990).
Prechtl, H. F. et al. An early marker for neurological deficits after perinatal brain lesions. The Lancet 349, 1361–1363 (1997).
Bruggink, J. L. et al. The quality of the early motor repertoire in preterm infants predicts minor neurologic dysfunction at school age. J. Pediatr. 153, 32–39 (2008).
Bruggink, J. L., Van Braeckel, K. N. & Bos, A. F. The early motor repertoire of children born preterm is associated with intelligence at school age. Pediatrics 125, e1356–e1363 (2010).
Hadders-Algra, M. General movements: A window for early identification of children at high risk for developmental disorders. J. Pediatr. 145, S12–S18 (2004).
Hadders-Algra, M., Bouwstra, H. & Groen, S. E. Quality of general movements and psychiatric morbidity at 9 to 12 years. Early Hum. Dev. 85, 1–6 (2009).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5 Vol. 5 (American psychiatric association, Washington, DC, 2013).
Brett, D., Warnell, F., McConachie, H. & Parr, J. R. Factors affecting age at ASD diagnosis in UK: No evidence that diagnosis age has decreased between 2004 and 2014. J. Autism Dev. Disorders 46, 1974–1984 (2016).
Leader, G. et al. Age of autism spectrum disorder diagnosis and comorbidity in children and adolescents with autism spectrum disorder. Dev. Neurorehabilitation 25, 29–37 (2022).
Steiner, A. M., Goldsmith, T. R., Snow, A. V. & Chawarska, K. Practitioner’s guide to assessment of autism spectrum disorders in infants and toddlers. J. Autism Dev. Disord. 42, 1183–1196 (2012).
Lord, C. et al. Autism from 2 to 9 years of age. Arch. Gen. Psychiatr. 63, 694–701 (2006).
Moore, V. & Goodson, S. How well does early diagnosis of autism stand the test of time? follow-up study of children assessed for autism at age 2 and development of an early diagnostic service. Autism 7, 47–63 (2003).
Adrien, J. L. et al. Blind ratings of early symptoms of autism based upon family home movies. J. Am. Acad. Child Adolesc. Psychiatr. 32, 617–626 (1993).
Baranek, G. T. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J. Autism Dev. Disord. 29, 213–224 (1999).
Klin, A., Lin, D. J., Gorrindo, P., Ramsay, G. & Jones, W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature 459, 257–261 (2009).
Elsabbagh, M. et al. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Curr. Biol. 22, 338–342 (2012).
Osterling, J. A., Dawson, G. & Munson, J. A. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev. Psychopathol. 14, 239–251 (2002).
Dawson, G., Osterling, J., Meltzoff, A. N. & Kuhl, P. Case study of the development of an infant with autism from birth to two years of age. J. Appl. Dev. Psychol. 21, 299–313 (2000).
Green, J. et al. Parent-mediated intervention versus no intervention for infants at high risk of autism: A parallel, single-blind, randomised trial. Lancet Psychiatr. 2, 133–140 (2015).
Dawson, G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev. Psychopathol. 20, 775–803 (2008).
Zwaigenbaum, L. et al. Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics 123, 1383–1391 (2009).
Esposito, G., Venuti, P., Maestro, S. & Muratori, F. An exploration of symmetry in early autism spectrum disorders: Analysis of lying. Brain Dev. 31, 131–138 (2009).
Esposito, G., Venuti, P., Apicella, F. & Muratori, F. Analysis of unsupported gait in toddlers with autism. Brain Dev. 33, 367–373 (2011).
Libertus, K., Sheperd, K. A., Ross, S. W. & Landa, R. J. Limited fine motor and grasping skills in 6-month-old infants at high risk for autism. Child Dev. 85, 2218–2231 (2014).
Landa, R. & Garrett-Mayer, E. Development in infants with autism spectrum disorders: a prospective study. J. Child Psychol. Psychiatr. 47, 629–638 (2006).
Teitelbaum, P., Teitelbaum, O., Nye, J., Fryman, J. & Maurer, R. G. Movement analysis in infancy may be useful for early diagnosis of autism. Proc. Natl. Acad. Sci. 95, 13982–13987 (1998).
Ozonoff, S. et al. Gross motor development, movement abnormalities, and early identification of autism. J. Autism Dev. Disord. 38, 644–656 (2008).
Bhat, A., Galloway, J. & Landa, R. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behav. Dev. 35, 838–846 (2012).
Sacrey, L.-A.R., Zwaigenbaum, L., Bryson, S., Brian, J. & Smith, I. M. The reach-to-grasp movement in infants later diagnosed with autism spectrum disorder: A high-risk sibling cohort study. J. Neurodev. Disord. 10, 1–11 (2018).
Einspieler, C. et al. Highlighting the first 5 months of life: General movements in infants later diagnosed with autism spectrum disorder or rett syndrome. Res. Autism Spectr. Disord. 8, 286–291 (2014).
Doi, H. et al. Prediction of autistic tendencies at 18 months of age via markerless video analysis of spontaneous body movements in 4-month-old infants. Sci. Rep. 12, 18045 (2022).
Tsuji, T. et al. Markerless measurement and evaluation of general movements in infants. Sci. Rep. 10, 1–13 (2020).
Silva, N. et al. The future of general movement assessment: The role of computer vision and machine learning-a scoping review. Res. Dev. Disabil. 110, 103854 (2021).
Groos, D., Adde, L., Støen, R., Ramampiaro, H. & Ihlen, E. A. Towards human-level performance on automatic pose estimation of infant spontaneous movements. Comput. Med. Imag. Graph. 95, 102012 (2022).
Marschik, P. B. et al. A novel way to measure and predict development: A heuristic approach to facilitate the early detection of neurodevelopmental disorders. Curr. Neurol. Neurosci. Rep. 17, 1–15 (2017).
Reich, S. et al. Novel AI driven approach to classify infant motor functions. Sci. Rep. 11, 9888 (2021).
Hashimoto, Y. et al. Automated classification of general movements in infants using two-stream spatiotemporal fusion network. In Proceedings of International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI), 753–762 (2022).
Denisova, K. & Zhao, G. Inflexible neurobiological signatures precede atypical development in infants at high risk for autism. Sci. Rep. 7, 1–17 (2017).
Blumberg, M. S., Marques, H. G. & Iida, F. Twitching in sensorimotor development from sleeping rats to robots. Curr. Biol. 23, R532–R537 (2013).
Blumberg, M. S., Dooley, J. C. & Sokoloff, G. The developing brain revealed during sleep. Curr. Opin. Physiol. 15, 14–22 (2020).
Sakurai, K. et al. Chiba study of mother and children’s health (C-MACH): Cohort study with omics analyses. BMJ Open 6, e010531 (2016).
Einspieler, C., Prechtl, H. F. R., Bos, A. F., Ferrari, F. & Cioni, G. Prechtl’s Method on the Qualitative Assessment of General Movements in Preterm, Term and Young Infants (Clinics in Developmental Medicine) (Mac Keith Press, 2004).
Robins, D. L. et al. Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics 133, 37–45 (2014).
Inada, N., Koyama, T., Inokuchi, E., Kuroda, M. & Kamio, Y. Reliability and validity of the Japanese version of the modified checklist for autism in toddlers (M-CHAT). Res. Autism Spectr. Disord. 5, 330–336 (2011).
Kamio, Y. et al. Brief report: Best discriminators for identifying children with autism spectrum disorder at an 18-month health check-up in japan. J. Autism Dev. Disord. 45, 4147–4153 (2015).
Kamio, Y. et al. Effectiveness of using the modified checklist for autism in toddlers in two-stage screening of autism spectrum disorder at the 18-month health check-up in japan. J. Autism Dev. Disord. 44, 194–203 (2014).
Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of 2nd International Symposium on Information Theory, 267–281 (Akadémiai Kiadó Location Budapest, Hungary, 1973).
Tordjman, S. et al. Autism as a disorder of biological and behavioral rhythms: Toward new therapeutic perspectives. Front. Pediatr. 3, 1 (2015).
Shultz, S., Klin, A. & Jones, W. Neonatal transitions in social behavior and their implications for autism. Trends Cognit. Sci. 22, 452–469 (2018).
Sokoloff, G. et al. Spatiotemporal organization of myoclonic twitching in sleeping human infants. Dev. Psychobiol. 62, 697–710 (2020).
Kanazawa, H. et al. Open-ended movements structure sensorimotor information in early human development. Proc. Natl. Acad. Sci. 120, e2209953120 (2023).
Whitehead, K., Meek, J. & Fabrizi, L. Developmental trajectory of movement-related cortical oscillations during active sleep in a cross-sectional cohort of pre-term and full-term human infants. Sci. Rep. 8, 17516 (2018).
Petersson, P., Waldenström, A., Fåhraeus, C. & Schouenborg, J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature 424, 72–75 (2003).
Stenberg, N. et al. Identifying children with autism spectrum disorder at 18 months in a general population sample. Paediatr. Perinat. Epidemiol. 28, 255–262 (2014).
Guthrie, W. et al. Accuracy of autism screening in a large pediatric network. Pediatrics 144, e20183963 (2019).
Carbone, P. S. et al. Primary care autism screening and later autism diagnosis. Pediatrics 146, e20192314 (2020).
Robins, D. L., Fein, D., Barton, M. L. & Green, J. A. The modified checklist for autism in toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. J. Autism Dev. Disord. 31, 131–144 (2001).
Xu, G. et al. Prevalence and treatment patterns of autism spectrum disorder in the United States, 2016. JAMA Pediatr. 173, 153–159 (2019).
Brazelton, T. B. & Nugent, J. K. Neonatal Behavioral Assessment Scale Vol. 137 (Cambridge University Press, 1995).
Bölte, S. et al. Sex and gender in neurodevelopmental conditions. Nat. Rev. Neurol. 19, 136–159 (2023).
Doi, H., Kanai, C. & Ohta, H. Transdiagnostic and sex differences in cognitive profiles of autism spectrum disorder and attention-deficit/hyperactivity disorder. Autism Res. 15, 1130–1141 (2022).
Lai, M.-C., Lombardo, M. V., Auyeung, B., Chakrabarti, B. & Baron-Cohen, S. Sex/gender differences and autism: Setting the scene for future research. J. Am. Acad. Child Adolesc. Psychiatr. 54, 11–24 (2015).
Supekar, K. & Menon, V. Sex differences in structural organization of motor systems and their dissociable links with repetitive/restricted behaviors in children with autism. Mol. Autism 6, 1–13 (2015).
Acknowledgements
We thank staffs of Yamaguchi Clinic for their help in recruiting participants and video-recording collection. This study was financed by JSPS KAKENHI Grant Number 16H01781 and 15H01584.
Author information
Authors and Affiliations
Contributions
H.D., A.F., and R.U. wrote the initial draft, and T.T. edited the manuscript. A.F. and R.U. prepared figures. M.Y., K.Sakurai, and C.M. conducted experiments and collected data. A.F. and R.U developed analysis methods. R.U. conducted the analysis. H.D. and K.Shimatani contributed to data interpretation. T.T. designed the study and conceived experiments. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doi, H., Furui, A., Ueda, R. et al. Spatiotemporal patterns of spontaneous movement in neonates are significantly linked to risk of autism spectrum disorders at 18 months old. Sci Rep 13, 13869 (2023). https://doi.org/10.1038/s41598-023-40368-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40368-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.