Abstract
We aimed to compare longitudinal brain atrophy in patients with neuromyelitis optica spectrum disorder (NMOSD) with healthy controls (HCs). The atrophy rate in patients with anti-aquaporin-4 antibody-positive NMOSD (AQP4 + NMOSD) was compared with age-sex-matched HCs recruited from the Japanese Alzheimer’s Disease Neuroimaging Initiative study and another study performed at Chiba University. Twenty-nine patients with AQP4 + NMOSD and 29 HCs were enrolled in the study. The time between magnetic resonance imaging (MRI) scans was longer in the AQP4 + NMOSD group compared with the HCs (median; 3.2 vs. 2.9 years, P = 0.009). The annualized normalized white matter volume (NWV) atrophy rate was higher in the AQP4 + NMOSD group compared with the HCs (median; 0.37 vs. − 0.14, P = 0.018). The maximum spinal cord lesion length negatively correlated with NWV at baseline MRI in patients with AQP4 + NMOSD (Spearman’s rho = − 0.41, P = 0.027). The annualized NWV atrophy rate negatively correlated with the time between initiation of persistent prednisolone usage and baseline MRI in patients with AQP4 + NMOSD (Spearman’s rho = − 0.43, P = 0.019). Patients with AQP4 + NMOSD had a greater annualized NWV atrophy rate than HCs. Suppressing disease activity may prevent brain atrophy in patients with AQP4 + NMOSD.
Similar content being viewed by others
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a severe form of the central nervous system (CNS) inflammation that typically affects the spinal cord and optic nerve. However, brain lesions can occur in NMOSD. Another feature of NMOSD is positivity for aquaporin-4 (AQP4) antibodies, which are present in the sera of 60%–90% of patients with NMOSD (AQP4 + NMOSD patients)1,2.
Disability in patients with NMOSD is reported to be attack-dependent3. Therefore, NMOSD is considered to not exhibit attack-independent neurodegeneration. However, previous reports observed decreases in the white matter volume in patients with NMOSD compared to that in healthy controls (HCs)4,5,6. Widespread occult damage in normal-appearing white matter was reported in NMOSD compared to the findings in HCs7. A recent study observed a spectrum of astrocytopathy that supports the concept of attack-independent structural changes in the NMOSD pathology8. Therefore, attack-independent neurodegeneration might occur in the white matter in patients with NMOSD.
Moreover, unlike multiple sclerosis (MS), NMOSD is not thought to exhibit longitudinal brain atrophy compared with HCs. However, only a few studies have investigated longitudinal brain atrophy in patients with NMOSD, including our study9,10. We compared longitudinal brain atrophy in patients with AQP4 + NMOSD to longitudinal brain atrophy in patients with MS and showed that brain atrophy silently progressed in patients with AQP4 + NMOSD, even in clinically inactive patients10. Patients with AQP4 + NMOSD and long cord lesions exhibited annualized brain gray matter volume atrophy rates that were higher than patients without long cord lesions10. Thus, we hypothesized that subclinical dying back degeneration caused by long cord lesion contributed to the brain atrophy in patients with AQP4 + NMOSD. However, HCs were absent in our previous study. Therefore, we compared the longitudinal brain atrophy rate between patients with NMOSD and HCs to overcome this limitation.
In this study, longitudinal brain atrophy was compared in patients with AQP4 + NMOSD to age-sex-matched HCs using magnetic resonance imaging (MRI) images obtained from the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) study and another study performed at Chiba University. We also investigated the clinical characteristics associated with brain volume at baseline in patients with AQP4 + NMOSD.
Materials and methods
Study design and patient populations
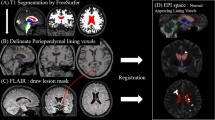
We expanded the previously published dataset10. Therefore, patients’ data overlapped with the previous study (82.8%)10. We reviewed the clinical records of 124 patients with AQP4 + NMOSD at Chiba University Hospital. Figure 1 demonstrates the patient enrolment and study design. Recruitment of patients with AQP4 + NMOSD was conducted as previously described10. First, we included patients with two MRI scans by the same scanner at an interval of > 1 year. We selected two MRI scans (MRI-1 and MRI-2) as the interval widened, as previously reported11. We excluded MRIs performed within 60 days of prednisolone pulse therapy or additional immunotherapies including plasma exchange to minimize pseudoatrophy12,13. All AQP4 + NMOSD patients fulfilled the 2015 international diagnostic criteria for NMOSD1 with AQP4 + cell-based assay results, as previously described14. In addition, patients were grouped by age (< 55 years vs. ≥ 55 years) and matched by age and sex with HCs.
Patients who were at least 55 years old were age-sex matched with HCs from the J-ADNI study (UMIN000001374)15. The public–private partnership, J-ADNI, was established in 2007 with Professor Takeshi Iwatsubo as the Principal Investigator. The J-ADNI study’s main objective was to ascertain serial magnetic MRI, positron emission tomography, other biological markers, and clinical and neuropsychological assessment could be integrated to track mild Alzheimer’s disease and late mild cognitive impairment in the Japanese population. The National Bioscience Database Center Human Database, Japan (Research ID: hum0043.v1, 2016) provided the J-ADNI data. All J-ADNI MRI data were published after distortion correction16.
Volunteer participants between 60 and 84 years old enrolled in the J-ADNI study. The eligibility criteria of the ADNI study was applied to the volunteer participants17. Subjects who scored 24–30 in the Mini-Mental State Examination scores without memory complaints were treated as HCs. We included subjects with two same-scanner MRI scans at an interval of > 1 year. The two MRI scans (MRI-1 and MRI-2) with the largest time interval were selected. The J-ADNI database information were obtained from the National Bioscience Database Center Human Database, Japan (Research ID: hum0043.v1, 2016)15. Other inclusion and exclusion criteria are described at https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000012764.
Patients less than 55 years old were age-sex-matched with HCs from another study performed at Chiba University by Professor Shimizu (Chiba-HCs). Chiba-HCs had no history of mental disorder, and they were confirmed to currently not meet the diagnostic criteria for any mental disorder by psychosomatic physicians or psychiatrists by a comprehensive structured interview based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Any subjects with claustrophobia, head trauma, neurological disorders, or substance abuse were excluded. Nine volunteers underwent two same-scanner MRI scans at an interval of > 1 year.
Patients with AQP4 + NMOSD and HCs were sorted by age. Younger patients with AQP4 + NMOSD and HCs with an age difference of ≤ 5 years were matched. The HC closest in age to the patient was matched. If there were several candidates, patients or controls were blindly selected by a doctor (Yosuke Onishi).
Demographic characteristics
Demographic characteristics at MRI-1 and MRI-2, including sex ratio and age, and clinical features, including disease duration, Kurtzke’s Expanded Disability Status Scale (EDSS) score, annualized relapse rate (ARR) from disease onset, years from the last attack, years of continuous prednisolone, and oligoclonal band positivity were investigated. Years of the relapse prevention treatment at the same dosage which was given at MRI-1 or MRI-2 were also examined. Histories of optic neuritis, myelitis, long cord lesion, brainstem lesion, area postrema syndrome and cerebral syndrome were also investigated.
The brain volumes at MRI-1 and MRI-2 and atrophy rates in patients with AQP4 + NMOSD and HCs were compared.
Association of brain volume and clinical characteristics in patients with AQP4 + NMOSD
The maximum spinal cord lesion length and brain volume at baseline were correlated. The associations between annualized brain atrophy rates and clinical characteristics, including treatment duration, EDSS and ARR were also analyzed. Long cord lesion was defined as > 3 vertebral segments. We measured the spinal cord lesion length (vertebral body segments) from the image showing the maximum spinal cord lesion length in all spinal cord images performed in the acute phase of previous myelitis before MRI-1, and analyzed the correlation between the length and annualized atrophy rate. The analysis of association between years of continuous prednisolone usage and brain atrophy rates were added when difference of brain atrophy rates was found between patients and HCs. Differences in the annualized NWV atrophy rate with or without a history of brainstem lesion or cerebral syndrome were investigated in patients with AQP + NMOSD. Clinical and brain volume difference between male and female patients with AQP4 + NMOSD were also investigated.
Brain MRI scan and brain volume measurements
The same MR scanner, a 1.5-Tesla Signa HDxT (GE Healthcare, Milwaukee, WI, USA), was used to obtain a conventional brain MRI, T1-weighted three-dimensional (3D) images, and fluid-attenuated inversion recovery (FLAIR) or multiplanar reconstruction (MPR) from the 3D-FLAIR from each patient. Supplementary Table S1 shows details of the MRI systems for patients.
Brain MRI imaging of HCs participating in the J-ADNI study was performed using a 1.5-Tesla15. The 3.0-Tesla Discovery MR750 (GE Healthcare, Milwaukee, WI, USA) was used for all Chiba-HCs. Supplementary Table S2 presents MRI system information for younger HCs.
Since previous studies demonstrated the different scanners at different time points significantly affected the brain atrophy measures in the longitudinal morphometric results18,19, the same MRI scanner was used for individual patients or Chiba-HCs to minimize the effect of the field strength difference between 3.0-Tesla for Chiba-HCs and 1.5-Tesla for all patients. Distortion correction was performed to all J-ADNI MRI data before the data publication20.
We calculated brain volumes using statistical parametric mapping-12 (SPM12) with MATLAB (Version R2016b; The MathWorks, Inc., Natick, MA, USA). Measuring brain volume in each patient was performed as described previously21,22. We employed a previously described technique to segment lesions and calculate the annualized atrophy rate10,23. Lesion Segmentation Tool (LST) toolbox version 2.0.15 (available in the public domain at www.statisticalmodelling.de/lst.html) was used for SPM23. As recommended by Schmidt et al.23, we used an initial threshold (κ) value of 0.30. Normalized brain (NBV), gray matter (NGV), lesion (NLV), and white matter (NWV) volumes were defined as previously reported10. Briefly, each volume was divided by the intracranial volume, which was the sum of the whole-brain gray matter, white matter, and cerebrospinal fluid volumes, to reduce interindividual variation24.
Statistical analysis
Statistical analyses were performed with SPSS version 27.0 (IBM Corporation, Armonk, NY, USA). Continuous data were compared by the Mann–Whitney U test. The Fisher’s exact test was used to evaluate categorical outcomes. Correlations were analyzed using the Spearman’s rank test. P < 0.05 was considered statistically significant. An analysis of covariance was performed when the annualized atrophy rate was determined using significant different items as covariates.
Ethical approval and consent to participate
The Chiba University School of Medicine ethic committee approved the study (No. 2555 and M10545). Informed consent was provided by all patients. The methods used in this study comply with the Declaration of Helsinki and its subsequent amendments, and were performed in accordance with the relevant guidelines and regulations.
Results
Demographics and clinical characteristics at MRI-1 and MRI-2
We enrolled 29 patients in each group. Twenty-three patients with AQP4 + NMOSD who were at least 55 years old were matched with HCs in J-ADNI. Of the 22 patients less than 55 years old, 6 patients were matched with Chiba-HCs. Three HCs in the Chiba study were not matched because of sex differences. Of the 29 patients who were enrolled in this study, twenty-four patients overlapped with our previous study10. Table 1 displays the clinical characteristics and demographics of patients with AQP4 + NMOSD and the HCs at MRI-1. Females accounted for 75.9% of the patients in both groups. The age difference between the two groups was not statistically significant (median: 59.0 vs. 61.0 years, interquartile range: 9.5 vs. 4.5, P = 0.30). The median disease duration at MRI-1 was 7.0 years (interquartile range: 13.4, range: 0.3–42.9). The median EDSS score and ARR from disease onset were 3.5 and 0.4, respectively (interquartile ranges: 4.0 and 0.4, ranges: 0.1–9.0 and 0.2–4.0, respectively). The median time from initiating continuous prednisolone therapy was 3.9 years (interquartile range: 3.2, range: 0.1–8.8). The median time since the last attack was 2.8 years (interquartile range: 3.3, range: 0.2–6.9). The median of years of the relapse prevention treatment was 1.3 at MRI-1 (interquartile range: 1.8, range: 0.1–5.2). Twenty patients with AQP4 + NMOSD received prednisolone alone; three patients received prednisolone plus azathioprine, one patient received prednisolone and eculizumab, and five patients did not receive any treatment at MRI-1. Dose range of prednisolone was from 1.25 to 20 mg/day at MRI-1.
The clinical features at MRI-2 in patients with AQP4 + NMOSD and HCs are shown in Table 2.
The median years from the last attack to MRI-2 was 5.2 years (interquartile range: 6.3, range: 0.9–10.3). In total, six patients showed the relapse between MRI-1 and MRI-2. Only one patient relapsed with cerebral syndrome between MRI scans. Years from the last attack to MRI-2 in the six patients were 0.90, 0.98, 1.26, 1.28, 1.96, and 2.64. The median of years of the relapse prevention treatment was 3.3 at MRI-2 (interquartile range: 4.4, range: 0.8–7.9). Ten patients changed the relapse prevention treatment between MRI-1 and MRI-2. One patient discontinued relapse prevention and one patient initiated the continuous prednisolone. Within other eight patients, four patients decreased the dosage of prednisolone between MRI-1 and MRI-2, two patients increased the prednisolone dosage, and two patients showed the same dosage of prednisolone. Nineteen patients with AQP4 + NMOSD received prednisolone alone; four patients received prednisolone plus azathioprine, one patient received prednisolone and eculizumab, and five patients received no treatment at MRI-2. Dose range of prednisolone was from 5.0 mg/day to 15 mg/day at MRI-2.
Linearity of changes in brain volume in patients with AQP4 + NMOSD
To establish the linearity of longitudinal brain atrophy in patients with AQP4 + NMOSD, brain volume changes and disease duration in each patient with AQP4 + NMOSD were analyzed. Most patients showed a similar slope for changes in brain atrophy between MRI-1 and MRI-2, regardless of the disease duration (Fig. 2).
Brain volume changes and disease duration in each patient with AQP4 + NMOSD. (A) NBV changes and disease durations. (B) NGV changes and disease durations. (C) NWV changes and disease durations. AQP4 + NMOSD anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorders, NBV normalized brain volume, NGV normalized gray matter volume, NWV normalized white matter volume.
Annualized NWV atrophy rate
Table 2 shows the brain volumes at MRI-1 and MRI-2 as well as the annualized atrophy rate between MRI-1 and MRI-2 in patients with AQP4 + NMOSD and HCs. Patients with AQP4 + NMOSD had significantly longer intervals between MRI-1 and MRI-2 than HCs (median: 3.2 vs. 2.9, P = 0.009). Patients with AQP4 + NMOSD had decreased NBV at MRI-1 and NGV at MRI-1 and MRI-2 compared to NBV and NGV in HCs. Although the annualized NGW and NBV atrophy rates were not significantly different between patients with AQP4 + NMOSD and HCs, the patients with AQP4 + NMOSD had an annualized NWV atrophy rate greater than that of HCs (P = 0.018). The annualized NWV atrophy rate was significantly related to the MRI-1 and MRI-2 time interval in patients with AQP4 + NMOSD and HCs, according to the parallelism test (P = 0.048). Therefore, an analysis of covariance using the MRI-1 and MRI-2 time interval as covariates could not be performed for the annualized atrophy rate of NWV. Figure 3 shows the percentage of NBV, NGV, and NWV changes in patients with AQP4 + NMOSD and HCs.
The percent brain volume changes between MRI-1 and MRI-2 in patients with AQP4 + NMOSD and HCs. (A) NBV changes and follow-up durations. (B) NGV changes and follow-up durations. (C) NWV changes and follow-up durations. The black dotted lines represent brain volume changes (in percentage) for each patient. The fitted average slopes in patients with AQP4 + NMOSD and HCs are shown by the black line. AQP4 + NMOSD anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorders, HCs healthy controls, NBV normalized brain volume, NGV normalized gray matter volume, NWV normalized white matter volume.
Correlation between spinal cord lesion length and NWV at MRI-1
In patients with AQP4 + NMOSD, we examined the relationships between the annualized atrophy rate of each brain volume and the spinal cord lesion length and brain volumes at MRI-1 and MRI-2. The spinal cord lesion length negatively correlated with NWV at MRI-1 (Spearman’s rho = − 0.41, P = 0.027, Fig. 4) but not with NWV at MRI-2, NBV at MRI-1 or MRI-2, NGV at MRI-1 or MRI-2, or NLV at MRI-1 or MRI-2. No associations were found between the spinal cord lesion length and annualized atrophy rate of each brain volumes including NBV, NGV, and NWV.
Correlation between brain volumes and spinal lesion cord length in patients with AQP4 + NMOSD. (A) NBV changes and maximum spinal cord lesion length. (B) NGV changes and maximum spinal cord lesion length. (C) NWV changes and maximum spinal cord lesion length. AQP4 + NMOSD anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorders, NBV normalized brain volume, NGV normalized gray matter volume, NWV normalized white matter volume.
Correlation between persistent prednisolone usage duration and the annualized NWV atrophy rate
The annualized NWV atrophy rate negatively correlated with the time from the initiation of persistent prednisolone usage to MRI-1 and MRI-2 in patients with AQP4 + NMOSD (Spearman’s rho = − 0.43 and − 0.46, P = 0.019 and 0.011, respectively, Fig. 5). EDSS at MRI-1, ΔEDSS (EDSS at MRI-2 minus EDSS at MRI-1), and ARR between MRI-1 and MRI-2 did not correlate with the annualized NBV, NGV or NWV atrophy rates. On the other hand, ARR at MRI-1 negatively correlated with the annualized NWV atrophy rate (Spearman’s rho = − 0.41, P = 0.026) but not with the annualized NBV or NGV atrophy rates. In patients with persistent prednisolone at MRI-1, ARR at MRI-1 negatively correlated with the duration of persistent prednisolone use (Spearman’s rho = − 0.44, P = 0.033). No correlation was found between the annualized NWV atrophy rate and disease duration at MRI-1, MRI-2, or the interval between MRI-1 and MRI-2.
Correlation between persistent prednisolone usage and the annualized NWV atrophy rate in patients with AQP4 + NMOSD. (A) The annualized NWV atrophy rates and persistent prednisolone usage durations at MRI-1. (B) The annualized NWV atrophy rates and persistent prednisolone usage durations at MRI-2. AQP4 + NMOSD anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorders, NWV normalized white matter volume.
Difference in the annualized NWV atrophy rate between patients with and without a history of brainstem lesion or cerebral syndrome
No difference was found in the annualized NWV atrophy rate between patients with (N = 6) and those without a history of brainstem lesion (N = 23). The annualized NWV atrophy rate was higher in patients with a history of cerebral syndrome than in those without history of cerebral syndrome (median: 1.38% vs. 0.20%, interquartile range: 1.13 vs. 1.30, N = 4 vs. N = 25, P = 0.043). Patients with a history of cerebral syndrome had higher NLV at MRI-1 than those without history of cerebral syndrome (median: 11.5. mL vs. 0.91 mL, interquartile range: 12.8 vs. 3.07, N = 4 vs. N = 25, P = 0.008). Patients without cerebral syndrome tended to have a higher annualized NWV atrophy rate than age- and sex-matched HCs (median: 0.20% vs. − 0.17%, interquartile range: 1.30 vs. 0.96, N = 25 vs. N = 25, P = 0.068). One patient had a history of both brainstem lesion and cerebral syndrome. Patients with a history of brainstem lesion or cerebral lesion (N = 9) showed no difference from those without these histories (N = 20).
Clinical and brain volume difference between male and female patients with AQP4 + NMOSD
Male patients showed shorter disease duration at MRI-1 (median: 2.4 vs. 9.0 years, P = 0.013) and MRI-2 (median: 5.6 vs. 11.9 years, P = 0.013) than female patients. The male patients had higher intracranial volume at MRI-1 (median: 1.52 vs. 1.32, P < 0.001) and MRI-2 (median: 1.52 vs. 1.32, P < 0.001), and age at disease onset (median: 54.0 vs. 46.5 years, P = 0.049) than female patients. No difference was found in NBV, NGV, NWV, and NLV at MRI-1 and MRI-2 between male and female patients. Annualized atrophy rates of NBV, NGV, and NWV were similar in male and female patients.
Discussion
Our results show that patients with AQP4 + NMOSD had significantly higher annualized NWV atrophy rates than age-sex-matched HCs. Spinal cord lesion length negatively correlated with NWV in patients with AQP4 + NMOSD. Persistent prednisolone usage negatively correlated with the annualized NWV atrophy rate. These findings suggest that suppressing disease activity may prevent longitudinal brain atrophy in patients with AQP4 + NMOSD.
Patients with AQP4 + NMOSD had higher annualized NWV atrophy rates than HCs. Our result corresponds with previous studies. Decreased brain white matter volume was reported in NMO compared with HCs4,5,6. Another study reported widespread occult damage in normal-appearing white matter in NMOSD compared with HCs7. These results clearly demonstrate brain white matter could be damaged in AQP4 + NMOSD. On the other hand, in HCs, previous studies showed brain white matter volume appeared to be relatively stable except in oldest participants, while brain gray matter volume loss appeared to be constant throughout the adult life25,26. Meanwhile, a previous study demonstrated patients with acute spinal cord injury showed faster atrophy rates in brain white matter and gray matter compared with HCs27. These findings may explain why we found differences only in NWV but not NGV between patients with AQP4 + NMOSD and HCs.
Our study revealed a negative correlation between the spinal cord lesion length and NWV in patients with NMOSD. Another study reported a lower lateral geniculate nucleus volume in patients with NMOSD and a history of ON than in patients with NMOSD without a history of ON and controls28. Oral prednisolone maintenance therapy was reported to be effective to prevent relapse in patients with AQP4 + NMOSD29. Moreover, some biological disease-modifying drugs were reported to lower disability or reduce the risk of disability progression in patients with AQP4 + NMOSD. Interleukin-6 receptor blockade decreases ARR and lowers disability in patients with NMOSD30,31. CD-19 blockade was also demonstrated to decrease the risk of 3-month EDSS-confirmed disability progression in patients with NMOSD32. In addition, MRI studies demonstrated decreased spinal cord MRI activity during tocilizumab therapy, particularly in patients with AQP4 + NMOSD30. The mean upper cervical cord area was associated with normalized brain volume in patients with MS33. Cortical atrophy following spinal cord injury was also reported34. Therefore, if silent progression by subclinical dying back degeneration occurs in patients with AQP4 + NMOSD, as we hypothesized, then biological disease-modifying drugs can prevent brain atrophy by decreasing the activity and lesion length in the spinal cord.
We demonstrated a negative correlation between annualized NWV atrophy rate and ARR at MRI-1. In addition, ARR at MRI-1 negatively correlated with persistent prednisolone duration at MRI-1 in patients undergoing persistent prednisolone treatment. These results suggest that inhibiting relapse and inflammation lowers subsequent brain white matter atrophy rates in patients with AQP4 + NMOSD. Autopsy results from a patient with AQP4 + NMO showed persistent microscopic active inflammatory lesions in the CNS35. The patient received oral prednisolone treatment for over 40 years and showed no relapse for more than five years before death. Microscopic active inflammatory lesions were found not only in the spinal cord but also in the white matter of the right frontal lobe, left amygdala and central pons. These subclinical microscopic active inflammatory lesions in the brain may result in higher longitudinal brain atrophy rates in patients with AQP4 + NMOSD. This hypothesis may explain our findings of a negative correlation between longer prednisolone usage and annualized NWV atrophy rate. Conversely, cerebral syndrome, such as higher brain dysfunction, tends not to be reflected in the EDSS score. Moreover, attack-independent structural changes were reported in the NMOSD pathology8. These facts could explain our results, in which ΔEDSS and ARR between MRI-1 and MRI-2 was not correlated with the annualized NBV, NGV, and NWV atrophy rates. Moreover, tiny structural changes in the brain without clinical relapse or EDSS changes might also contribute to brain atrophy in patients with NMOSD. Therefore, not only dying back degeneration but also subclinical active lesions may cause brain atrophy in patients with AQP4 + NMOSD. However, another study reported two cases of progressive cerebral atrophy in patients with NMO36. The authors speculated that progressive cerebral cortical atrophy is induced by severe intrathecal inflammation in patients with NMO. Meanwhile, previous studies reported that chronic steroid use might contribute to the loss of brain tissue37,38. These reports are not consistent with our findings, in which persistent prednisolone usage had a negative correlation with the annualized NWV atrophy rate. However, chronic steroid use could inhibit the silent progression in NMOSD as we hypothesized in our previous study. Therefore, further investigation is required regarding the underlying mechanisms of brain atrophy in patients with AQP4 + NMOSD.
Our study has several limitations. First, this study included the same patient data as our previous report (24/29, 82.8%)10. Therefore, further study using different patients is required. Second, MRI imaging performed in Chiba-HCs did not include FLAIR or MPR. FLAIR or MRP images are required to perform lesion filling. Thus, lesion filling could not be performed only in the younger Chiba-HCs. In general, lacking lesion filling should result in decreased brain volumes. Therefore, if the subclinical cerebral lesions occur between MRI-1 and MRI-2 in Chiba-HCs, the brain volume of Chiba-HCs at MRI-2 should be calculated as lower, resulting in the higher brain atrophy rates in Chiba-HCs. However, higher brain atrophy rates in Chiba-HCs would not affect our conclusion. Third, we used 3.0-Tesla MRI imaging only in younger HCs for the comparison of brain volumes between MRI-1 and MRI-2. Validation is required, particularly for the cross-sectional study, when using different scanners or different magnetic field strength imaging. Therefore, the use of different scanners may have affected our cross-sectional study. However, because individual patients or HCs underwent MRI with the same scanner for the longitudinal study, our results concerning atrophy rates should not be affected. Moreover, a previous study reported that different tesla did not affect brain atrophy results39. Fourth, linear brain atrophy was hypothesized in our study. Therefore, the annualized NWV atrophy rate exhibited interaction with the MRI interval according to the parallelism test. If non-linear atrophy occurs, studies from other groups or several time points may be required. Fifth, six patients in our study demonstrated a relapse between MRI-1 and MRI-2. The proportions of patients with relapse between MRI scans may influence the result. Finally, the interval between MRIs was different in the AQP4 + NMOSD and HC groups. No differences in NVWs at either MRI-1 or MRI-2 were observed between patients with AQP4 + NMOSD and HCs, while a higher annualized NWV atrophy rate was observed in patients with AQP4 + NMOSD. A prospective study comparing patients with AQP4 + NMOSD and HCs with the same MRI duration is required to identify the best MRI follow-up interval for detecting NWV differences.
Our study demonstrated that patients with AQP4 + NMOSD had greater rates of longitudinal brain white matter atrophy than HCs. Not only dying back degeneration but also subclinical active lesions may be involved in brain white matter atrophy pathogenesis in patients with AQP4 + NMOSD. Previous studies demonstrated some differences in clinical and demographic features in patients with NMOSD among different ethnic or geographic groups40,41. Confounding factors including smoking may be involved in the brain atrophy in MS and NMOSD42. Future studies with a higher number of patients, different ethnicity groups, adjusted confounding factors, and a unified MRI scanner are required. Evaluating differences in atrophy rates in patients with or without biological disease-modifying drugs, such as anti-interleukin-6 receptor therapy, may be necessary to determine whether preventing MRI activation in the spinal cord or subclinical active lesions prevents brain atrophy in patients with AQP4 + NMOSD.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Wingerchuk, D. M. et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85, 177–189. https://doi.org/10.1212/wnl.0000000000001729 (2015).
Jarius, S. et al. Neuromyelitis optica. Nat. Rev. Dis. Primers 6, 85. https://doi.org/10.1038/s41572-020-0214-9 (2020).
Kawachi, I. & Lassmann, H. Neurodegeneration in multiple sclerosis and neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry 88, 137–145. https://doi.org/10.1136/jnnp-2016-313300 (2017).
Blanc, F. et al. White matter atrophy and cognitive dysfunctions in neuromyelitis optica. PLoS ONE 7, e33878. https://doi.org/10.1371/journal.pone.0033878 (2012).
Chanson, J. B. et al. White matter volume is decreased in the brain of patients with neuromyelitis optica. Eur. J. Neurol. 20, 361–367. https://doi.org/10.1111/j.1468-1331.2012.03867.x (2013).
Duan, Y. et al. White matter atrophy in brain of neuromyelitis optica: a voxel-based morphometry study. Acta Radiol. 55, 589–593. https://doi.org/10.1177/0284185113501815 (2014).
Kim, S. H. et al. Diffusion tensor imaging of normal-appearing white matter in patients with neuromyelitis optica spectrum disorder and multiple sclerosis. Eur. J. Neurol. 24, 966–973. https://doi.org/10.1111/ene.13321 (2017).
Guo, Y. et al. Spectrum of sublytic astrocytopathy in neuromyelitis optica. Brain 145, 1379–1390. https://doi.org/10.1093/brain/awab394 (2022).
Liu, Y. et al. Different patterns of longitudinal brain and spinal cord changes and their associations with disability progression in NMO and MS. Eur. Radiol. 28, 96–103. https://doi.org/10.1007/s00330-017-4921-x (2018).
Masuda, H. et al. Silent progression of brain atrophy in aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder. J. Neurol. Neurosurg. Psychiatry 93, 32–40. https://doi.org/10.1136/jnnp-2021-326386 (2022).
Masuda, H. et al. Comparison of brain atrophy in patients with multiple sclerosis treated with first-versus second-generation disease modifying therapy without clinical relapse. Eur. J. Neurol. 27, 2056–2061. https://doi.org/10.1111/ene.14335 (2020).
Pelletier, D., Garrison, K. & Henry, R. Measurement of whole-brain atrophy in multiple sclerosis. J. Neuroimaging 14, 11S-19S. https://doi.org/10.1177/1051228404266264 (2004).
Zivadinov, R. et al. Interferon beta-1a slows progression of brain atrophy in relapsing-remitting multiple sclerosis predominantly by reducing gray matter atrophy. Mult. Scler. 13, 490–501. https://doi.org/10.1177/1352458506070446 (2007).
Sugimoto, K. et al. The accuracy of flow cytometric cell-based assay to detect anti-myelin oligodendrocyte glycoprotein (MOG) antibodies determining the optimal method for positivity judgement. J. Neuroimmunol. 336, 577021. https://doi.org/10.1016/j.jneuroim.2019.577021 (2019).
Iwatsubo, T. et al. Japanese and North American Alzheimer’s disease neuroimaging initiative studies: Harmonization for international trials. Alzheimer Dement. 14, 1077–1087. https://doi.org/10.1016/j.jalz.2018.03.009 (2018).
Maikusa, N. et al. Improved volumetric measurement of brain structure with a distortion correction procedure using an ADNI phantom. Med. Phys. 40, 062303. https://doi.org/10.1118/1.4801913 (2013).
Petersen, R. C. et al. Alzheimer’s disease neuroimaging initiative (ADNI): Clinical characterization. Neurology 74, 201–209. https://doi.org/10.1212/WNL.0b013e3181cb3e25 (2010).
Sinnecker, T. et al. Brain atrophy measurement over a MRI scanner change in multiple sclerosis. NeuroImage Clin. 36, 103148. https://doi.org/10.1016/j.nicl.2022.103148 (2022).
Takao, H., Hayashi, N. & Ohtomo, K. Effects of the use of multiple scanners and of scanner upgrade in longitudinal voxel-based morphometry studies. J. Magn. Reson. Imaging 38, 1283–1291. https://doi.org/10.1002/jmri.24038 (2013).
Fujishima, M. et al. Sample size estimation for Alzheimer’s disease trials from Japanese ADNI serial magnetic resonance imaging. J. Alzheimers Dis. 56, 75–88. https://doi.org/10.3233/jad-160621 (2017).
Masuda, H. et al. Comparison of brain atrophy in patients with multiple sclerosis treated with first- versus second-generation disease modifying therapy without clinical relapse. Eur. J. Neurol. https://doi.org/10.1111/ene.14335 (2020).
Masuda, H. et al. Relapse numbers and earlier intervention by disease modifying drugs are related with progression of less brain atrophy in patients with multiple sclerosis. J. Neurol. Sci. 403, 78–84. https://doi.org/10.1016/j.jns.2019.06.011 (2019).
Schmidt, P. et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 59, 3774–3783 (2012).
Whitwell, J. L., Crum, W. R., Watt, H. C. & Fox, N. C. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am. J. Neuroradiol. 22, 1483–1489 (2001).
Ge, Y. et al. Age-related total gray matter and white matter changes in normal adult brain. Part I: Volumetric MR imaging analysis. AJNR. Am. J. Neuroradiol. 23, 1327–1333 (2002).
Gunning-Dixon, F. M., Brickman, A. M., Cheng, J. C. & Alexopoulos, G. S. Aging of cerebral white matter: A review of MRI findings. Int. J. Geriatr. Psychiatry 24, 109–117. https://doi.org/10.1002/gps.2087 (2009).
Freund, P. et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: A prospective longitudinal study. Lancet. Neurol. 12, 873–881. https://doi.org/10.1016/s1474-4422(13)70146-7 (2013).
Papadopoulou, A. et al. Attack-related damage of thalamic nuclei in neuromyelitis optica spectrum disorders. J. Neurol. Neurosurg. Psychiatry 90, 1156–1164. https://doi.org/10.1136/jnnp-2018-320249 (2019).
Takai, Y. et al. Optimal management of neuromyelitis optica spectrum disorder with aquaporin-4 antibody by oral prednisolone maintenance therapy. Mult. Scler. Relat. Disord. 49, 102750. https://doi.org/10.1016/j.msard.2021.102750 (2021).
Ringelstein, M. et al. Interleukin-6 receptor blockade in treatment-refractory MOG-IgG-associated disease and neuromyelitis optica spectrum disorders. Neurol. Neuroimmunol. Neuroinflamm. https://doi.org/10.1212/nxi.0000000000001100 (2022).
Du, C. et al. Early initiation of tocilizumab treatment against moderate-to-severe myelitis in neuromyelitis optica spectrum disorder. Front. Immunol. 12, 660230. https://doi.org/10.3389/fimmu.2021.660230 (2021).
Marignier, R. et al. Disability outcomes in the n-momentum trial of inebilizumab in neuromyelitis optica spectrum disorder. Neurol. Neuroimmunol. Neuroinflamm. 8, 1–10. https://doi.org/10.1212/nxi.0000000000000978 (2021).
Daams, M. et al. Mean upper cervical cord area (MUCCA) measurement in long-standing multiple sclerosis: Relation to brain findings and clinical disability. Mult. Scler. 20, 1860–1865. https://doi.org/10.1177/1352458514533399 (2014).
Freund, P. et al. Disability, atrophy and cortical reorganization following spinal cord injury. Brain 134, 1610–1622. https://doi.org/10.1093/brain/awr093 (2011).
Fujii, C. et al. Persistent microscopic active inflammatory lesions in the central nervous system of a patient with neuromyelitis optica treated with oral prednisolone for more than 40 years. Neurol. Sci. 11, 17–19. https://doi.org/10.1016/j.ensci.2018.05.005 (2018).
Warabi, Y., Takahashi, T. & Isozaki, E. Progressive cerebral atrophy in neuromyelitis optica. Mult. Scler. 21, 1872–1875. https://doi.org/10.1177/1352458515600246 (2015).
Zivadinov, R. Steroids and brain atrophy in multiple sclerosis. J. Neurol. Sci. 233, 73–81. https://doi.org/10.1016/j.jns.2005.03.006 (2005).
van der Meulen, M., Amaya, J. M., Dekkers, O. M. & Meijer, O. C. Association between use of systemic and inhaled glucocorticoids and changes in brain volume and white matter microstructure: A cross-sectional study using data from the UK Biobank. BMJ Open 12, e062446. https://doi.org/10.1136/bmjopen-2022-062446 (2022).
Chow, N. et al. Comparing 3T and 1.5T MRI for mapping hippocampal atrophy in the Alzheimer’s disease neuroimaging initiative. AJNR Am. J. Neuroradiol. 36, 653–660. https://doi.org/10.3174/ajnr.A4228 (2015).
Asseyer, S. et al. AQP4-IgG autoimmunity in Japan and Germany: Differences in clinical profiles and prognosis in seropositive neuromyelitis optica spectrum disorders. Mult. Scler. J. Exp. Transl. Clin. 7, 20552173211006864. https://doi.org/10.1177/20552173211006862 (2021).
Kitley, J. L., Leite, M. I., George, J. S. & Palace, J. A. The differential diagnosis of longitudinally extensive transverse myelitis. Mult. Scler. 18, 271–285. https://doi.org/10.1177/1352458511406165 (2012).
Lie, I. A. et al. The effect of smoking on long-term gray matter atrophy and clinical disability in patients with relapsing-remitting multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 9, e20009. https://doi.org/10.1212/nxi.0000000000200008 (2022).
Acknowledgements
The authors thank Dr. Yosuke Onishi for the blind selection of patients and HCs for age-sex matching. J-ADNI was supported by the following grants: Translational Research Promotion Project from the New Energy and Industrial Technology Development Organization of Japan; Research on Dementia, Health Labor Sciences Research Grant; Life Science Database Integration Project of Japan Science and Technology Agency; Research Association of Biotechnology (contributed by Astellas Pharma Inc., Bristol-Myers Squibb, Daiichi-Sankyo, Eisai, Eli Lilly and Company, Merck-Banyu, Mitsubishi Tanabe Pharma, Pfizer Inc., Shionogi & Co., Ltd., Sumitomo Dainippon and Takeda Pharmaceutical Company), Japan and a grant from an anonymous Foundation. Recruiting Chiba-HCs were supported by the following grants: AMED Brain/MINDS Beyond program Grant No. JP18dm0307002 and JSPS KAKENHI Grants No. 19K03309, 22H01090.
Author information
Authors and Affiliations
Consortia
Contributions
H.M. drafted the first manuscript. M.M., S.H., and S.K. revised the manuscript and gave final approval of the current submission. H.M., M.M., S.H., and S.K. contributed to the conception and design of the study; H.M., M.M., A.U., T.U., M.M., R.O., and R.A. contributed to the acquisition and analysis of data in patients with AQP4 + NMOSD; Y.H. contributed to the acquisition of data in Chiba-HCs; H.M. collected clinical and MRI data of healthy control with help from Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI); H.M. drafted the text and prepared the figures. The authors have approved the manuscripts submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Masuda, H., Mori, M., Hirano, S. et al. Higher longitudinal brain white matter atrophy rate in aquaporin-4 IgG-positive NMOSD compared with healthy controls. Sci Rep 13, 12631 (2023). https://doi.org/10.1038/s41598-023-38893-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38893-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.