Abstract
Temporal processing, the ability to mentally represent and process the dynamical unfolding of events over time, is a fundamental feature of cognition that evolves with advancing age. Aging has indeed been associated with slower and more variable performance in timing tasks. However, the role of depressive symptoms in age-related changes in temporal processing remains to be investigated. Therefore, the present work aims to shed light on the link between temporal processing and depressive symptoms, which are frequent with advancing age. We relied on the multicentric “Blursday Project” database, providing measures of temporal processing together with questionnaires investigating psychological wellbeing. Results reveal that aging influences several timing abilities, from the reproduction of short time intervals to verbal estimations of longer temporal distances. Furthermore, the slowing down of felt passage of time regarding the last few days with age was fully mediated by the intensity of depressive symptoms. Overall, these findings suggest that depressive symptoms may play a pivotal role in age-related temporal processing changes.
Similar content being viewed by others
Introduction
Aging is associated with several cognitive changes involving memory, attention, and executive control1. A slowing down of the speed of information processing, as well as decline in working memory and episodic memory, are among the most frequently reported features of cognitive aging2. However, age-related cognitive changes are highly heterogenous, as several factors interact in individual cognitive trajectories3. As an example, the presence of even subclinical forms of depression (that is, that do not meet diagnostic criteria for clinical depression disorder) have been associated with higher risk of cognitive decline in cognitively unimpaired older adults4,5.
Temporal processing, the processing and maintenance in memory of the dynamical unfolding of events over time, are essential to the functioning of humans and animals6. The precision of temporal representations has important implications for cognitive functioning, well-being, and daily life activities7: However, the sense of time in aging could be affected by several factors, such as task load8, mental states9, and levels of arousal induced by the context10,11. Detecting such factors could contribute to further our understanding of the heterogeneity of age-related trajectories of cognitive decline. Here, we investigated how the perception and representation of time evolve throughout the lifespan across several temporal processes and time scales, while considering differences across individuals, such as the presence of depressive symptoms.
A wealth of experimental research has investigated temporal processing, greatly advancing our understanding of psychological and neural mechanisms underlying the perception and encoding of temporal information, yet a standard conceptual framework is still lacking12. Here, we will use the term “Temporal Processing” to cover different temporal scales and four main temporal aspects13: duration processing, temporal order and simultaneity, feeling of the passage of time, mental time travel14. Duration processing is typically assessed on short time scales (i.e., milliseconds, seconds), and can involve productions of specific durations (e.g., pressing a button for two seconds), reproductions of stimuli durations, or comparisons between stimuli durations (i.e., determining which stimulus is the shortest/longest). Order and simultaneity can be assessed when participants perform tapping tasks to produce or pursue an initiated rhythm or stay in synchrony with a cued rhythm. The Passage Of Time Judgements (POTj) and Mental Time Travel (MTT) (i.e., the ability to relive past experience and to imagine future events) can notably be quantified using questionnaires15 assessing the subjective temporal distance at larger time scales (i.e., weeks, months, years) for both past and future events16. These different paradigms require the efficient functioning of several cognitive functions, which are supported by distinct neural underpinnings17,18. For instance, performance in duration processing tasks has been associated with attention, processing speed, and working memory abilities19. At the neural level, duration processing has been associated with activations of frontal and parietal cortices including the insular, superior/inferior frontal gyri, the superior temporal gyrus, and the striatum20. M/EEG studies reported an association between sustained synchronized activity between frontal and parietal regions and the maintenance of temporal representations and the detection of deviations21. Regarding the feeling of passage of time, previous work reported associations with episodic memory22 and consciousness23. Similarly, the mental time travel to the past (episodic autobiographical memory) and to the future (episodic future thinking are thought to rely on a partially shared set of brain regions including medial temporal and frontal lobes, posterior cingulate and retrosplenial cortex, and lateral parietal and temporal areas24. From a neural point of view, sustained brain oscillations and ramping brain activity over time have been interpreted as reflecting accumulation of information over time25. Ramping activity over time has been associated with activity of the supplementary motor area, and the posterior insula26.
Gradual changes of temporal processing with advancing age have been previously reported27,28,29, with older individuals showing reduced precision across temporal processing tasks17,30. Moreover, older individuals produce longer inter-tap intervals (ITI) than younger adults when required to tap at their preferred rate for 30 s (i.e., spontaneous motor tapping task (SMT), resulting in a slowing down of the motor tempo31,32. However, contrasted findings have been reported regarding the evolution of the subjective feeling of the passage of time with advancing age33. As an example, some studies investigating the passage of time found no or little evidence for changes in the rate of the feeling of time passing with age, over different periods (i.e., weeks, months, years, decades)34,35, while others reported a slower passage of time feeling in people older than 75 years old36.
Although there is still a lack of consensus in the previous literature regarding the reason why time may pass differently with age, the effect of negative emotions on temporal judgements is well known37 notably in the older population and could thus play a role in this process. For instance, Droit-Volet7 used an Experience Sampling Methodology (ESM) and found that both young and older participants reported a slowing down of the passage of present time when they felt sad. While this relationship should be further investigated, this suggests a pivotal role of affective states on the felt passage of time38.
It has been suggested that altered temporal estimations in older adults may either be related to an attention deficit linked to an insufficient focus of attention on temporal information39, or to noisy duration representations related to reduced working memory capacities40. Nonetheless, aging effects and the interplay of individual characteristics on the mechanisms supporting temporal processing changes remain to be clarified, with a need to further consider the heterogeneity of changes across timing abilities and temporal scales with advancing age. One major factor contributing to cognitive decline in the aging population is the presence of depressive symptoms41,42. Moreover, recent behavioral meta-analyses43,44 revealed alterations in the encoding and representation of short and longer durations in individuals with depressive symptoms relative to controls. Depressed individuals indeed tend to overproduce short durations and underproduce long duration intervals (i.e., from 400 ms to 30 s)45. Detecting such changes of temporal processing, notably in the aged population, could be insightful to uncover the presence of subclinical depressive syndromes, thereby contributing to the prevention of cognitive decline5.
To shed light on the interplay between aging effects and depressive symptoms on the evolution of temporal processing, we relied on the Blursday database46, which includes repeated temporal processing measures together with a variety of questionnaires investigating the psychological well-being of 2840 participants in over nine countries, ranging from 18 to 80 years. This project documents the effects of social isolation of lockdown measures on the subjective experience of time as Covid lockdown measures significantly affected on temporal processing47,48. However, as our goal was to investigate the effect of depressive symptoms on the relationship between temporal processing and aging as independently of these events as possible, analyses were restricted to the control session (i.e., outside lockdown) of this database. To our knowledge, no study to date has integrated several temporal processing tasks across different time scales to assess the effects of aging, and the contributing role of depressive symptoms. In the light of previous findings on aging and time estimation, we could anticipate a decrease of precision and a larger variability on tasks tied to duration processing with advancing age17. Moreover, we also expected changes in temporal aspects of cognition with aging, to be driven by the presence and intensity of depressive symptoms. Since temporal processing and cognitive functions are intertwined49, disentangling the link between age-related temporal processing changes from concurrent factors could contribute to explaining the inter-individual differences observed between different trajectories of cognitive decline across individuals.
Methods
To investigate the relationships between temporal processing and depressive symptoms with aging, we relied on the Blursday Project’s repository whose data is freely accessible under the Gorilla Open Materials Attribution Non-Commercial Research-Only licensing: https://app.gorilla.sc/openmaterials/278377. An informed consent was obtained from all participants.
Sample
Data were extracted from a sample of 388 participants from the control session of the study (naive participants tested outside the most severe measures of lockdowns). However, 17 participants were excluded due to a lack of basic demographic information (i.e., age and gender). After removing outliers in the age variable by excluding values above 2.5 SD of the mean (n = 2), we obtained a sample of 369 participants aged between 18 and 69 years (Females = 261, MAge ± SDAge: 31.9 ± 13.9; Males = 108, MAge ± SDAge: 35.7 ± 14), living in France (n = 172; Females: 130, Males: 42; MAge ± SDAge:31.5 ± 12.4), Germany (n = 75; Females: 62, Males: 13; MAge ± SDAge: 24 ± 5), Italy (n = 47; Females: 33, Males: 14; MAge ± SDAge: 32.6 ± 15.8), and Japan (n = 75; Females: 36, Males: 39; MAge ± SDAge: 45.6 ± 13.6).
As the Control Session of the Blursday Database from which the data have been extracted (n = 369) contained a great number of participants between 20 and 30 years old (n = 218) and between 30 and 40 years old (n = 60), we extracted a subsample by the “rand()” function in Excel from each age subgroup, but respecting the proportion of the world origin and sex (n = 148, Mage = 45.9 ± 14.420, M = 70, F = 78), to compute the robust One-way ANOVAs, divided in age groups of equal sizes (χ2 4 = 1.65, p = 0.799), composed as follows: n (20–30) = 28; n (30–40) = 27; n (40–50) = 28, n (50–60) = 35; n (60–80) = 30. We thus conducted additional analyses on a sub-sample balanced based on these factors, as probed by the Chi-square test for proportions with continuity correction applied (χ2 4 = 1.65, p = 0.799), to control for multiple comparisons. The contingency tables showing the frequencies of country and sex are showed in Supplementary Tables S1A and S1B. A graphical representation of the cultural composition of the sample is provided in Fig. 1.
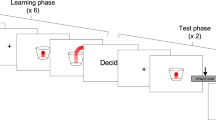
Graphical representation of behavioral tasks and questionnaires adapted from “The Blursday Project”. (a) Foreperiod Implicit Timing Task. (b) Retrospective Duration. (c) Spontaneous Tapping. (d) Synchronization-Continuation Task (e) Passage of Time. (f) Subjective Temporal Distance of the next week. (g) Subjective Temporal Distance of the next month.
Unfortunately, not every subject participating to the Control Session of the Blursday Project completed all tasks and questionnaires, resulting in a different number of participants for each of the tasks (shown in Table 1): Foreperiod Implicit Timing (n = 124), Retrospective duration (n = 73), Spontaneous Tapping (n = 130), Synchronization-Continuation (n = 145), Passage of Time (n = 135), Subjective Temporal Distance (Weeks) (n = 122), Subjective Temporal Distance (Months)(n = 122). However, all the tasks were analyzed separately using robust One-Way ANOVAs, and missing values were automatically excluded for each one of the mediations analyses.
Task description
The enrolled subjects to the Blursday Project, first completed the questionnaires administered in a random order across participants, most of which they had to take once per session. After the questionnaires, they accomplished a series of diverse pseudo-randomly ordered (latin-square design) behavioral tasks. Individual scores on The Hospital Anxiety and Depression Scale50 (HADS-A and D) were considered to quantify self-perceived depressive and anxiety symptoms in the last weeks. In addition, we extracted information about previous lockdown stringency51: A composite score of nine governmental response indicators including school closures, workplace closures and travel bans, was rescaled to values ranging from 0 to 100, with 100 being the strictest stringency on the restrictions index.
The following temporal processing tasks were considered (Fig. 1): the Foreperiod Implicit Timing task52, an auditory reaction time task in which participants were asked to respond as fast as possible to a series of target tones, which were preceded by cue tones. The Foreperiod (i.e., time interval between the cue and target tone (2 runs) was one of three durations (500 ms, 1750 ms and 3000 ms), presented as either fixed (3 trials) or variable (3 trials) throughout one block. In total, participants realized 6 blocks of 9 trials each. To quantify the imprecision and to avoid confounding slowing effects on sensorimotor response due to physiological aging, we computed the standard deviation of the reaction time to the fixed Foreperiod condition. Then, the retrospective duration task considers the individual estimation of elapsed time to perform the tests on the Blursday platform, spanning from several minutes to hours. The estimates were transformed to seconds to allow for comparisons. In the spontaneous finger tapping task (2 runs) participants had to press the spacebar at their preferred rate for 90 s32. To assess the internal production of time intervals, we considered the total time divided by the number of taps, thus obtaining a mean of the individual intertap interval. In the Synchronization-Continuation task53,54 (2 runs) participants were asked to synchronize themselves with an audio/visual stimulus presented on a screen at a rate of 1.3 Hz (78 Beats per minute) for 60 s. The standard deviation of the cue’s fixed interval (1 s) during the synchronization phase and the intertap interval (ITI) during continuation was considered as an index of asynchrony55.
The “passage of time” judgment about how fast or slow individuals’ passage of time appeared in the last few days, was expressed by participants on a Visual Analog Scale (VAS) ranging from 0 (“very slow”) to 100 (“very fast”). Finally, the subjective temporal distance from the next week and month was assessed by asking participants, “How far does a day a week from now seem to you?” and “How far does a day a month from now seem to you?” on a VAS from 0 to 100, where 0 corresponded to “very close” and 100 to “very far”.
An overview of the runs and stimuli used are described in Supplementary Fig. 2 and Table 1 of the original paper of the Blursday Project’s Database (Chaumon et al.46, Supplementary Information). Both provide a comprehensive description of the content of each session.
Statistical analyses
All analyses were performed with JASP (v. 0.16.4) and R (cran.r-project.org/). After conducting descriptive statistical analyses, the data’s normality was assessed using the Shapiro–Wilk test. Variables were z-score transformed prior to statistical analyses, to obtain a unique scale, therefore accounting for the different response type and time scales (spanning from milliseconds to months) between the analyzed tasks. Because normality assumptions were violated for several variables, we used robust statistical methods56 to assess differences between the 5 age-groups on temporal tasks performances. More specifically, we computed seven One-Way Robust Analyses of the Variance (Robust ANOVAs) using the functions “t1waybt” and “mcppb20” (for post-hoc comparisons), implemented in the packages “robustbase” and “WRS2” in R. We applied corrections for outliers by trimming the 20% of the upper and lower bounds of the data and we dealt with the violations of the assumptions for parametric tests by bootstrapping method57 with 2000 repetitions58.
To control for possible cultural variations in the expression of sadness feelings, we computed a parametric 5*2 parametric ANOVA between Age Groups and Region of the world on the HADS-D mean score (Supplementary Table S2A) as normality test was not significant (Shapiro–Wilk’s = 0.986, p = 0.142) as well as the Levene’s test for homogeneity of the variances (F(9,138) = 1.33, p = 0.255). Post-hoc analyses were corrected for multiple comparisons by applying the Bonferroni correction59 and shown in the Supplementary Table S2B.
Then, to investigate whether the intensity of depressive symptoms could mediate the relationship between the evolution of temporal productions and time estimations abilities across the lifespan we estimated the total effect (Path c) of aging (X = continuous predictor) on temporal processing (Y) and the indirect effects of the depressive symptoms (Path ab, mediator = HADS-D) for each one of the tasks considered: Foreperiod Implicit Timing (n = 299), Retrospective Duration (n = 175), Spontaneous Tapping (n = 321), Synchronization-Continuation (n = 364), Passage of Time (n = 306), Subjective Temporal Distance (Weeks) (n = 290), Subjective Temporal Distance Months (n = 290). As our primary purpose was to study the role of depression, we controlled for anxiety and confinement index as continuous background confounding variables.
As the whole sample (n = 369) did not respect the proportions of biological sex (F = 0.707; M = 0.293, χ2 = 62.8, p < 0.001) nor for the origin of the world [Europe (n = 294), Asia (n = 75), χ2 = 129, p < 0.001)] we conducted the mediation analyses on the balanced subsample of participants while controlling proportions of both biological sex and region of the world. Coefficients were completely standardized, and direct and indirect effects were computed with Bias-Corrected (BC) bootstrap, 95% confidence intervals based on 5000 bootstrap samples.
Results
Statistical descriptive analyses including the mean, standard deviation, and p values of the Shapiro–Wilk’s Test for normality on the temporal tasks and to the HADS scores, are shown in Table 1. Results of ANOVAs investigating cultural differences on HADS-D scores between age groups are shown in Supplementary Tables S2A–2C.
Temporal processing abilities across different age groups
The One-Way robust ANOVA probing the effect of age (5 age groups) on the performances to the 7 timing tasks revealed a statistically significant difference in the Asynchrony in performing the Synchronization-Continuation Task (Ftest = 3.110, p = 0.05, effect size = 0.382, Table 2). Bonferroni’s Test for multiple comparisons found that the asynchrony was significantly greater in the oldest group (60–80) compared to the adult one (40–50) (\(\widehat{\psi }\)= − 1.017, 95% CI [− 2.233; 0.166], p = 0.017) and detected a significant difference between the 30–40 compared to the 40–50 years old group (\(\widehat{\psi }\) = 0.716, 95% CI [0.001; 1.429], p = 0.004) (Fig. 2; Supplementary Table S3).
GLM mediation analysis
Direct effect of aging on temporal processing (c’ path)
The analysis of the direct effect of age on temporal processing performance highlighted a positive relationship with asynchrony, suggesting that the ability to maintain the pace after observing a cue, decreases with advancing age (β = 0.126, z = 2.043, p = 0.041, 95% CI [0.004; 0.223], Fig. 3, panel a). An effect of aging was also observed on the subjective temporal distance of the following week (β = − 0.206, z = − 2.972, p = 0.003, 95% CI [− 0.332; − 0.081], Fig. 3, panel b) and month (β = − 0.139, z = − 2.016, p = 0.044, 95% [− 0.265; − 0.007], Fig. 3, panel c) which were perceived as shorter by older participants.
Direct Effects of Age on Temporal Processing. Dots represent participants’ data. For illustrative purposes, variables are not scaled in the plot. Panel (a) Linear Regression of the effect of age (x-axis) on Asynchrony (y-axis) at the Synchronization-Continuation task. Panel (b) Linear Regression of the effect of age (x-axis) on Subjective Temporal Distance of the next week Panel (c) Linear Regression of the effect of age (x-axis) on Subjective Temporal Distance of the next month.
Direct effect of aging on depressive symptoms (path a)
Results of simple regression analyses for each path coefficient, notably highlighted that aging positively affected the HADS-D score (β = 0.176, z = 3.060, p = 0.002, 95% CI [0.070;0.382]). Results of total effects and all path coefficients computed are shown in Supplementary Materials, Tables S4 and S5. The path plot showing these path coefficients is depicted in Fig. 4 (Table 3).
Investigating the role of depressive symptoms on the relationship between temporal processing and aging (path ab)
By analyzing the indirect effects computed using the GLM mediation to further elucidate the role of depressive symptoms in the relationship between temporal processing and aging (Table 4), we observed that the felt passage of time was fully mediated by the intensity of depressive symptoms (Fig. 5)(β = − 0.028, z = − 2.011, p = 0.044, 95% CI [− 0.068; − 0.008]). Furthermore, we replicated the indirect effect of depression on the slowdown of the passage of time in the subsampled controlled based on gender and culture (n = 148) (β = 0.03, z = 2.131, p = 0.03, 95% CI [0.004; 0.223]), Supplementary Tables S6A–6D).
Plot of the mediated effect of the HADS-D on the relationship between Aging and Passage of Time. The distance between the horizontal lines represents the total effect of Aging (X) on Passage Of Time (Y), \(\widehat{c}\) and the distance between the two vertical lines is equal to \(\widehat{a}\). The slope of each of these lines is equal to b. The distance from the intersection point to the Y = i1 + \(\widehat{c}(\)MeanX) horizontal line is equal to the mediated effect, ab. Note that SD stands for the standard deviation of X. For convenience, hats were not included over the parameter estimates in the plot.
Discussion
The present study aimed at further specifying the effects of age and depressive symptoms on temporal processing. Our primary goal was to identify the aspects of time processing showing the most significant age-related changes. Then, because of its prevalence in the older population60 and its impact on cognitive functions42 we wanted to elucidate the interplay between aging and depressive symptoms effects on various aspects of temporal processing.
To this end, we relied on data from the large-scale Blursday Project, which provided several temporal processing measures and psychological well-being questionnaires46. A general linear model of mediation was carried out to consider the performance of each temporal processing measure, their association with age, and the mediation effect of depressive symptoms’ intensity.
Analyses of the relationship between age and temporal processing revealed age-related effects on several aspects of time processing. Overall, previous findings observed that the timing abilities in the hundreds to milliseconds range relies on attention and working memory processes19 which are known to decline with physiological aging2. We observed that the precision at the continuation-synchronization task decreased with advancing age. These results have also been confirmed by the analysis of variance investigating between groups differences on the timing tasks, highlighting a significant difference between the adult and the oldest group. The Sensorimotor synchronization (SMS), defined as the ability to synchronize to regularities in an external temporal structure61 is crucial not only for producing music or dancing, but also in a wide variety of interpersonal contexts, allowing to predict the events in the environment62. Regularity and precision of temporal coordination with an external rhythm, were observed to be reduced in healthy older adults63. Moreover, Bangert and Balota (2012) compared the continuous tapping accuracy of young and older adults, and individuals in the earliest stages of dementia. They found that young adults outperformed both older adult groups in both measures of accuracy and response variability64.
Traditional models of interval timing65,66,67 are composed of three stages: 1) At the onset of a to-be-timed interval, a pacemaker or internal clock encodes time units; 2) These time units are stored in a memory system; 3) When a decision needs to be made about whether a given interval is similar to an earlier perceived interval, these time units are compared, and a decision is made. Following cognitive investigations68,69 work on the neural bases of time processing led researchers to several frameworks70. According to the striatal beat frequency (SBF)71 model, frequently discussed in the neural interpretation of behavioral differences across groups17 the start signal to time a given stimulus or event involves oscillatory cortical synchrony. Such phase synchrony would then be detected by the striatum, the central clock in the model, which would then project its activity to the thalamus, the hippocampus, and the cortex72. This model thus strongly relies on the precision of synchronized brain couplings73. Alternative, distributed models have been proposed, without the presence of a single region detecting coincident events between oscillators as an internal clock25,74. These models notably involve state-dependent networks, where temporal information is reflected in the intrinsic and distributed synchronized dynamics of brain connectivity 70. However, these models were mainly developed based on findings in animals and in young adults and the evolution of such complex interactions with advancing age remains seldom investigated.
Results also revealed a negative direct effect of aging on Subjective Temporal Distances. We observed that the older people were, the closer they judged the weeks and months ahead. Decoupling from the present and exploring other times75, involves multiple cognitive processes, such as episodic memory and the abstraction of temporal relations between events75,76, which are also known to decline with advancing age77. In summary, the present work specifies that aging mainly affects the aspects tied to order and simultaneity13 (on a time scale of the order of 1 s) and the subjective future temporal distance14,16, of the next week and month. Conversely, in line with previous systematic questionnaires studies reporting subtle or no changes in the perceived passage of time with age34,35, we did not observe a unique effect of age on passage of time judgments concerning the last few days. While a slowing down of the flow of time in presence of feelings like boredom78 and sadness37,79 has indeed been observed and documented by previous studies, the association with aging effects was not previously been considered.
Here, we observed for the first time that the intensity of depressive symptoms fully mediates the feeling of the passage of time across the lifespan, suggesting these symptoms could play a pivotal role in age-related changes in the subjective experience of the felt passage of time over the last few days. Temporal processing is significantly altered in major depression80,81 with reports of a slowing both duration processing and sense of time flow. Dopaminergic deficits and altered nigro-striatal-prefrontal connectivity have been reported in presence of depressive symptoms82, and in association with impairments of attention and working memory performance83. Changes in the passage of time could thus constitute an indicator of altered cognitive functioning or mental health issues. The present results suggest that aging effects on felt passage of time largely result from the presence of depressive symptoms that may slow down the encoding of time units84. More prominently with advancing age, the presence of depressive symptoms could be associated with a lower drift rate or neural trajectory over time. These findings could also reflect a slowing down of the internal clock, and a less precise maintenance and encoding of temporal information with advancing age, associated with decreased memory and attentional capacities40. Aging effects on time processing may emerge as the result of even subtle dysfunction of fronto-striatal circuits85, which are known to be affected in Major Depression Disorder86.
The association between age, depressive symptoms and passage of time is also relevant for frameworks of cognitive aging3. For instance, a slower feeling of the passage of time has been showed to distinguish between clinical and sub-clinical depression in the young population87. Distortions of temporal aspects of cognition, such as the subjective feeling of the passage of time, could contribute to better identify depressive symptoms in the aging population, and be sensitive to cognitive changes with advancing age. This work extends our knowledge on cognitive changes associated with the presence of depressive symptoms in the aging population and suggest this aspect should be further considered in neuropsychological assessments13.
Future implications
Our results could help to improve the neuropsychological assessment of cognitive changes with advancing age by including easy tasks involving synchronizing with an external rhythm. Resynchronization is important in aging to enhance interpersonal interactions as well as to interact with the environment63. Most importantly, the observed slowdown of the passage of time in aging associated with the presence of depressive symptoms could help to detect even sub-clinical depressive symptoms in the aging population, thus clarifying the inter-individual heterogeneity of the trajectories of cognitive decline3. However, further longitudinal neuroimaging studies are needed to bridge the gap between the changes of cerebral mechanisms supporting temporal processing in the latest stages of life, and the influence of dopamine neurotransmission changes related to depressive symptoms on temporal processing.
Limitations
Despite the variety of temporal processing tasks included in the Blursday Project, not every subject enrolled accomplished all the tasks, resulting in different sample sizes for each task. Also, it is worth mentioning that education levels and general cognitive performance measures were not collected in the Blursday Project, which prevented the investigation of the association of the reported results with these variables. Thus, future longitudinal studies will further investigate the variability of aging effects on temporal processing and their associations with other cognitive processes.
Data availability
All data are available on the Blursday platform33.
References
Anderson, N. D. & Craik, F. I. M. 50 years of cognitive aging theory. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 72, 1–6 (2017).
Craik, F. I. M., Salthouse, T. A., Braver, T. S. & West, R. The Handbook of Aging and Cognition 3rd edn, 311–323 (Psychology Press, 2008).
Reuter-Lorenz, P. A. & Park, D. C. How does it STAC up? Revisiting the Scaffolding theory of aging and cognition. Neuropsychol. Rev. 24, 355–370. https://doi.org/10.1007/s11065-014-9270-9 (2014).
Touron, E. et al. Depressive symptoms in cognitively unimpaired older adults are associated with lower structural and functional integrity in a frontolimbic network. Mol. Psych. 27, 5086–5095 (2022).
Wilson, R. S. et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 59(3), 364–370. https://doi.org/10.1212/wnl.59.3.364 (2002).
Xu, R. & Church, R. M. Age-related changes in human and nonhuman timing. Timing Time Percept 5, 261–279. https://doi.org/10.1163/22134468-00002092 (2017).
Droit-Volet, S. Time perception, emotions and mood disorders. J. Physiol. Paris 107(255–264), 2013. https://doi.org/10.1016/j.jphysparis.2013.03.005 (2013).
Polti, I., Martin, B. & van Wassenhove, V. The effect of attention and working memory on the estimation of elapsed time. Sci. Rep. 8, 6690. https://doi.org/10.1038/s41598-018-25119-y9 (2018).
Droit-Volet, S. & Meck, W. H. How emotions color our perception of time. Trends Cognit. Sci. 11, 504–513 (2007).
Gil, S. & Droit-Volet, S. Emotional time distortions: The fundamental role of arousal. Cognit. Emot. 26, 847–862 (2012).
Droit-Volet, S., Fayolle, S. L. & Gil, S. Emotion and time perception: Effects of film-induced mood. Front Integr. Neurosci. 5, 33 (2011).
Thönes, S. & Stocker, K. A standard conceptual framework for the study of subjective time. Conscious Cognit. 71, 114–122. https://doi.org/10.1016/j.concog.2019.04.004 (2019).
Hinault, T. et al. Time processing impairments in neurological and psychiatric disorders. https://doi.org/10.31234/osf.io/kdc7e nd.
Suddendorf, T. & Corballis, M. C. The evolution of foresight: What is mental time travel, and is it unique to humans?. BBS 30, 299–313 (2007).
Wearden, J. H. Passage of time judgements. Conscious Cogn 38, 165–171 (2015).
D’Argembeau, A. Zooming in and out on one’s life: Autobiographical representations at multiple time scales. J. Cognit. Neurosci. 32, 2037–2055 (2020).
Turgeon, M., Lustig, C. & Meck, W. H. Cognitive aging and time perception: Roles of Bayesian optimization and degeneracy. Front Aging Neurosci. 8, 1. https://doi.org/10.3389/fnagi.2016.00102 (2016).
Nani, A. et al. The neural correlates of time: A meta-analysis of neuroimaging studies. J. Cognit. Neurosci. 31, 1796–1826 (2019).
Matthews, W. J. & Meck, W. H. Temporal cognition: Connecting subjective time to perception, attention, and memory. Psychol. Bull. 142, 865–907 (2016).
Naghibi, N. et al. Embodying time in the brain: A multi-dimensional neuroimaging meta-analysis of 95 duration processing studies. Neuropsychol. Rev. https://doi.org/10.1007/s11065-023-09588-1 (2023).
Kononowicz, T. W., Sander, T., Van Rijn, H. & van Wassenhove, V. Precision timing with α–β oscillatory coupling: Stopwatch or motor control?. J. Cognit. Neurosci. 32, 1624–1636 (2020).
Martinelli, N. & Droit-Volet, S. What factors underlie our experience of the passage of time? Theoretical consequences. Psychol. Res. 86, 522–530 (2022).
Nyberg, L., Kim, A. S. N., Habib, R., Levine, B. & Tulving, E. Consciousness of subjective time in the brain. Proc. Natl. Acad. Sci. U. S. A. 107, 22356–22359 (2010).
Schacter, D. L., Benoit, R. G. & Szpunar, K. K. Episodic future thinking: mechanisms and functions. Curr. Opin. Behav. Sci. 17, 41–50. https://doi.org/10.1016/j.cobeha.2017.06.002 (2017).
van Wassenhove, V., Herbst, S. K. & Kononowicz, T. W. Timing the brain to time themind: Critical contributions of time-resolved neuroimaging for temporal cognition. In Magnetoencephalography: From Signals to Dynamic Cortical Networks: Second Edition 855–905 (Springer International Publishing, 2019). https://doi.org/10.1007/978-3-030-00087-5_67.
Wittmann, M. The inner sense of time: How the brain creates a representation of duration. Nat. Rev. Neurosci. 14, 217–223. https://doi.org/10.1038/nrn3452 (2013).
Winkler, I. et al. Has it really been that long? Why time seems to speed up with age. Timing Time Percept. 5, 168–189 (2017).
McCormack, T., Brown, G. D. A., Maylor, E. A., Richardson, L. B. N. & Darby, R. J. Effects of aging on absolute identification of duration. Psychol. Aging 17, 363–378 (2002).
Wearden, J. H. The wrong tree: Time perception and time experience in the elderly. in Measuring the Mind Speed, control, and age (Oxford University Press, 2012). https://doi.org/10.1093/acprof:oso/9780198566427.003.0006.
Lamotte, M. & Droit-Volet, S. Aging and time perception for short and long durations: A question of attention?. Timing Time Percept. 5, 149–167 (2017).
Baudouin, A., Vanneste, S., Pouthas, V. & Isingrini, M. Age-related changes in duration reproduction: Involvement of working memory processes. Brain Cognit. 62, 17–23 (2006).
Vanneste, S., Pouthas, V. & Wearden, J. H. Temporal control of rhythmic performance: A comparison between young and old adults. Exp. Aging Res. 27, 83–102 (2001).
Wearden, J. H. Passage of time judgements. Conscious Cognit. 38, 165–171 (2015).
Wittmann, M. & Lehnhoff, S. Age effects in perception of time. Psychol. Rep. 97, 921–935 (2005).
Friedman, W. J. & Janssen, S. M. J. Aging and the speed of time. Acta Psychol. (Amst.) 134, 130–141 (2010).
Droit-Volet, S. Time does not fly but slow down in old age. Time Soc. 28, 60–82 (2019).
Gil, S. & Droit-Volet, S. Time perception, depression and sadness. Behav. Proc. 80, 169–176 (2009).
Droit-Volet, S. & Wearden, J. H. Experience Sampling Methodology reveals similarities in the experience of passage of time in young and elderly adults. Acta Psychol. (Amst.) 156, 77–82 (2015).
Zakay, D. & Block, R. A. The role of attention in time estimation processes. Adv. Cognit. Psychol. 115, 143–164 (1996).
Mioni, G., Cardullo, S., Ciavarelli, A. & Stablum, F. Age-related changes in time discrimination: The involvement of inhibition, working memory and speed of processing. Curr. Psychol. 40, 2462–2471 (2021).
Weisenbach, S. L., Boore, L. A. & Kales, H. C. Depression and cognitive impairment in older adults. Curr. Psych. Rep. 14, 280–288 (2012).
Lam, R. W., Kennedy, S. H., Roger, M. S. & Atul, K. Cognitive dysfunction in major depressive disorder: Effects on psychosocial functioning and implications for treatment. Can. J. Psych. 59, 649–654 (2014).
Kent, L., Van Doorn, G. & Klein, B. Time dilation and acceleration in depression. Acta Psychol. 194, 77–86. https://doi.org/10.1016/j.actpsy.2019.02.003 (2019).
Thönes, S. & Oberfeld, D. Time perception in depression: A meta-analysis. J. Affect. Disord. 175, 359–372. https://doi.org/10.1016/j.jad.2014.12.057 (2015).
Mioni, G., Stablum, F., Prunetti, E. & Grondin, S. Time perception in anxious and depressed patients: A comparison between time reproduction and time production tasks. J. Affect. Disord. 196, 154–163 (2016).
Chaumon, M. et al. The Blursday database as a resource to study subjective temporalities during COVID-19. Nat. Hum. Behav. https://doi.org/10.1038/s41562-022-01419-2 (2022).
Droit-Volet, S. et al. The persistence of slowed time experience during the COVID-19 pandemic: Two longitudinal studies in France. Front Psychol 12, 721716 (2021).
Grondin, S., Mendoza-Duran, E. & Rioux, P. A. Pandemic, quarantine, and psychological time. Front Psychol. 11, 2749 (2020).
Capizzi, M., Visalli, A., Faralli, A. & Mioni, G. Explicit and implicit timing in older adults: Dissociable associations with age and cognitive decline. PLoS ONE https://doi.org/10.31234/osf.io/wrfhc (2021).
Crawford, J. R., Henry, J. D., Crombie, C. & Taylor, E. P. Normative data for the HADS from a large non-clinical sample. B J. Clin. Psychol. 40, 429–434 (2001).
Hale, T. et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 5, 529–538 (2021).
Nobre, A. C. & van Ede, F. Anticipated moments: Temporal structure in attention. Nat. Rev. Neurosci. 1, 9. https://doi.org/10.1038/nrn.2017.141 (2018).
Repp, B. H. & Su, Y. H. Sensorimotor synchronization: A review of recent research (2006–2012). Psychon. Bull. Rev. 20, 403–452 (2013).
Wing, A. M. & Kristofferson, A. B. Response delays and the timing of discrete motor responses. Percept. Psychophys. 14, 5–12 (1973).
Chen, Y., Ding, M. & Scott Kelso, J. A. Origins of timing errors in human sensorimotor coordination. J. Mot. Behav. 33, 3–8 (2001).
Mair, P. & Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 52, 464–488 (2020).
Bradley, E. & Robert, T. An Introduction to the Bootstrap (Chapman & Hall, 1994).
Field, A. P. & Wilcox, R. R. Robust statistical methods: A primer for clinical psychology and experimental psychopathology researchers. Behav. Res. Ther. 98, 19–38 (2017).
Bonferroni, C. E. Teoria Statistica Delle Classi e Calcolo Delle Probabilità/Carlo E. Bonferroni (Seeber, 1936).
Hu, T. et al. Prevalence of depression in older adults: A systematic review and meta-analysis. Psych. Res. https://doi.org/10.1016/j.psychres.2022.114511 (2022).
Schwartze, M., Keller, P. E., Patel, A. D. & Kotz, S. A. The impact of basal ganglia lesions on sensorimotor synchronization, spontaneous motor tempo, and the detection of tempo changes. Behav. Brain Res. 216, 685–691 (2011).
Bispham, J. Rhythm in music: What is it? Who has it? And why?. Music Percept. 24, 125–134 (2006).
von Schnehen, A., Hobeika, L., Huvent-Grelle, D. & Samson, S. Sensorimotor synchronization in healthy aging and neurocognitive disorders. Front Psychol. 1, 3. https://doi.org/10.3389/fpsyg.2022.838511 (2022).
Bangert, A. S. & Balota, D. A. Keep up the pace: Declines in simple repetitive timing differentiate healthy aging from the earliest stages of Alzheimer’s disease. J. Int. Neuropsychol. Soc. 18, 1052–1063 (2012).
Treisman, M. Temporal discrimination and the indifference interval. Implications for a model of the ‘internal clock’. Psychol. Monogr. 77, 1–31 (1963).
Gibbon, J., Malapanits, C., Dale, C. L. & Gallistep, C. R. Toward a neurobiology of temporal cognition: Advances and challenges. Curr. Opin. Neurobiol. 7, 170–184 (1997).
Gibbon, J., Church, R. M. & Meck, W. M. Scalar timing in memory. Ann. N. Y. Acad. Sci. 423, 52–77 (1984).
Allman, M. J., Teki, S., Griffiths, T. D. & Meck, W. H. Properties of the internal clock: First- and second-order principles of subjective time. Ann. Rev. Psychol. 65, 743–771. https://doi.org/10.1146/annurev-psych-010213-115117 (2014).
Kononowicz, T. W. & van Wassenhove, V. In search of oscillatory traces of the internal clock. Front Psychol. 7, 224 (2016).
Hass, J. & Durstewitz, D. Time at the center, or time at the side? Assessing current models of time perception. Curr. Opin. Behav. Neurosci. 8, 238–244. https://doi.org/10.1016/j.cobeha.2016.02.030 (2016).
Matell, M. S. & Meck, W. H. Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Cognit. Brain Res. 21, 139–1700. https://doi.org/10.1016/j.cogbrainres.2004.06.012 (2004).
Buhusi, C. V. & Meck, W. H. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765. https://doi.org/10.1038/nrn1764 (2005).
Agostino, P. V. & Cheng, R. K. Contributions of dopaminergic signaling to timing accuracy and precision. Curr. Opin. Behav. Neurosci. 8, 153–160. https://doi.org/10.1016/j.cobeha.2016.02.013 (2016).
Salet, J. M., Kruijne, W., van Rijn, H., Los, S. A. & Meeter, M. f MTP: A unifying computational framework of temporal preparation across time scales. Psychol. Rev. 129, 911–948 (2022).
Schacter, D. L. & Addis, D. R. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos. Trans. R. Soc. B Biol. Sci. 362, 773–786 (2007).
D’Argembeau, A., Ortoleva, C., Jumentier, S. & Van Der Linden, M. Component processes underlying future thinking. Mem. Cognit. 38, 809–819 (2010).
Schacter, D. L., Devitt, A. L. & Addis, D. R. Episodic future thinking and cognitive aging. In Oxford Research Encyclopedia of Psychology (Oxford University Press, 2018). https://doi.org/10.1093/acrefore/9780190236557.013.380.
Zakay, D. Psychological time as information: The case of boredom. Front Psychol. 5, 917 (2014).
Benau, E. M., DeLoretta, L. C. & Moelter, S. T. The time is “right:” Electrophysiology reveals right parietal electrode dominance in time perception. Brain Cognit. 123, 92–102 (2018).
Ratcliffe, M. Varieties of temporal experience in depression. J. Med. Philos. (U. K.) 37, 114–138 (2012).
Bech, P. Depression: Influence on time estimation and time experience. Acta Psychiatr. Scand. 51, 42–50 (1975).
Belujon, P. & Grace, A. A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychofarmacol. 20, 1036–1046. https://doi.org/10.1093/ijnp/pyx056 (2017).
Perini, G. et al. Cognitive impairment in depression: Recent advances and novel treatments. Neuropsychiatr. Dis. Treat. 15, 1249–1258 (2019).
Kent, L., Van Doorn, G. H., Hohwy, J. & Klein, B. Time perception and depression symptoms: A perceived delay cues feelings of hopelessness. https://doi.org/10.31234/osf.io/b5dkv90. (2020).
Wild-Wall, N., Willemssen, R., Falkenstein, M. & Beste, C. Time estimation in healthy ageing and neurodegenerative basal ganglia disorders. Neurosci. Lett. 442, 34–38 (2008).
Furman, D. J., Hamilton, J. P. & Gotlib, I. H. Frontostriatal functional connectivity in major depressive disorder. Biol. Mood Anxiety Disord. 1, 11 (2011).
Kapitány-Fövény, M., Bokk, O., Kiss, A. & Sulyok, M. Time perception at resting state and during active motion: The role of anxiety and depression. J. Psychiatr. Res. 155, 186–193 (2022).
Acknowledgements
Authors thank Maximilien Chaumon, Virginie Van Wassenhove, and all collaborators of the Blursday project.
Author information
Authors and Affiliations
Contributions
G.B. extracted, analyzed the data and wrote the paper. T.H. designed the research, supervised the project, and wrote the paper. A.A. and F.E. helped analyze the data and write the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buzi, G., Eustache, F., D’Argembeau, A. et al. The role of depressive symptoms in the interplay between aging and temporal processing. Sci Rep 13, 11375 (2023). https://doi.org/10.1038/s41598-023-38500-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38500-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.