Abstract
The present work shows the possibility of use of gallic acid (GA) and methyl gallate (MG) as natural antioxidants replacing the powerful synthetic antioxidant TBHQ in frying process. Oxidative stability index (OSI) and the kinetics of change in lipid-peroxidation conjugated dienes (LCD), carbonyls (LCO), and acid value were adopted for the evaluation purposes. GA alone (1.2 mM) and in combination with MG (75:25) provided OSI values comparable to that of TBHQ (18.5–19.0 h). The GA/MG 75:25 exerted a frying performance quite better than TBHQ (rn = 0.1351 vs. 0.1784 h−1) in preventing the LCD formation. From the LCO formation standpoint, the GA/MG 75:25 (rn = 0.0758 h−1) and then MG (rn = 0.1004 h−1) provided better performances than TBHQ (rn = 0.1216 h−1). Lipid hydrolysis was also inhibited well by GA (AVm = 8.6) and GA/MG 75:25 (AVm = 7.9), respectively (AVm = 9.2 for TBHQ).
Similar content being viewed by others
Introduction
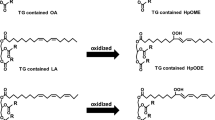
Frying of foods in an edible oil exposed to high temperatures (170–200 °C) for a long while has always been accompanied by serious sensory and nutritional concerns related to the probable oxidative and hydrolytic deteriorations. The total content of lipid-peroxidation polar compounds is the most well-known analytical measure to evaluate the health of used frying oils1. However, this measure has been shown to correlate well with a simpler, quicker, and less expensive measure to determine, namely the total content of lipid-peroxidation conjugated dienes (LCD)2. LCD are basically a range of the primary oxidation products resulting from the double bond shifts in polyunsaturated fatty acids3. Their content at the initial stage of the reaction is roughly the same as that of the total conjugated and non-conjugated hydroperoxides. After prolonged frying times, however, the total LCD content becomes smaller than the total content of the conjugated and non-conjugated isomers, due to the secondary LCD oxidation leading to the loss of conjugation4. Moreover, the total LCD content reaches a plateau resulting from the Diels–Alder reaction (Fig. 1), which is a dimerization between a conjugated di-olefin and a mono-olefin group to form a cyclohexene ring5. Such a pattern is in line with the kinetic model of the accumulation of lipid hydroperoxides developed recently by the author (see the section “Kinetic data analyses”)6,7.
From a sensory as well as a nutritional point of view, the total content of lipid-peroxidation carbonyls (LCO), comprising a numerous variety of volatile and non-volatile secondary oxidation products, has been considered as a valuable quantitative measure to evaluate the quality of used frying oils8. A sigmoidal pattern has frequently been observed for the change in the total LCO content during frying, namely an initial slow increasing phase followed by a rapid rising phase terminated to a maximum value. Afterwards, its level show constant or reduced amounts8,9 due basically to the further degradations of the primary carbonyls to non-carbonyls and/or more volatile products8,9,10.
Acid value (AV) is the other well-known frying measure to detect the progressive hydrolysis of triacylglycerols to free fatty acids and glycerol11. Lower levels of AV make frying oils less prone to the strong off-flavors caused by the degradation volatile and/or non-volatile products arising from the free fatty acids, which are inherently of higher oxidative reactivity than the parent triacylglycerols12. Furthermore, the quite toxic component acrolein (an oral LD50 in rats of only 46 mg/kg of body weight) with a very pungent, irritating smell13 is produced from the dehydration of glycerol easily at temperatures as low as 180 °C14 and more quickly at higher temperatures reaching the smoke point at which an oil starts to smoke13. Acrolein is so volatile (boiling point 52 °C) and does not significantly remain in frying oil13 but its trace amounts clearly appear as the blue haze above the smoking oil14.
Incorporating antioxidants into the matrix of frying oils has frequently been adopted as a major treatment to protect them from the deteriorative reactions of oxidative and/or hydrolytic natures. Synthetic antioxidants, including butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG), and tert-butylhydroquinone (TBHQ), have often been used to enhance the frying performance of edible oils15. TBHQ has also been recognized to be the most powerful one employed widely in the food industry as well as highly resistant to thermal decomposition and/or volatilization16. Nevertheless, synthetic antioxidants have been questioned due to their probable contribution to increasing health risks such as cancer and carcinogenesis17. Addition of natural antioxidants, hence, has always been considered to be a safer and consumer-friendly way to improve the frying performance of edible oils.
The two natural phenolic compounds gallic acid (3,4,5-trihydroxybenzoic acid, GA) and methyl gallate (MG) have fully been recognized due to many of their valuable biological effects18,19. A limited number of studies in recent years have indicated the powerful antioxidant activity of GA and MG in some storage and rather harsh conditions20,21,22,23. However, there is no analytical data on their protective effect under frying processes. Hence, this study aimed to investigate the kinetics of oxidative and hydrolytic deteriorations over a frying process protected by GA and MG compared with the powerful synthetic antioxidant TBHQ.
Materials and methods
Materials
Refined, bleached, and deodorized sunflower and palm olein oils with no added antioxidants were supplied by Segol factory in Nishabour, Iran. The oil samples were stored at − 18 °C until analysis. Agria potato variety was purchased from the farmers in Fariman, Iran. All the chemicals and solvents used in the study were of analytical reagent grade and purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (St. Louis, MO, USA).
Deep-fat frying procedure
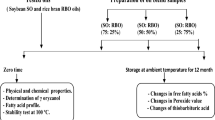
Peeled and cut (4.0 × 0.5 × 0.5 cm) potatoes were submerged in water (25 °C) until frying. After rinsing with cold water and drying by a fan as well as a clean towel, they (30 g) were fried (180 °C) in 1 L of the oil treatments (sunflower/palm olein 65:35, as control oil, containing a total concentration of 1.2 mM of TBHQ, GA, MG, or GA/MG in the ratios of 75:25, 50:50, and 25:75) with no replenishment by a bench-top fryer (Hamilton DF-535T, PRC). The potato pieces were fried at 5-min intervals for 8 h. At 1 h intervals, 45 g of the oils were filtered into a screw-cap vial and immediately stored in the dark at − 3 °C until analysis. Frying processes were carried out in four replications8.
Chemical properties of the frying oil
To determine fatty acid composition, fatty acid methyl esters (FAME) were prepared by shaking a solution of the oil sample in hexane (0.3 g in 7 mL) with 7 mL of 2 N methanolic KOH at 50–55 °C for 15 min. After settling the solution for 5 min, the upper layer was mixed with anhydrous Na2SO4 and then filtered. FAME were injected into a gas–liquid chromatograph (Hewlett-Packard, Santa Clarita CA, USA) equipped with a FID and a BPX 70 capillary column (60 m × 0.22 mm I.D., 0.2 mm film thickness), using He as the carrier gas at a flow rate of 0.7 mL min−1. The oven temperature was maintained at 198 °C and those of the injector and detector at 280 °C and 250 °C, respectively. The analysis was carried out in duplicate and data was reported as relative area percentages24.
Peroxide value (PV), expressed as milliequivalents of O2 per kilogram of the oil sample, was measured according to the thiocyanate method described by Shantha and Decker25. Total tocopherols (TT) content was measured according to the colorimetric method described by Wong et al.26. A calibration curve of α-tocopherol in toluene was prepared in a concentration range of 0–240 μg mL−1. TT content was reported as milligrams of α-tocopherol per kilogram of the oil sample. Total phenolics (TP) content was measured spectrophotometrically using Folin–Ciocalteau’s reagent as described by Capannesi et al.27. A calibration curve of GA in CH3OH was prepared in a concentration range of 0.04–0.40 mg mL−1. TP content was reported as milligrams of GA per kilogram of the oil sample.
Oxidative stability index (OSI)
A Metrohm Rancimat model 743 (Herisau, Switzerland) was used to measure OSI (h). The tests were carried out with 3 g of the oil samples at 110 °C and an airflow rate28 of 15 L h−1.
Total LCD content
The oil samples were dissolved in hexane (1:600) and their absorbance were read at 234 nm against HPLC grade hexane as blank. An extinction coefficient of 29,000 mol/L was used to calculate millimoles of LCD per liter29.
Total LCO content
The content of total LCO was measured according to the method developed by Endo et al.30 using 2-propanol and 2,4-decadienal as solvent and standard, respectively. Total LCO content was reported as micromoles of 2,4-decadienal per gram of the oil sample.
Acid value (AV)
Ten grams of the oil samples were dissolved in 50 ml of chloroform-ethanol (50:50 v/v) and titrated with 0.1 N ethanolic KOH. AV was the milligrams of KOH required to neutralize the free fatty acids in 1 g of the oil sample31.
Kinetic data analyses
Kinetic curves of LCD accumulation were drawn by plotting the changes in the total content of LCD (mM) versus time t (h). The sigmoidal Eq. (1) was fitted on the kinetic data points of LCD accumulation6,7:
where a, b, and c are the equation parameters. The finite value LCDmax is calculated from the ratio a/b. The maximum rate of LCD accumulation (rmax, mM h−1) equals 0.25a2/b. Its normalized form (rmax/LCDmax = rn, h−1) is given by 0.25a. The parameter b represents the pseudo-second order rate constant kd (mM−1 h−1) for the LCD lost through chemical decomposition or the Diels–Alder reaction. The time at which the total content of LCD practically approaches LCDmax (tmax, h) is obtained from the ratio (2 + ac − lnb)/a.
The method developed recently by the author was employed to calculate the LCO-based kinetic parameters32. Kinetic curves of LCO (μmol g−1) accumulation were drawn by plotting the changes in the total content of carbonyls versus time t (h). The sigmoidal Eq. (2) was fitted on the kinetic data points of LCO accumulation:
where a, b, c, and d are the equation parameters. The finite value LCOmax, where the rate of LCO accumulation reaches zero at infinity, equals a + b. At the equation’s turning point with the coordinates tT = c and LCOT = a + 0.5b, the rate of LCO accumulation (μmol g−1 h−1) reaches the maximum value rmax = 0.25b/d. Its normalized form (rmax/LCOmax = rn, h−1) is calculated from the ratio b/4d(a + b). The time at which carbonyls practically approach LCOmax (tmax, h) is obtained from c + 2d.
Kinetic curves of organic acids (mg g−1) accumulation were drawn by plotting the changes in AV versus time t (h). The power Eq. (3) was fitted on the kinetic data points:
where a, b, and c are the equation parameters. From a mathematical point of view, the ratio 1/bc (AVm), which quantitatively represents the change pattern of organic acids over time, was used as an empirical measure of hydrolytic stability of the frying system.
Statistical analysis
All determinations were carried out in triplicate and data were subjected to analysis of variance (ANOVA). ANOVA and regression analyses were performed according to the MStatC (developed by the Department of Plant Science and Soil of Michigan State University, USA) and SlideWrite version 7.0 (Advanced Graphics Software, Inc., Toronto, Canada), respectively. Significant differences between means were determined by Duncan’s multiple range tests. P values less than 0.05 were considered statistically significant.
Results and discussion
Chemical composition of the oil samples
Table 1 shows the fatty acid composition (Fig. 2), the initial quality indicators (PV and AV), and the TT and TP contents of the oils studied. The oil samples had the fatty acid compositions in agreement with those usually reported in literature. They could be differentiated from each other by mainly the contents of palmitic (C16:0), oleic (C18:1), and linoleic (C18:2) acids. Due to the considerably higher levels of C16:0 (38.9 vs. 6.95%) and C18:1 (42.0 vs. 25.5%), the palm olein oil was of about 3.7- and 1.6-fold contents of saturated (SFA) and monounsaturated (MUFA) fatty acids, respectively, compared with the sunflower oil. In contrast, the sunflower oil possessed a polyunsaturated fatty acids (PUFA, mainly C18:2: 61.8 vs. 12.2%) content of almost five times that of the palm olein oil. This naturally makes the sunflower oil a rather unstable frying medium with respect to the relative rate of oxidation for C18:3, C18:2, C18:1, and C18:0 as 2500:1200:100:133. Therefore, a blend of the sunflower and palm olein oils (65:35), indicating better qualitative parameters than each of the oils alone over frying in overall34, was used to evaluate the frying performance of the antioxidant treatments.
The PV and AV of the oils and their blend were all less than 2 meq kg−1 and 0.3 mg g−1, respectively, reflecting that they were unoxidized and of high initial quality (Table 1). The indigenous tocopherols and phenolic compounds, which are basically recognized for their biologically useful effects as well as antioxidant activities, were within their normal quantitative range in the corresponding refined, bleached, and deodorized oils35.
OSI values
The control oil showed an OSI value of 11.4 h (Fig. 3), indicating its time resistance to the dramatically increased formation of some volatile acids (mostly formic acids, with lesser amounts of acetic, propionic, and other acids) as secondary oxidation products at thermally (~ 110–130 °C) and oxidatively (bubbling air into the oil) harsh conditions36. Interestingly, GA provided an OSI value comparable to that of the powerful synthetic antioxidant TBHQ (~ 18.5 h). However, its methyl ester derivative MG was not able to significantly stabilize the control oil. Replacing 25 percent of the GA concentration with MG insignificantly increased the value of OSI to 19.0 h, although the higher MG percentages led to the reduced OSI values 15.9 and 13.7 h for the GA/MG combinations 50:50 and 25:75, respectively.
Oxidative stability index (OSI, h) of the control oil (sunflower/palm olein 65:35) in the presence of 1.2 mM of tert-butylhydroquinone (TBHQ) or gallic acid (GA)/methyl gallate (MG) combinations. Means ± SD (standard deviation) with the same lowercase letters are not significantly different at p < 0.05.
Kinetics of change in the total LCD and LCO contents and AV
The sigmoidal (1 and 2) and power (3) equations fitted very well (R2 > 0.97) the changes in the total LCD and LCO contents and AV, respectively, over the frying process at 180 °C (Fig. 4A–C). The kinetic data resulted from the corresponding accumulation curves are shown in Tables 2, 3, and Fig. 5, respectively.
Kinetic curves of the accumulation of lipid-peroxidation conjugated dienes (LCD, A), carbonyls (LCO, B), and organic acids (AV, C) during the frying of the control oil (sunflower/palm olein 65:35) containing 1.2 mM of gallic acid/methyl gallate (50:50) at 180 °C, and the kinetic parameters from the Eqs. (1)–(3) fitted on the whole range of the data points. LCOT/LCDT: total LCO/LCD contents at the turning point of the sigmoidal equations with the x-coordinates tT; LCOmax/LCDmax: the maximum total LCO/LCD contents attained at the values of tmax; rmax: maximum rate of LCO/LCD accumulations; AVm: the quantitative measure of hydrolytic stability of the frying system.
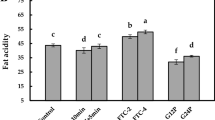
The quantitative measure of hydrolytic stability (AVm) resulted from the accumulation curve of lipid-peroxidation organic acids over the frying of the control oil (sunflower/palm olein 65:35) at 180 °C in the presence of 1.2 mM of tert-butylhydroquinone (TBHQ) or gallic acid (GA)/methyl gallate (MG) combinations. Means ± SD (standard deviation) with the same lowercase letters are not significantly different at p < 0.05.
The control oil was of the significantly highest value of LCDmax (Table 2), which the level of that is expected to be affected by the rates of LCD formation (represented in overall by the value of rmax) and LCD loss through chemical decompositions and/or the Diels–Alder reaction (represented in overall by the value of kd). The maximum level of total conjugated and non-conjugated lipid hydroperoxides has been shown to correlate very well with the rmax/kd ratio7. The powerful synthetic antioxidant TBHQ significantly decreased the value of LCDmax, being consistent with the remarkably reduced rmax value. There was no significant difference between the LCDmax value of TBHQ and those of all the GA/MG combinations. However, GA and its combinations with MG provided the significantly lower values of rmax compared with TBHQ. The value of rn, which unifies the two kinetic parameters, provided a better oxidative measure to compare the frying performance of the antioxidants. On this basis, as shown in Table 2, all the GA/MG combinations with a better synergistic effect observed for the GA/MG 75:25 treatment demonstrated frying performances significantly higher than that found for TBHQ. Moreover, the value of tmax as a time parameter indicating the resistance against the formation of conjugated dienes revealed the best frying performance for the GA/MG 75:25 treatment as well.
The kinetics of change in carbonyls were found to be similar, to a large extent, to those in conjugated dienes in the presence of the antioxidants. As shown in Table 3, the significantly highest LCOmax value of the control oil was almost halved by TBHQ, which was in line with the corresponding reduced value of rmax. However, the GA/MG 75:25 treatment and then MG alone provided better performances than TBHQ against the progressive generation of carbonyls over the frying process. This can clearly be seen in their significantly lower and higher values of rn and tmax, respectively. Considering roughly the same rmax values, the better performances of the formers could be explained by their lower degradation rates of primary carbonyls to the fragments of lower molecular weight, escaping more easily from the system, or to non-carbonyls undetectable by the LCO assay8,9,10.
Figure 5 exhibits the empirical measure of hydrolytic stabilities AVm for the control oil as affected by the antioxidant treatments. TBHQ and GA with no statistically significant difference were the most efficient antioxidants to prevent hydrolysis of the frying medium and, as a result, the following nutritional and toxicological degradations12,13. Although MG significantly improved the hydrolytic resistance of the control oil, it did not exert the same anti-hydrolysis activity as GA did. Besides, increasing its contribution to the GA/MG treatments decreased the ability of GA in protecting the frying medium against hydrolysis.
Conclusions
The present study was the first attempt to kinetically evaluate frying performance of the two well-known natural antioxidants gallic acid (GA) and methyl gallate (MG). Given the inhibition of the primary and secondary oxidation products during the frying process, GA alone and in combination with its methyl ester (GA/MG 75:25) exerted the same or even better frying performances compared with the powerful synthetic antioxidant TBHQ. Lipid hydrolysis was also inhibited very well by the natural antioxidants. This enables edible oil industry to supply healthy frying oils of reduced risk due to replacing TBHQ with the natural ones, although some complimentary studies in a number of different frying media used to fry some other foodstuffs could be helpful for a better assessment of the efficiency of GA and MG.
Data availability
All data generated or analysed during this study are included in this published article.
References
Pantzaris, T. P. Comparison of monounsaturated and polyunsaturated oils in continuous frying. Gras. Aceit. 49, 319–352 (1998).
Farhoosh, R. & Moosavi, S. M. R. Evaluating the performance of peroxide and conjugated diene values in monitoring quality of used frying oils. J. Agric. Sci. Technol. 11, 173–179 (2009).
Houhoula, D. P., Oreopoulou, V. & Tzai, C. A kinetic study of oil deterioration during frying and a comparison with heating. J. Am. Oil Chem. Soc. 79, 133–137 (2002).
Fukuzumi, S., Tanii, K. & Tanaka, T. Peroxidation of polyunsaturated fatty acids, initiated by the direct interaction between a cobalt porphyrin complex and polyunsaturated fatty acids. J. Chem. Soc. Perkin Trans. 12, 2035–2039 (1989).
White, P. J. Methods for measuring changes in deep-fat frying oils. Food Technol. 45, 75–80 (1991).
Farhoosh, R. Reliable determination of the induction period and critical reverse micelle concentration of lipid hydroperoxides exploiting a model composed of pseudo-first and -second order reaction kinetics. LWT Food Sci. Technol. 98, 406–410 (2018).
Farhoosh, R. A reconsidered approach providing kinetic parameters and rate constants to analyze the oxidative stability of bulk lipid systems. Food Chem. 327, 127088 (2020).
Farhoosh, R. & Moosavi, S. M. R. Carbonyl value in monitoring of the quality of used frying oils quality. Anal. Chim. Acta 617, 18–21 (2008).
Damanik, M. & Murkovic, M. Formation of potentially toxic carbonyls during oxidation of triolein in the presence of alimentary antioxidants. Monatsh. Chem. 148, 2031–2035 (2017).
Frankel, E. N. Hydroperoxide decomposition. In Lipid Oxidation (ed. Frankel, E. N.) 67–98 (Woodhead Publishing Limited, 2012).
Choe, E. & Min, D. B. Chemistry of deep-fat frying oils. J. Food Sci. 72, R77–R86 (2007).
Frega, N., Mozzon, M. & Lercker, G. Effect of free fatty acids on the oxidative stability of vegetable oils. J. Am. Oil Chem. Soc. 76, 325–329 (1999).
Crosby, G. Do cooking oils present a health risk?. Food Technol. 72, 50–57 (2018).
Katragadda, H. R., Fullana, A., Sidhu, S. & Carbonell-Barrachina, A. A. Emissions of volatile aldehydes from heated cooking oils. Food Chem. 120, 59–65 (2010).
Chen, J. et al. The formation, determination and health implications of polar compounds in edible oils: Current status, challenges and perspectives. Food Chem. 364, 130451 (2021).
Ying, Z. et al. Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem. 118, 656–662 (2010).
Olajide, T. M., Liu, T., Liu, H. & Weng, X. Antioxidant properties of two novel lipophilic derivatives of hydroxytyrosol. Food Chem. 315, 126197 (2020).
Rahman, N., Jeon, M. & Kim, Y. S. Methyl gallate, a potent antioxidant inhibits mouse and human adipocyte differentiation and oxidative stress in adipocytes through impairment of mitotic clonal expansion. BioFactors 42, 716–726 (2016).
Rajan, V. K. & Muraleedharan, K. A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, gallic acid. Food Chem. 220, 93–99 (2017).
Farhoosh, R., Sharif, A., Asnaashari, M., Johnny, S. & Molaahmadibahraseman, N. Temperature-dependent mechanism of antioxidant activity of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid in fish oil. J. Am. Oil Chem. Soc. 93, 555–567 (2016).
Farhoosh, R. & Nystrom, L. Antioxidant potency of gallic acid, methyl gallate and their combinations in sunflower oil triacylglycerols at high temperature. Food Chem. 244, 29–35 (2018).
Mansouri, H., Farhoosh, R. & Rezaie, M. Interfacial performance of gallic acid and methyl gallate accompanied by lecithin in inhibiting bulk phase oil peroxidation. Food Chem. 328, 127128 (2020).
Shojaee, M. S., Moeenfard, M. & Farhoosh, R. Kinetics and stoichiometry of gallic acid and methyl gallate in scavenging DPPH radical as affected by the reaction solvent. Sci. Rep. 12, 8765 (2022).
Farhoosh, R., Niazmand, R., Rezaei, M. & Sarabi, M. Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur. J. Lipid Sci. Technol. 110, 587–592 (2008).
Shantha, N. C. & Decker, E. A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 77, 421–424 (1994).
Wong, M. L., Timms, R. E. & Goh, E. M. Colorimetric determination of total tocopherols in palm oil, olein and stearin. J. Am. Oil Chem. Soc. 65, 258–261 (1988).
Capannesi, C., Palchetti, I., Mascini, M. & Parenti, A. Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem. 71, 553–562 (2000).
Farhoosh, R. & Moosavi, S. M. R. Rancimat test for the assessment of used frying oils quality. J. Food Lipids 14, 263–271 (2007).
Saguy, I. S., Shani, A., Weinberg, P. & Garti, N. Utilization of jojoba oil for deep-fat frying of foods. Leb. Wiss. u.-Technol. 29, 573–577 (1996).
Endo, Y., Li, C. M., Tagiri-Endo, M. & Fugimoto, K. A modified method for the estimation of total carbonyl compounds in heated and frying oils using 2-propanol as a solvent. J. Am. Oil Chem. Soc. 10, 1021–1024 (2001).
AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society (AOCS Press, 1993).
Farhoosh, R. Quantitative criteria characterizing the time change pattern of total lipid-peroxidation carbonyls. Sci. Rep. 12, 22345 (2022).
Hsieh, R. J. & Kinsella, J. E. Oxidation of polyunsaturated fatty acids: Mechanisms, products, and inhibition with emphasis on fish. Adv. Food Nutr. Res. 33, 233–341 (1989).
De Marco, E. et al. Frying performance of a sunflower/palm oil blend in comparison with pure palm oil. Eur. J. Lipid Sci. Technol. 109, 237–246 (2007).
Gunstone, F. D. Vegetable sources of lipids. In Modifying Lipids for Use in Food (ed. Gunstone, F. D.) 11–27 (Woodhead Publishing Limited, 2006).
Shahidi, F. & Zhong, Y. In Edible Oil and fat Products, Chemistry, Properties and Health Effects, Lipid Oxidation: Measurement Methods: Bailey’s Industrial Oil and Fat Products (ed. Shahidi, F.) 370–371 (Wiley, 2005).
Author information
Authors and Affiliations
Contributions
M.H. performed the experiments. R.F. designed research, interpreted results, and drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hosseinkhani, M., Farhoosh, R. Kinetics of chemical deteriorations over the frying protected by gallic acid and methyl gallate. Sci Rep 13, 11059 (2023). https://doi.org/10.1038/s41598-023-38385-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38385-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.