Abstract
Prognostic value of hematologic indices and their association with the tumor microenvironment (TME) remain unclear in advanced soft tissue sarcoma (STS). We aimed to evaluate their prognostic value and correlation with the TME status in advanced STS treated with first-line doxorubicin (DXR) therapy. Clinical data and three hematological indices, including lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio, and neutrophil-to-lymphocyte ratio, were collected from 149 patients with advanced STS. The TME status was pathologically examined by CD3, CD68, and CD20 staining of resected tumor slides. In a multivariate Cox analysis, low LMR and absence of primary tumor resection were independently associated with worse overall survival (OS) (HR 3.93, p = 0.001; HR 1.71, p = 0.03). A prognostic model using these variables predicted OS with greater area under curves than those obtained using Systemic Inflammatory Score and Glasgow Prognostic Score. The LMR significantly correlated with the tumoral CD3/CD68-positive cell ratio in surgical specimens (R = 0.959, p = 0.04). In conclusion, LMR was a prognostic factor in advanced STS treated with first-line DXR therapy. LMR could partially reflect anti-tumor immunity in the TME and have the prognostic value. The potential role of LMR as an indicator of TME status warrants further investigation.
Similar content being viewed by others
Introduction
Soft tissue sarcoma (STS) is a rare, heterogeneous tumor of mesenchymal origin with an incidence of approximately 1% in adults. There are at least 100 different histological and molecular subtypes of STS that show variable clinical behaviors1. Except for subtypes with unique histological presentations, such as Ewing sarcoma or rhabdomyosarcoma that often affect children, treatment has not been individualized for most subtypes of STS. Despite advances in multidisciplinary treatment, the 5-year survival rate of patients with STS remains approximately 60%2,3. Furthermore, 25% patients develop distant metastasis after curative resection of the primary tumor1. Unfortunately, the prognosis of patients with metastasis is poor, with a 5-year survival rate of only 15%3. The standard first-line systemic therapy for advanced STS includes doxorubicin (DXR) monotherapy4,5. Phase 3 trials have shown a median progression-free survival (PFS) of 4–7 months and a response rate of 15–18% for patients treated with first-line DXR monotherapy6,7.
Prognostication of advanced STS is challenging. Patients treated with DXR therapy possess significantly different overall survivals (OS) with an interquartile range of 19.7 months (9.9–35.5 months), potentially due to the heterogeneity of STS7. Active agents beyond DXR with the capacity to treat certain histological subtypes are being developed8,9,10,11. Specifying accurate prognostic markers can help to predict patient outcomes, and, thus, facilitates individual treatment options. However, the current TNM staging system classifies STS according to tumor size, depth, nodal involvement, distant metastases, and malignancy grade12, and does not consider the histological diversity of STS13,14. Furthermore, this staging system is based on the outcomes of patients post-surgery and does not necessarily predict the survival of those treated with chemotherapy instead. An improvement in prognostic classification is warranted for monitoring patients with STS15.
As cancer alters immune system function16, immune cell counts can be used to predict patient outcomes. Certain hematological indices, such as the lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR), are derived from differential white blood cell (WBC) counts and reflect systemic immunity. The prognostic role of these indices has been examined in patients with STS pre-surgery as well as during a variety of treatments17,18,19,20,21,22,23,24,25,26,27,28,29,30,31; however, these studies have rarely examined the indices of patients prior to chemotherapy. Therefore, the performance of current prognostic models based on these studies may not be representative of patient outcomes after chemotherapy. Despite the prevalence of advanced STS1, which is often treated with chemotherapy, a limited number of studies have addressed the role of hematological indices in the prognosis of this advanced disease22,24,26,29. It is hypothesized that analyzing the association between the indices measured before chemotherapy and patient outcomes of those treated with a single first-line chemotherapy could yield a prognostic model that is more robust and applicable to these patients.
Evaluating immune cells in the tumor microenvironment (TME) can also be used to estimate the outcome of cancer patients. The presence of tumor-infiltrating lymphocytes (TILs) or tumor-associated macrophages (TAMs) in the TME impacts the prognosis of patients32,33,34,35,36,37,38,39. In patients with hepatocellular carcinoma undergoing liver transplantation, the blood LMR correlated with the CD3-positive to CD68-positive cell ratio (potentially TILs to TAMs ratio) in the resected specimens40. Moreover, NLR inversely correlated with the density of CD8-positive TILs in lung cancer41. These findings suggest that anti-tumor immunity in the TME could be noninvasively monitored through these hematological indices.
In this study, we primarily determined which of the indices (LMR, NLR, and PLR) was most associated with increased survival in patients with advanced STS treated with first-line DXR therapy, and secondarily explored the relationship between the prognostic index and the histopathological TME status.
Results
Patient characteristics
Between August 2009 and December 2018, 153 patients were treated with first-line DXR at the National Cancer Center Hospital. Since four patients with missing differential WBC counts were excluded, 149 patients were enrolled in the study. Among these patients, 145 experienced disease progression (PD) before the day of data cut-off.
The patients’ characteristics are summarized in Table 1. Eleven patients (7.4%) had Eastern Cooperative Oncology Group performance status (PS) of 2 or 3. The primary tumor was resected and recurred in 108 (72.5%) patients. Tumors were classified as abdominal or thoracic visceral in 74 patients (49.7%) according to the American Joint Committee on Cancer (AJCC) staging system 8th edition12. Leiomyosarcoma and liposarcoma affected 57 (38.3%) and 31 (20.8%) patients, respectively.
Variation of the hematological indices
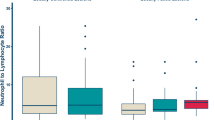
Interpatient variation in the hematological indices among the selected subgroups is depicted in Fig. 1. Patients who relapsed after primary tumor resection had a significantly higher baseline LMR (4.47 vs. 3.27, p = 0.004) and lower baseline NLR (4.00 vs. 5.15, p = 0.001) than those who did not receive surgery. Patients with PS of 0 or 1 had higher LMR (4.29 vs. 2.45, p = 0.002), lower NLR (4.15 vs. 6.27, p = 0.0003), and lower PLR (245.3 vs. 350.2, p = 0.044) than patients with higher PS. The pairwise correlations between each of the variables are shown in Supplementary Fig. 1. Notably, LMR negatively correlated with PS (ρ = − 0.43), NLR (− 0.71), PLR (− 0.61), lactose dehydrogenase (LDH) (− 0.28), and C-reactive protein (CRP) (− 0.65) values, and positively correlated with albumin (0.57) levels.
Variation in hematological indices among selected patient subgroups. Box plots showing indices according to age, histology, tumor site, presence of primary tumor resection, PS, and presence of metastasis. Each point on the scatter plot represents an individual patient within a specified subgroup. The overlaid box plot presents the median and interquartile range of the indices for all patients. DDLPS, dedifferentiated liposarcoma; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PS, performance status; UPS, undifferentiated polymorphic sarcoma. *p < 0.05; **p < 0.01; ***p < 0.001.
Prognostic value of LMR for overall survival
The optimal cutoff values for the hematological indices were determined using receiver operating characteristic (ROC) analyses. Based on Youden’s index, the cutoff values for the LMR, NLR, and PLR in predicting OS were 2.1, 5.5, and 264.0, respectively. To estimate PFS, the cutoff values for the LMR, NLR, and PLR were set to 3.3, 4.3, and 239.0, respectively.
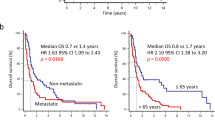
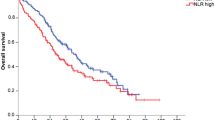
In the Cox regression model, univariate analysis showed that low LMR (hazard ratio [HR] 6.17, p < 0.0001), high NLR (HR 2.68, p = 0.0001), and high PLR (HR 2.92, p < 0.0001) were associated with worse OS. Furthermore, lower OS was also associated with a PS ≥ 2 (HR 2.42, p = 0.019), absence of primary tumor resection (HR 2.21, p = 0.0008), decreased albumin level (HR 1.98, p = 0.001), and elevated LDH (HR 2.07, p = 0.002) and CRP (HR 2.39, p < 0.0001) levels (Table 2). Multivariate analysis indicated that low LMR (HR 3.93, p = 0.001) and absence of primary tumor resection (HR 1.71, p = 0.034) were independent risk factors for OS. In the Kaplan–Meier analysis, the median OS for the overall study population was 24.3 months (Supplementary Fig. 2A), and patients with a low LMR (8.6 vs. 27.9 months, p < 0.0001) (Fig. 2A), a high NLR (8.9 vs. 27.9 months, p < 0.0001), and a high PLR (10.1 vs. 32.3 months, p < 0.0001) had shorter OS than their respective opposing groups (Supplementary Fig. 2B).
OS stratified by LMR (A) and the LMR prognostic score (B). (C) The prognostic potential of the LMR prognostic score was compared with that of SIS or GPS in ROC analyses for OS at 1 year (left), 2 years (middle), and 3 years (right). AUC, area under curve; CI, confidence interval; DXR, doxorubicin; GPS, Glasgow prognostic score; LMR, lymphocyte-to-monocyte ratio; OS, overall survival; SIS, Systemic Inflammatory Score.
Development of LMR prognostic score
Based on the results of the multivariate analysis, we developed a prognostic scoring model utilizing LMR and a history of primary tumor resection (Table 3). Patients with an LMR of less than 2.1 or those who did not undergo primary tumor resection had a partial score of 1, which yielded a total score ranging from 0 to 2. As shown in Table 4, and in common with SIS and GPS, the LMR-based model was associated with worse OS (score of 1, HR 2.26, p = 0.004; score of 2, HR 8.09, p < 0.0001). Kaplan–Meier analysis showed that compared with patients with a prognostic score of 0, those with scores of 1 and 2 had shorter OS (median 32.6, 12.0, and 8.6 months, p < 0.0001) (Fig. 2B). ROC analysis showed that the area under curves (AUCs) for LMR prognostic score were 0.711 (95% CI, 0.624 to 0.798), 0.712 (0.636 to 0.788), and 0.728 (0.677–0.79) for 1-year, 2-year, and 3-year survival, respectively (Fig. 2C). To internally validate the LMR-based scoring model, we performed a resampling analysis using the bootstrap method (that is, the model was resampled 1000 times) and found minimal overfitting (mean values were overoptimistic by 0.0005 for 1-year survival, 0.0002 for 2-year survival, and 0.0004 for 3-year survival). Although there was no significant statistical difference, the LMR-based scoring model showed larger AUCs than those of Systemic Inflammation Score (SIS)42 or Glasgow Prognostic Score (GPS)43 across 1 year, 2-year, and 3-year survival (Supplementary Table 1), which indicates that discrimination accuracy of the LMR prognostic score is sufficient.
Predictive value of LMR for the efficacy of DXR therapy
The median PFS of patients treated with DXR therapy was 4.3 months (Supplementary Fig. 3A). Those with low LMR (3.1 vs. 5.3 months, p = 0.005), high NLR (3.3 vs. 4.9 months, p = 0.011), and high PLR (3.2 vs. 5.5 months, p = 0.004) possessed shorter PFS (Supplementary Fig. 3B). However, in the multivariate Cox regression analysis no indices were significantly associated with PFS (low LMR, HR 1.09, p = 0.721; high NLR, HR 1.06, p = 0.792; high PLR, HR 1.01, p = 0.972) (Supplementary Table 2) and the efficacy of DXR therapy (LMR, p = 0.113; NLR, p = 0.521; PLR, p = 0.507) (Supplementary Fig. 3C). Together, these data suggest a limited predictive value of the indices for the efficacy of DXR therapy.
Association between LMR and TME
To explore whether the LMR correlated with TILs status in the TME, we performed immunohistochemical (IHC) analysis for CD3, CD68, and CD20 using surgical specimens (Fig. 3A). As a pilot analysis, we selected four representative samples of leiomyosarcoma and liposarcoma from 26 cases with available surgical samples. The CD3-positive cells were more abundant in the high-LMR tumor (upper images) than in the low-LMR tumor (lower images), whereas the CD68-positive cells were less abundant in the high-LMR tumor (upper images) than in the low-LMR tumor (lower images). The correlations between the LMR and the densities of tumor-infiltrating immune cells are shown in Fig. 3B. LMR significantly correlated with the tumoral CD3/CD68 ratio (R = 0.959, p = 0.041).
(A) Immunohistochemical analysis of the selected surgical samples. The upper images show a leiomyosarcoma with a high LMR of 7.19. The HE-stained (left), CD3-positive (middle left), CD68-positive (middle right), and CD20-positive (right) cells are shown. The lower images show a dedifferentiated liposarcoma with a low LMR of 0.94. Scale bars: 250 μm. (B) Correlation between LMR and the densities of positive cells. Pearson correlation coefficients are shown. HE, hematoxylin and eosin; LMR, lymphocyte-to-monocyte ratio.
Discussion
In this study, we evaluated the roles of three hematological indices (LMR, NLR, and PLR) in the prognosis of advanced STS and identified the predictive value of the LMR measured before DXR therapy. Overall, low LMR was independently associated with shorter OS, and the LMR-based scoring model was able to discriminate high-risk patients. Furthermore, the LMR correlated with the tumoral CD3/CD68 ratio in the surgical samples, suggesting that the LMR partly reflects anti-tumor immunity governing the TME.
Staging systems, such as the AJCC TNM system, are widely used in the prognosis of patients with STS12. However, the separation of patients into accurate prognostic cohorts in this way is hindered by the histological diversity of STS44, prompting the identification of new prognostic factors outside of the nature of the tumor itself. Studies have reported that age, chemotherapeutic regimen, PS, and levels of albumin, CRP, and hemoglobin are prognostic factors for survival in patients with advanced STS45,46,47,48,49,50. Additionally, GPS, a cumulative score based on CRP and albumin levels43, was also prognostic for OS in patients with advanced STS51. While hematological indices were prognostic for STS17,18,19,20,21,22,23,24,25,26,27,28,29,30,31, most of these studies only considered resectable STS and analyzed preoperative parameters, with a focus on the NLR17,18,19,20,21,23,25,27. Finally, some studies on these prognostic factors have excluded these indices in survival analysis altogether45,46,47,48,49,50. Our study revealed the prognostic value of the LMR for patients with advanced STS treated with DXR, which is in line with previous findings that the LMR or peripheral monocyte ratio is prognostic for OS in patients with metastatic STS21,22.
In this study, the LMR level was associated with the tumoral CD3/CD68 ratio. The mechanism behind this relationship may be partly explained in terms of the TME. Accumulating evidence shows that the TME dynamically regulates tumor progression and influences therapeutic outcomes52. Some TILs function as cytotoxic T cells that suppress tumor growth, and a high density of TILs has been linked to improved clinical outcomes in solid tumors53,54,55. TAMs are partly derived from circulating monocytes and recruited to the tumor site by tumor-derived chemotactic factors. TAMs can inhibit cytotoxic T cell responses through immune checkpoint engagement, production of cytokines, metabolic activities, and modulation of the TME56. Patients with high infiltration of TAMs had worse OS57,58. The peripheral monocyte count can be representative of the TAMs because a high peripheral monocyte count is a major risk factor in patients with solid tumors59 and is associated with a high density of TAMs60. Given the opposing functions of TILs and TAMs, it is plausible that the LMR can represent the dynamic equilibrium between immune cells in the TME. The correlation between LMR and CD3/CD68 ratio has also been reported in patients with hepatocellular carcinoma40. Collectively, low LMR was associated with worse survival, possibly due to an insufficient anti-tumor immune response in the TME.
The use of DXR therapy in our cohort may be another possible reason for the prognostic role of the LMR. DXR drives immunogenic cell stress by activating an adaptive immune response and eliciting immunological memory in immunocompetent hosts, resulting in long-lasting protective antitumor immunity61,62,63,64. This immune response can only be properly executed in a permissive TME that contains abundant cytotoxic TILs and/or scarce immunosuppressive TAMs61. In this context, tumors with higher CD3/CD68 ratios might favor the occurrence of immunogenic cell stress and be more sensitized to cytotoxic T cells, which could be the reason for longer survival after DXR therapy in high-LMR patients.
Contrary to the prognostic value of the LMR, its predictive value for the efficacy of DXR therapy remains unclear because the LMR was not associated with PFS or treatment response in our study. The mode of drug-induced immune response can partly explain the reason for this discrepancy. Trabectedin, an approved anticancer agent, not only triggers cell-cycle arrest and apoptosis in tumor cells but also depletes TAMs in the TME65,66. Its immunomodulatory property has been thought to elicit a delayed response with prolonged stabilization of disease67. Indeed, in a phase 3 trial of advanced STS, most patients who benefited from trabectedin experienced durable stable disease rather than tumor shrinkage9. PFS and treatment response indicate an early therapeutic efficacy compared to OS and thus cannot encompass the long-term aspect of DXR-induced immune response, which might lead to better OS in high-LMR patients. Additionally, the inhibition of the vascular endothelial growth factor (VEGF) pathway normalizes the tumor blood vessels and reprograms the immunosuppressive TME into an immunostimulatory milieu in solid tumors68. Pazopanib, a VEGF receptor inhibitor, induces immune activation by influencing the differentiation and maturation of dendritic cells69. Future studies should highlight the prognostic and predictive role of LMR, characterizing the TME with more phenotypic markers of TILs and TAMs in patients treated with immunomodulatory anticancer agents.
Primary tumor resection was independently associated with a longer OS and higher LMR. Moreover, patients with resected STS had higher LMRs and NLRs before DXR therapy than those with unresected STS, suggesting that primary tumor resection impacted immunological control of the disease16. Tumor resection is thought to induce immunosuppression after surgery70. However, recent studies have shown that successful tumor resection largely reverts systemic immune dysfunction, including the cytotoxicity of T cells, and restored immunocompetence, even in patients with metastatic disease71,72. Theoretically, primary tumor resection could rescue the systemic immunity and facilitate the adaptive immune response, potentially augmented by DXR therapy. This could contribute to better survival after DXR therapy in patients receiving surgery than those who did not. Another possible reason for the favorable surgical result is the paucity of effective systemic therapy for advanced STS. For patients with metastatic renal cell carcinoma, cytoreductive nephrectomy has long been the sole standard of care. However, a phase 3 trial has shown that sunitinib alone was not inferior to cytoreductive nephrectomy, followed by sunitinib, in patients who were classified as having intermediate- or poor-risk disease73, and only patients with good PS and local symptoms are currently recommended to undergo upfront surgery74. This suggests that the efficacy of promising systemic therapies outweighs the survival advantage of surgery. However, no promising therapy has been introduced for patients with advanced STS. Our study emphasized the significance of primary tumor resection for patients with STS.
The LMR prognostic score, based on the LMR level and tumor resection history, reliably identified high-risk patients. The accurate prediction of survival can help future care and provide opportunities for patients and their families to focus on what is relevant to them when time is limited75. Our model can help avoid harm and inappropriate therapies in vulnerable patients76 and enhance patients’ autonomy77. In addition to being a prognostic estimate, the LMR score can offer an insight into the TME status. The inferred TME status has been reported to be associated with outcomes in multiple types of sarcomas78 and predictive of response to immunotherapy79. Thus, the LMR scoring model can facilitate decision making regarding optimal treatment.
This study was limited by its retrospective nature as well as the enrollment of patients from only a single institution. Since we included patients with unresectable and recurrent STS treated with DXR therapy, we had a limited number of surgical samples available and could not fully capture the pathological data on the specimens. The opportunity to sample additional tumor tissues before chemotherapy was limited by a local health insurance scheme in clinical practice. Additionally, data on the tumor grade were lacking. According to the definition of the La Fédération Nationale des Centres de Lutte Contre le Cancer grading system80,81, we scored only 10 of the 26 resected samples available and thus excluded the grade in the survival analysis. We obtained the results of IHC analysis from only a few resected samples because we excluded biopsied samples from staining and secondarily evaluated the relationship between the LMR and TME status. The IHC results from the resected tumor samples reflected the TME status at the time of primary tumor resection, which might have been altered before DXR therapy. We enrolled patients treated with DXR therapy, excluding other types of chemotherapy, because DXR has been the most common agent for advanced STS among the few drugs covered by the local healthcare system. Differences in characteristics between patients treated with DXR and those treated with other agents (paclitaxel and pazopanib) during the study period are not shown because some data were unavailable. Our patient selection according to the treatment regimen introduced results that should be generalized with caution to all patients with advanced STS. Finally, although the benefit of histology-tailored chemotherapy for STS has been debatable82,83, developing subtype-specific or biomarker-driven strategies is essential. The diverse tumor histologies affected the variation of the indices; thus, the results should be interpreted cautiously.
In conclusion, the LMR was an independent prognostic factor for OS in patients with advanced STS treated with first-line DXR therapy, and the LMR prognostic scoring model reliably predicted OS. Additionally, measuring peripheral LMR can provide clues to the anti-tumor immunity status in the TME. Further investigation is needed to examine the underlying mechanisms of the relationship between the LMR and TME.
Patients and methods
Patients
Retrospectively, we reviewed patients with unresectable or recurrent STS who received first-line DXR therapy at the National Cancer Center Central Hospital between August 2009 and December 2018. The eligibility criteria for this study were histologically confirmed STS and availability of laboratory data that were measured before DXR therapy and included differential WBC counts. This study was approved by the ethics committee of the National Cancer Center Hospital (approval numbers: 2012-335, 2016-086). Informed consent was obtained from all participants and/or their legal guardians. All methods were performed in accordance with the relevant guidelines and regulations.
Clinical characteristics and hematological indices
Clinical characteristics and laboratory data before DXR therapy were taken from the medical records. The parameters collected included patients’ age, sex, PS before DXR therapy, and comorbidity; history of primary tumor resection, radiotherapy, and perioperative (adjuvant and neoadjuvant) chemotherapy prior to first-line DXR therapy, disease status, and time to recurrence; primary tumor site; histological type of STS; and presence of metastasis, pleural effusion, and ascites. Laboratory data included absolute counts of lymphocytes, neutrophils, monocytes, and platelets, as well as albumin, LDH, and CRP levels.
LMR, NLR, and PLR were defined as the ratio of absolute lymphocyte count divided by absolute monocyte count, absolute neutrophil count divided by absolute lymphocyte count, and absolute platelet count divided by absolute lymphocyte count, respectively. Optimal cutoffs for the indices were determined using Youden’s index in ROC analyses. Cutoff values for albumin, LDH, and CRP were determined as the upper and lower limits of the normal ranges for the variables in our institution.
The Charlson Comorbidity Index84, SIS42, and GPS43 were calculated as previously described, and patients were categorized into subgroups according to their indices.
Patient outcomes
The tumor was evaluated using computed tomography (CT) before DXR therapy and again after two or three cycles of DXR therapy. After the first evaluation, CT scans were performed when clinically necessary. The efficacy of DXR therapy was determined according to the Response Evaluation Criteria in Solid Tumors, version 1.185.
OS was defined as the period from the date of pathological diagnosis of STS in patients with unresectable tumors or from the date of recurrence in patients with resected tumors to the date of death from any cause or the last follow-up. PFS was defined as the period from the date of initiation of DXR therapy to the date of documentation of PD, death from any cause, or the last follow-up. Data cut off occurred on November 30, 2021.
Immunohistochemistry
Tissue specimens from patients with STS were available from pathology files at the National Cancer Center. All the tissues were formalin-fixed and embedded in paraffin (FFPE). The histology of each case was evaluated by a board-certified sarcoma pathologist (A.Y.). If patients experienced postoperative recurrence during follow-up in external hospitals and were referred to us, the diagnosis of STS subtypes was confirmed using the external FFPE samples. Diagnostic rebiopsy or tumor resection was performed only if clinically required. IHC analysis of the tumor was performed in resected samples because we excluded biopsied samples from being immunohistochemically stained and secondarily evaluated the relationship between the LMR and TME status. The IHC staining was performed according to the manufacturer’s instructions using an autostainer (Dako Autostainer Link 48 and Omnis staining platform; Dako, Glostrup, Denmark) and the following monoclonal antibodies: CD3 (PS1, Leica Biosystems, Nussloch, Germany), CD68 (PGM1, Dako), and CD20 (L26, Thermo Fisher Scientific, MA, US).
Evaluation of tumor-infiltrating immune cells
The stained slides were examined using a high-resolution digital slide scanner (NanoZoomer 2.0-HT whole-slide imager; Hamamatsu Photonics, Hamamatsu, Japan). Immune cell infiltration was evaluated by two observers (S.W. and A.Y.). Seven independent 0.0625-mm2 areas containing immune cells in the central tumor were selected, and the number of positively-stained immune cells was averaged for each patient. The density of tumor-infiltrating immune cells was calculated as the average number of stained cells divided by the examined area (per 1 square millimeter). The CD3/CD68 ratio was calculated as the density of CD3-positive cells divided by the density of CD68-positive cells.
Statistical analysis
Variation in hematological indices among patient subgroups was compared using the Wilcoxon rank-sum test. The correlation between each laboratory data and PS was estimated using a Spearman’s rank correlation coefficient. The correlation between the indices and the densities of tumor-infiltrating immune cells was analyzed using Pearson’s correlation coefficients. Association of the indices with the efficacy of DXR therapy was tested using the chi-squared test.
OS and PFS were examined using the Kaplan–Meier method and differences in survival between patient subgroups were compared using the log-rank test. HRs and 95% confidence intervals were estimated using the Cox regression analysis. All significant variables identified by univariate analysis were further evaluated by multivariate analysis. Based on the results of multivariate analysis, a prognostic model for survival was constructed and internally validated by resampling using the bootstrap method86. The prognostic performance of the model was evaluated by calculating the area AUC in the ROC analysis, and the difference in AUCs between the models was examined using the DeLong test.
Statistical analyses were performed using JMP version 12.0.1 (SAS Institute Inc., NC, US) and R ver. 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were two-sided, and a p-value less than 0.05 was regarded as statistically significant.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to sensitivity of human data but are available from the corresponding author on reasonable request.
References
Gamboa, A. C., Gronchi, A. & Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J. Clin. 70, 200–229. https://doi.org/10.3322/caac.21605 (2020).
Cancer Research UK. Soft tissue sarcoma survival statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/soft-tissue-sarcoma/survival.
American Cancer Society. Survival rates for soft tissue sarcoma. https://www.cancer.org/cancer/soft-tissue-sarcoma/detection-diagnosis-staging/survival-rates.
National Comprehensive Cancer Network. Soft tissue sarcoma, version 22022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 20, 815–833. https://doi.org/10.6004/jnccn.2022.0035 (2022).
Gronchi, A. et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 32, 1348–1365. https://doi.org/10.1016/j.annonc.2021.07.006 (2021).
Judson, I. et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 15, 415–423. https://doi.org/10.1016/S1470-2045(14)70063-4 (2014).
Tap, W. D. et al. Effect of doxorubicin plus olaratumab vs. doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: The ANNOUNCE randomized clinical trial. JAMA 323, 1266–1276. https://doi.org/10.1001/jama.2020.1707 (2020).
van der Graaf, W. T. et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379, 1879–1886. https://doi.org/10.1016/S0140-6736(12)60651-5 (2012).
Demetri, G. D. et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J. Clin. Oncol. 34, 786–793. https://doi.org/10.1200/JCO.2015.62.4734 (2016).
Schöffski, P. et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 387, 1629–1637. https://doi.org/10.1016/S0140-6736(15)01283-0 (2016).
Berry, V. et al. REGOSARC: Regorafenib versus placebo in doxorubicin-refractory soft-tissue sarcoma−A quality-adjusted time without symptoms of progression or toxicity analysis. Cancer 123, 2294–2302. https://doi.org/10.1002/cncr.30661 (2017).
Amin, M. B. et al. AJCC Cancer Staging Manual (Springer, 2017).
Nathan, H. et al. Predictors of survival after resection of retroperitoneal sarcoma: A population-based analysis and critical appraisal of the AJCC staging system. Ann. Surg. 250, 970–976. https://doi.org/10.1097/SLA.0b013e3181b25183 (2009).
Massarweh, N. N., Dickson, P. V. & Anaya, D. A. Soft tissue sarcomas: Staging principles and prognostic nomograms. J. Surg. Oncol. 111, 532–539. https://doi.org/10.1002/jso.23851 (2015).
Casali, P. G. et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv51–iv67. https://doi.org/10.1093/annonc/mdy096 (2018).
Hiam-Galve, K. J., Allen, B. M. & Spitzer, M. H. Systemic immunity in cancer. Nat. Rev. Cancer. 21, 345–359. https://doi.org/10.1038/s41568-021-00347-z (2021).
Idowu, O. K., Ding, Q., Taktak, A. F. G., Chandrasekar, C. R. & Yin, Q. Clinical implication of pretreatment neutrophil to lymphocyte ratio in soft tissue sarcoma. Biomarkers 17, 539–544. https://doi.org/10.3109/1354750X.2012.699554 (2012).
Nakamura, T. et al. The combined use of the neutrophil-lymphocyte ratio and c-reactive protein level as prognostic predictors in adult patients with soft tissue sarcoma. J. Surg. Oncol. 108, 481–485. https://doi.org/10.1002/jso.23424 (2013).
Szkandera, J. et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br. J. Cancer 108, 1677–1683. https://doi.org/10.1038/bjc.2013.135 (2013).
Choi, E. S., Kim, H. S. & Han, I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann. Surg. Oncol. 21, 778–785. https://doi.org/10.1245/s10434-013-3418-3 (2014).
Szkandera, J. et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int. J. Cancer. 135, 362–370. https://doi.org/10.1002/ijc.28677 (2014).
Jiang, L. et al. Prognostic value of monocyte and neutrophils to lymphocytes ratio in patients with metastatic soft tissue sarcoma. Oncotarget 6, 9542–9550. https://doi.org/10.18632/oncotarget.3283 (2015).
Que, Y. et al. Preoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC Cancer 15, 648. https://doi.org/10.1186/s12885-015-1654-6 (2015).
Kobayashi, H. et al. Neutrophil-to-lymphocyte ratio after pazopanib treatment predicts response in patients with advanced soft-tissue sarcoma. Int. J. Clin. Oncol. 23, 368–374. https://doi.org/10.1007/s10147-017-1199-6 (2017).
Maretty-Kongstad, K., Aggerholm-Pedersen, N., Keller, J. & Safwat, A. A validated prognostic biomarker score for adult patients with nonmetastatic soft tissue sarcomas of the trunk and extremities. Transl. Oncol. 10, 942–948. https://doi.org/10.1016/j.tranon.2017.09.002 (2017).
Chan, J. Y. et al. Biological significance and prognostic relevance of peripheral blood neutrophil-to-lymphocyte ratio in soft tissue sarcoma. Sci. Rep. 8, 11959. https://doi.org/10.1038/s41598-018-30442-5 (2018).
Liang, Y. et al. Combined use of the neutrophil-lymphocyte and platelet-lymphocyte ratios as a prognostic predictor in patients with operable soft tissue sarcoma. J. Cancer 9, 2132–2139. https://doi.org/10.7150/jca.24871 (2018).
Cheng, Y., Mo, F., Pu, L., Li, Q. & Ma, X. Pretreatment inflammatory indexes as prognostic predictors of survival in patients suffering from synovial sarcoma. Front. Oncol. 9, 955. https://doi.org/10.3389/fonc.2019.00955 (2019).
Vinal, D. et al. Prognostic value of neutrophil-to-lymphocyte ratio and other inflammatory markers in patients with high-risk soft tissue sarcomas. Clin. Transl. Oncol. 22, 1849–1856. https://doi.org/10.1007/s12094-020-02324-8 (2020).
Liu, G., Ke, L. & Sun, S. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in patients with soft tissue sarcoma: A meta-analysis. Medicine 97, e12176. https://doi.org/10.1097/MD.0000000000012176 (2018).
Li, L. et al. Meta-analysis of hematological biomarkers as reliable indicators of soft tissue sarcoma prognosis. Front. Oncol. 10, 30. https://doi.org/10.3389/fonc.2020.00030 (2020).
Sorbye, S. W. et al. Prognostic impact of lymphocytes in soft tissue sarcomas. PLoS One 6, e14611. https://doi.org/10.1371/journal.pone.0014611 (2011).
Fujii, H. et al. CD8+ tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int. J. Cancer 134, 2393–2402. https://doi.org/10.1002/ijc.28581 (2014).
Boxberg, M. et al. PD-L1 and PD-1 and characterization of tumor-infiltrating lymphocytes in high grade sarcomas of soft tissue - prognostic implications and rationale for immunotherapy. Oncoimmunology 7, e1389366. https://doi.org/10.1080/2162402X.2017.1389366 (2018).
Kostine, M. et al. Increased infiltration of M2-macrophages, T-cells and PD-L1 expression in high grade leiomyosarcomas supports immunotherapeutic strategies. Oncoimmunology 7, e1386828. https://doi.org/10.1080/2162402X.2017.1386828 (2018).
Que, Y. et al. LAG-3 expression on tumor-infiltrating T cells in soft tissue sarcoma correlates with poor survival. Cancer Biol. Med. 16, 331–340. https://doi.org/10.20892/j.issn.2095-3941.2018.0306 (2019).
Lee, C. H. et al. Prognostic significance of macrophage infiltration in leiomyosarcomas. Clin. Cancer Res. 14, 1423–1430. https://doi.org/10.1158/1078-0432.CCR-07-1712 (2008).
Nabeshima, A. et al. Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3K/Akt pathways. Br. J. Cancer. 112, 547–555. https://doi.org/10.1038/bjc.2014.637 (2015).
Smolle, M. A. et al. Influence of tumor-infiltrating immune cells on local control rate, distant metastasis, and survival in patients with soft tissue sarcoma. Oncoimmunology 10, 1896658. https://doi.org/10.1080/2162402X.2021.1896658 (2021).
Mano, Y. et al. Lymphocyte-to-monocyte ratio is a predictor of survival after liver transplantation for hepatocellular carcinoma. Liver Transpl. 24, 1603–1611. https://doi.org/10.1002/lt.25204 (2018).
Shirasawa, M. et al. Prognostic impact of peripheral blood neutrophil to lymphocyte ratio in advanced-stage pulmonary large cell neuroendocrine carcinoma and its association with the immune-related tumour microenvironment. Br. J. Cancer 124, 925–932. https://doi.org/10.1038/s41416-020-01188-7 (2021).
Chang, Y. et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br. J. Cancer 113, 626–633. https://doi.org/10.1038/bjc.2015.241 (2015).
Forrest, L. M., MacMillan, D. C., McArdle, C. S., Angerson, W. J. & Dunlop, D. J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 89, 1028–1030. https://doi.org/10.1038/sj.bjc.6601242 (2003).
Callegaro, D., Miceli, R., Mariani, L., Raut, C. P. & Gronchi, A. Soft tissue sarcoma nomograms and their incorporation into practice. Cancer 123, 2802–2820. https://doi.org/10.1002/cncr.30721 (2017).
Van Glabbeke, M. et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens—A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J. Clin. Oncol. 17, 150–157. https://doi.org/10.1200/JCO.1999.17.1.150 (1999).
Karavasilis, V. et al. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: Retrospective analysis and identification of prognostic factors in 488 patients. Cancer 112, 1585–1591. https://doi.org/10.1002/cncr.23332 (2008).
Sleijfer, S. et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: An exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur. J. Cancer 46, 72–83. https://doi.org/10.1016/j.ejca.2009.09.022 (2010).
Iqbal, N. et al. Prognostic factors affecting survival in metastatic soft tissue sarcoma: An analysis of 110 patients. Clin. Transl. Oncol. 18, 310–316. https://doi.org/10.1007/s12094-015-1369-9 (2016).
Nakamura, T. et al. Analysis of factors for predicting survival in soft-tissue sarcoma with metastatic disease at initial presentation. Anticancer Res. 37, 3137–3141 (2017).
Young, R. J., Litiére, S. & Lia, M. Predictive and prognostic factors associated with soft tissue sarcoma response to chemotherapy: A subgroup analysis of the European Organisation for Research and Treatment of Cancer 62012 study. Acta Oncol. 56, 1013–1020. https://doi.org/10.1080/0284186X.2017.1315173 (2017).
Fang, E., Wang, X., Feng, J. & Zhao, X. The prognostic role of Glasgow Prognostic Score and c-reactive protein to albumin ratio for sarcoma: A system review and meta-analysis. Dis. Markers. 2020, 8736509. https://doi.org/10.1155/2020/8736509 (2020).
Bejarano, L., Jordao, M. J. C. & Joyce, J. A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 11, 933–959. https://doi.org/10.1158/2159-8290.CD-20-1808 (2021).
Loi, S. et al. Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers. J. Clin. Oncol. 37, 559–569. https://doi.org/10.1200/JCO.18.01010 (2019).
Idos, G. E. et al. The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: A systematic review and meta-analysis. Sci. Rep. 10, 3360. https://doi.org/10.1038/s41598-020-60255-4 (2020).
Orhan, A. et al. The prognostic value of tumour-infiltrating lymphocytes in pancreatic cancer: A systematic review and meta-analysis. Eur. J. Cancer 132, 71–84. https://doi.org/10.1016/j.ejca.2020.03.013 (2020).
DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369–382. https://doi.org/10.1038/s41577-019-0127-6 (2019).
Zhang, Q. W. et al. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS One 7, e50946. https://doi.org/10.1371/journal.pone.0050946 (2012).
Komohara, Y., Jinushi, M. & Takeya, M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 105, 1–8. https://doi.org/10.1111/cas.12314 (2014).
Wen, S. et al. Elevated peripheral absolute monocyte count related to clinicopathological features and poor prognosis in solid tumors: Systematic review, meta-analysis, and meta-regression. Cancer Med. 10, 1690–1714. https://doi.org/10.1002/cam4.3773 (2021).
Shibutani, M. et al. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: A retrospective study. BMC Cancer 17, 404. https://doi.org/10.1186/s12885-017-3395-1 (2017).
Kroemer, G., Galassi, C., Zitvogel, L. & Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 23, 487–500. https://doi.org/10.1038/s41590-022-01132-2 (2022).
Galluzzi, L. et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 8, e000337. https://doi.org/10.1136/jitc-2019-000337 (2020).
Fabian, K. P., Wolfson, B. & Hodge, J. W. From Immunogenic cell death to immunogenic modulation: Select chemotherapy regimens induce a spectrum of immune-enhancing activities in the tumor microenvironment. Front. Oncol. 11, 728018. https://doi.org/10.3389/fonc.2021.728018 (2021).
Zhou, J. et al. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell. Mol. Med. 23, 4854–4865. https://doi.org/10.1111/jcmm.14356 (2019).
D’Incalci, M. & Galmarini, C. M. A review of trabectedin (ET-743): A unique mechanism of action. Mol. Cancer Ther. 9, 2157–2163. https://doi.org/10.1158/1535-7163.MCT-10-0263 (2010).
Germano, G. et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 23, 249–262. https://doi.org/10.1016/j.ccr.2013.01.008 (2013).
Dossi, R. et al. Antiangiogenic activity of trabectedin in myxoid liposarcoma: involvement of host TIMP-1 and TIMP-2 and tumor thrombospondin-1. Int. J. Cancer. 136, 721–729. https://doi.org/10.1002/ijc.29023 (2015).
Patel, S. A. et al. Molecular mechanisms and future implications of VEGF/VEGFR in cancer therapy. Clin. Cancer Res. 29, 30–39. https://doi.org/10.1158/1078-0432.CCR-22-1366 (2023).
Zizzari, I. G. et al. TK inhibitor pazopanib primes DCs by downregulation of the beta-catenin pathway. Cancer Immunol. Res. 6, 711–722. https://doi.org/10.1158/2326-6066.CIR-17-0594 (2018).
Tang, F., Tie, Y. & Tu, C. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin. Transl. Med. 10, 199–223. https://doi.org/10.1002/ctm2.24 (2020).
Allen, B. M. et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat. Med. 26, 1125–1134. https://doi.org/10.1038/s41591-020-0892-6 (2020).
Danna, E. A. et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Can. Res. 64, 2205–2211. https://doi.org/10.1158/0008-5472.CAN-03-2646 (2004).
Mejean, A. et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N. Engl. J. Med. 379, 417–427. https://doi.org/10.1056/NEJMoa1803675 (2018).
Mejean, A. et al. Sunitinib alone or after nephrectomy for patients with metastatic renal cell carcinoma: Is there still a role for cytoreductive nephrectomy?. Eur. Urol. 80, 417–424. https://doi.org/10.1016/j.eururo.2021.06.009 (2021).
Stone, P. et al. Prognostic evaluation in patients with advanced cancer in the last months of life: ESMO Clinical Practice Guideline. ESMO Open 8, 101195. https://doi.org/10.1016/j.esmoop.2023.101195 (2023).
Earle, C. C. et al. Trends in the aggressiveness of cancer care near the end of life. J. Clin. Oncol. 22, 315–321. https://doi.org/10.1200/JCO.2004.08.136 (2004).
Fischer, G. S., Tulsky, J. A. & Arnold, R. M. Communicating a poor prognosis. In Topics in Palliative Care (eds Portenoy, R. K. & Bruera, E.) 75–89 (Oxford University Press, 2000).
The Cancer Genome Atlas Research Network. Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell 171, 950-965.e928. https://doi.org/10.1016/j.cell.2017.10.014 (2017).
Bagaev, A. et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 39, 845–865. https://doi.org/10.1016/j.ccell.2021.04.014 (2021).
Trojani, M. et al. Soft-tissue sarcomas of adults; Study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer 33, 37–42. https://doi.org/10.1002/ijc.2910330108 (1984).
Guillou, L. et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J. Clin. Oncol. 15, 350–362. https://doi.org/10.1200/jco.1997.15.1.350 (1997).
Gronchi, A. et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 18, 812–822. https://doi.org/10.1016/S1470-2045(17)30334-0 (2017).
Gronchi, A. et al. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J. Clin. Oncol. 38, 2178–2186. https://doi.org/10.1200/JCO.19.03289 (2020).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383. https://doi.org/10.1016/0021-9681(87)90171-8 (1987).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247. https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
Harrell, F. E. Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Acknowledgements
This work was supported by a grant from the Foundation for the Promotion of Cancer Research (S.W.).
Author information
Authors and Affiliations
Contributions
S.W. and T.S. designed the research, obtained and analyzed the clinical data. A.Y. performed immunohistochemical staining of the pathological specimens. S.W. and A.Y. analyzed the specimens. S.W., T.S., and A.K. wrote the manuscript. All authors contributed to data acquisition and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
T.N. received institutional support from Daiichi-Sankyo, institutional grants or contracts from AstraZeneca, and honoraria for speakers’ bureaus from AstraZeneca, Chugai, Takeda, MSD, Eisai, and Taiho Pharmaceutical. A.K. received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, and educational events from Daiichi-Sankyo and Taiho Pharmaceutical. K.Y. received grants for an advisory role from Novartis, Eisai, AstraZeneca, Chugai, Takeda, Genmab, and OncXerna; honoraria for lectures from Pfizer, Eisai, AstraZeneca, Eli Lilly, Takeda, Chugai, Fuji Film Pharma, MSD, Boehringer Ingelheim, Ono Pharmaceutical, and Daiichi-Sankyo; and institutional financial support from MSD, Daiichi-Sankyo, AstraZeneca, Taiho Pharmaceutical, Pfizer, Novartis, Takeda, Chugai, Ono Pharmaceutical, Sanofi, Seattle Genetics, Eisai, Eli Lilly, Genmab, Boehringer Ingelheim, Kyowa Hakko Kirin, Nihon Kayaku, and Haihe. All other authors declare no competing financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watanabe, S., Shimoi, T., Nishikawa, T. et al. Lymphocyte-to-monocyte ratio as a prognostic and potential tumor microenvironment indicator in advanced soft tissue sarcoma treated with first-line doxorubicin therapy. Sci Rep 13, 10734 (2023). https://doi.org/10.1038/s41598-023-37616-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37616-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.