Abstract
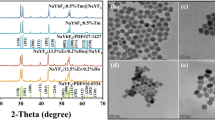
GdVO4-based dual-mode phosphors were successfully synthesized via a hydrothermal approach. The X-ray diffraction analysis determined the tetragonal structure as well as I41/amd space group of products by comparing with a reference pattern no. ICDD #01-072-0277. The morphology of yielded phosphors was confirmed by transmission electron microscopy and scanning electron microscopy. Detailed spectroscopy analysis revealed tunable luminescence properties with an increasing Yb3+ content in series of GdVO4: x% Yb3+, y% Tm3+, 5% Eu3+ (x = 5, 10, 15, 20; y = 0.1, 0.5, 1) phosphors. For Yb3+, Tm3+, and Eu3+- codoped phosphors we observed bands related to the 1G4 → 3H6 and 1G4 → 3F4 transitions of Tm3+ ions, occurred through the cooperative up-conversion mechanism, where two nearby Yb3+ ions were involved in near-infrared absorption. Moreover, the GdVO4: 20% Yb3+, 0.5% Tm3+, 5% Eu3+ showed the most outstanding color tunability from red color (x = 0.6338, y = 0.3172) under UV to blue color (x = 0.2640, y = 0.1988) under NIR excitation, which can be applied in anti-counterfeiting activity.
Similar content being viewed by others
Introduction
Inorganic materials doped with lanthanide ions (Ln3+) play a significant role in many fields of everyday life, based on their numerous applications as lasers, thin film phosphors, in drug delivery, bioimaging, or anti-counterfeiting1,2,3,4,5,6,7. The last-mentioned group consists of several media in which anti-counterfeiting tags are implemented, such as bar codes, inks, holograms, RFID (Radio Frequency Identification). Every aforementioned security approach has its limitations and thus cannot be applied in i.e., clothes or document markings, which may be regularly treated with water, washing agents, or UV radiation. Recently developed cellulose fibers modified- with inorganic phosphors8,9,10,11,12 are prepared via an environmentally friendly NMMO (N-Methylmorpholine-N-Oxide) method. As an outcome, the so-called Tencel fibers may be used for paper modification or as a part of the fabric. Also, during this rigorous process of fibers preparation, the luminescent modifier used has to outstand with its excellent stability. In our research, we chose GdVO4-based dual-mode phosphors based on their strong energy absorption as well as the high efficiency of energy transfer processes13,14,15. Another advantage of vanadate-based phosphors as an alternative type of material versus fluoride-based phosphors is their high thermal stability, beneficial in i.e. light-emitting diode application, in which operating temperature exceeds 100 °C16. In comparison to the vanadate materials, commonly used in phosphor applications fluoride materials are not only sensitive to high temperatures but also to surface contamination which may accidentally influence spectroscopic properties such as luminescence lifetime or emission color17.
Contrary to the accidental influence, the intentional impact on the intensity and different luminescence color under UV or NIR excitation is caused by the specified Ln3+ dopant concentration. Rare earth-doped materials exhibiting dual-mode luminescence possess vast potential for various applications18,19,20,21. When a combination of ions capable of absorbing energy with diverse energy values based on their electron structure is used, a unique luminescence color tunability can be achieved. The perfect combination for obtaining tunable luminescence is the system comprising Yb3+, Tm3+, and Eu3+ ions. Here, the NIR excitation of 980 nm can be absorbed by Yb3+ and after transferring two or more photons towards Tm3+ and Eu3+ ions, a visible up-conversion emission is observed, which is highly dependent on Tm3+ and Eu3+ ratio. Also, under UV irradiation, there is a possibility of direct Eu3+ excitation or energy transfer from the orthovanadate matrix to dopant ions—in both cases, a red emission associated with Eu3+ is observed22,23.
In this study, we aim to provide a comprehensive understanding of the properties and applications of the luminescent marks based on doped orthovanadates. All of above-mentioned features of the GdVO4: Yb3+, Tm3+, Eu3+ phosphors synthesized by the feasible hydrothermal approach, designate as perfect for the anti-counterfeiting applications as the color tunability within the same material is difficult to falsify. The study introduces dual-mode orthovanadates as a viable substitute for fluoride-based materials in the realm of anti-counterfeiting tags. Through precise selection of dopant ions and host matrix, the resulting luminophores undergo up-conversion processes and demonstrate robust emission capabilities owing to charge transfer phenomena between O2- and Eu3+ ions. These distinct mechanisms give rise to diverse color emissions and various wavelength excitation. Moreover, the orthovanadate materials exhibit remarkable resilience to harsh environmental conditions, including elevated temperatures, thereby rendering them advantageous by comparison to the aforementioned fluorides, which are more prone to decomposition.
A potential application of the implementation of a orthovanadates modifier into cellulose fibers for paper and fabric markings has been confirmed by the patent application submitted43,44. By showcasing the real-world application of our material, we establish its potential for practical implementation and highlight its relevance to various industries. Finally, according to our knowledge, this is the first time that Yb3+, Tm3+, Eu3+ dopants are incorporated into the orthovanadate matrix, and its structural followed by spectroscopic properties are determined. It fills a gap in the existing literature by presenting an innovative approach that has practical implications and opens new avenues for future research and development.
Experimental section
Materials
Ammonium metavanadate (NH4VO3, Sigma Aldrich, 99.9%), gadolinium(III) oxide (Gd2O3, Standford Materials, 99.99%), ytterbium(III) oxide (Yb2O3, Standford Materials, 99.99%), thulium(III) oxide (Tm2O3, Standford Materials, 99.99%), europium(III) oxide (Standford Materials, 99.99%) and acetic acid (CH3COOH, POCH, 99,95%) used in the synthesis of the materials.
Methods
A series of GdVO4: x% Yb3+, y% Tm3+, 5% Eu3+ (x = 5, 10, 15, 20; y = 0.1, 0.5, 1) was obtained in hydrothermal conditions. The concentration and the type of dopants was altered based on our knowledge and the literature in order to observe efficient emission processes24,25,26,27,28. In addition, the concentration of Yb3+, Tm3+ and Eu3+ dopant ions in prominent GdVO4 host was altered to provide the intense, dual-mode luminescence under UV and NIR irradiation for anticounterfeiting applications. The composition of Ln3+ ions used was selected to ensure the emission color dependent on excitation wavelength thus considered material is more difficult to replicate.
The synthesis was performed in Berghof autoclave (max. pressure 200 bar, additional stirring). All of the substrates were used as water solutions. The stoichiometric combination of 0.25 M Ln(CH3COO)3 was mixed with the 0.1 M NH4VO3 added dropwise under continuous stirring for 30 min. Resultant transparent mixture (pH 4.7) was then transferred to Teflon vessel and put for hydrothermal process under 180 °C for 3 h which yielded with yellow powder. Next, when the autoclave was naturally cooled to room temperature, the product was collected by centrifugation, washed with 1:1 mixture of deionized water and ethanol. Finally, the product was dried at 80ºC for 24 h for further analysis.
Characterization
The structural analysis was conducted with Bruker AXS D8 Advance powder X-ray diffractometer equipped with Johansson's monochromator and Lynx Eye strip detector, whereas the measurements were performed with Cu-Kα1 λ = 15,418 Å radiation within the 10–60 2Θ range, 0.05°/s step size. Morphology of studied materials was investigated with the use of transmission electron microscopy, TEM (JEOL 1400 with acceleration voltage of 80 kV) as well as scanning electron microscopy, SEM (Quanta 250 FEG, FEI equipped with EDAX detector). Luminescence properties were studied in terms of photoluminescence and upconversion luminescence, i.e. under UV or NIR excitation. The former was studied with the use of Hitachi F-7000 spectrofluorimeter, equipped with xenon lamp excitation source. The latter phenomenon was studied in terms of emission, luminescence decay and the number of photons involved in the process, with the use of PIXIS:256E Digital CCD Camera equipped with SP-2156 Imaging Spectrograph (Princeton Instruments), Mixed Domain Oscilloscope—200 MHz—Tektronix MDO3022 as well as the excitation source of CNI NIR 2W LASER 975 nm. All of the spectroscopic measurements were conducted at 293 K.
Results and discussion
Structure and morphology
Behind every specific luminescence feature, there are structural and morphological reasons as well. As seen in Fig. 1, the replacement of 25.5% of Gd3+ ions with dopant ions in the host structure did not cause severe lattice distortions since the ionic radius of Eu3+ is similar, while the Yb3+ and Tm3+ radii are smaller than the one of Gd3+29. The synthesized compounds are confirmed to be of GdVO4 I41/amd tetragonal zircon-type (ZrSiO4) structure with the cell parameters of a = b = 7.2126 Å, c = 6.3483 Å, according to the reference pattern no. ICDD #01-072-027730,31. The tetragonal and polyhedral structure of GdVO4 are presented in Fig. 2c. Here, vanadium atom of [VO4]3− is tetrahedrally coordinated with O2− ions, whereas the Ln3+ are surrounded by eight oxygen atoms in a distorted dodecahedron structure32. The presence of sharp, narrow reflexes indicates discussed materials as highly crystalline and bulk. By the fact that none of the additional peaks are observed, synthesized powders are monophased and the replacement of Gd3+ by Ln3+ dopants was successful. What has to be said, an increased grain size growth as well as excellent crystallinity are caused by inevitable high temperature annealing (900 °C) which was applied to induce the UC luminescence, initially diminished by structure defects, typical for materials synthesized in hydrothermal conditions33. These features were further confirmed with the use of TEM and SEM methods (Fig. 2a,b). The obtained orthovanadate crystals were observed to be agglomerated, displaying an irregular morphology. The average grain size, determined from the broadest fraction, was found to be approximately 1.5 µm. These observations confirm the microstructural properties of the orthovanadate crystals and provide quantitative data regarding their size distribution illustared on histogram (inset Fig. 2a). Despite the large grain size, it was possible to disperse the powder in water for further use in cellulose fiber modification.
Spectroscopic properties
According to the Figs. 3 and 4, GdVO4: 20% Yb3+, 0.5% Tm3+, 5% Eu3+ sample was chosen amongst the series as the most promising sample thus used for the fabric preparation based not only on its diverse luminescence color under UV and NIR excitation, but also the outstanding UC emission in the visible range.
Photoluminescence
Based on the excitation spectrum in the UV range Fig. 4, the O2−–Eu3+ charge transfer band (CT) with the maximum at 310 nm was chosen to observe visible luminescence at 621 nm, assigned to the 5D0 → 7F2 transition. What has to be mentioned, the broad CT band is in fact combined of O2−–V5+ and O2−–Eu3+; however, based on the small difference in between O2− and V5+, as well as the large charge difference, O2−–V5+ in [VO4]3− is easier observed34. Also, there are additional weak f–f transitions typical for Eu3+ ions in the 200–500 nm range35,36. Based on the ionic radii difference34, Gd3+ is being replaced by Eu3+ thus in the GdVO4: Yb, Eu, Tm system, Eu3+ has D2d symmetry as it is surrounded by eight O2- ions. Relative intensity of 5D0 → 7F1 and 5D0 → 7F2 is altered based on the local site symmetry of the Eu3+ ions11,37,38. In this research, the intensity of hypersensitive 5D0 → 7F2 is the highest among the Eu3+ emission bands which indicated the low symmetry around Eu3+ ions38. What is more, with an increasing Yb3+ concentration, the intensity of both excitation and emission curves is decreasing in terms of the Eu3+ → Yb3+ energy transfer, since the distance between these ions is shortened38. This phenomenon is further confirmed by the calculated Eu3+ luminescence lifetime values (Table 1) monitored under 310 nm excitation. With the increasing Yb3+ concentration, the energy is migrating from Eu3+ excited states to Yb3+ ions.
According to the chromaticity diagram Fig. 5, under 310 nm excitation the outcoming luminescence color is not altered by the incorporated Tm3+ ions.
Upconversion luminescence
It is essential for dual-mode luminescence (ergo inimitable anti-counterfeiting materials) to be intense both under UV and NIR irradiation. According to the upconversion spectrum depicted in Fig. 4, the energy transfer between Eu3+ and Tm3+ ions is observed as variety of Eu3+ and Tm3+ emission bands are present in the spectrum. What has to be noted, the population of Eu3+ via inefficient phononassisted- Yb3+–Eu3+ is barely observed; here, Tm3+ acts as an energy mediator between the sensitizer (Yb3+) and emitter (Eu3+)22. According to that, several emission bands associated to the Tm3+ and Eu3+ are observed in the spectrum, which vary with intensity, namely 1G4 → 3H6 (Tm3+, ~ 478 nm), 5D1 → 7F1 (Eu3+, ~ 521 nm), 5D1 → 7F2 (Eu3+, ~ 552 nm), 5D0 → 7F1 (Eu3+, ~ 590 nm), 5D0 → 7F2 (Eu3+, ~ 615 nm), 1G4 → 3F4 (Tm3+, ~ 650 nm), 5D0 → 7F4 (Eu3+, ~ 700 nm) as well as the most intense 3H4 → 3H6 (Tm3+, ~ 800 nm) band. The last-mentioned transition is observed in NIR region of spectrum thus it does not influence the emission color. What has to be mentioned, throughout changing Yb3+ concentration, the chromaticity coordinates of synthesized materials change, according to the Fig. 5 and Table 2. With an increasing Yb3+ content, the luminescence color shifts from the red towards purple and blue region of CIE chromaticity diagram. By the gradual substitution of Gd3+ by Yb3+, the distance between sensitizer and emitters such as Tm3+, Eu3+ decrease. As mentioned before, the efficiency of Yb3+–Eu3+ transfer is low thus the Yb3+–Tm3+ transfer is favored here. In this case, the competitive Tm3+–Eu3+ absorption is decreased, which is also connected with the lower intensity of Eu3+ emission bands in the UC spectrum, as well as the red component of the luminescence color23. Also, when Yb3+–Tm3+ is greatly enhanced, the relative intensity between blue and red emissions of Tm3+ is increased, which results with the outcoming blue upconversion luminescence40,41.
The population of Eu3+ and Tm3+ excited levels, as well as the energy transfer between these species were studied in terms of luminescence decay. Here, due to the color change, the most important bands are the ones in the blue and red region of spectrum. Based on that, the greatest attention was put to the lifetime of 1G4 → 3H6 (Tm3+, ~ 478 nm), 5D0 → 7F2 (Eu3+, ~ 615 nm) and 1G4 → 3F4 (Tm3+, ~ 650 nm). As shown in Table 3, the luminescence lifetime of Eu3+ is decreasing with an increasing Yb3+ content. Based on that, the possibility of Tm3+–Eu3+ is diminished, whereas Yb3+–Tm3+ transfer is favored. It is also confirmed by the enhanced blue emission of Tm3+ since less energy is transferred towards Eu3+ site of lattice. Moreover, in order to derive the average number of photons (n) involved in the upconversion process, a laser power dependent luminescence study was performed (Fig. 6). Interestingly, the slopes (n) of 1G4 → 3H6 and 1G4 → 3F4 transitions suggest the involvement of two photons regardless the Yb3+ concentration. In the contrary to the common understanding of the 1G4 population mechanism, i.e. through threephoton- absorption, meant also as sequential sensitization, in the case of GdVO4: x% Yb3+, 0.5% Tm3+, 5% Eu3+ a cooperative sensitization is in fact happening. In this mechanism there are two Yb3+ ions involved, which absorbs photons in order to promote themselves towards 2F5/2 excited state. Then, a coupled cluster state of two Yb3+ ions formed transfers energy towards Tm3+ which results with the population of its 1G4 level42.
To summarize all studied processes, a mechanism of upconversion in GdVO4: x% Yb3+, 0.5% Tm3+, 5% Eu3+ systems can be proposed in Fig. 7. The whole phenomenon begins under 975 nm CW excitation, when the energy is first absorbed by two nearby Yb3+ ions. This results with the promotion of sensitizers from their 2F7/2–2F5/2 level. At this point, two simultaneous processes are happening. Due to the formation of coupled Yb3+ cluster state, another photon is absorbed and transferred towards 1G4, from which 478 nm and 650 nm emissions occur. Also, there is a photon transferred from this level towards Eu3+ 5D1, where after energy dissipation to 5D0, several emissions associated with Eu3+ are observed in the spectrum. What is more, the Tm3+ 3H5 is populated via photon transfer from Yb3+ 2F5/2. After nonradiative relaxation to 3H4, an 800 nm emission is observed.
Based on its pristine tunable luminescence properties, sample composed of GdVO4: 20% Yb3+, 0.5% Tm3+, 5% Eu3+ was chosen for fibers preparation. Then luminescent fibers were used for paper modification and fabric production as an example of anti-counterfeiting application43,44. Regardless the medium, the outcoming luminescence color as well as its intensity remain unchanged. These properties recommend GdVO4: 20% Yb3+, 0.5% Tm3+, 5% Eu3+ phosphor for anticounterfeiting applications performed in patent proposal submission. In Fig. 8, an actual luminescence color under different excitation sources is present.
Conclusions
To conclude, the pre-eminent, spectroscopic properties of GdVO4: 20% Yb3+, 0.5% Tm3+, 5% Eu3+ define this material as the excellent one for anti-counterfeiting purposes. In our study, we investigated different Yb3+ concentration and its influence on structural and spectroscopic properties. With an increased sensitizer content, the upconversion luminescence is more intense whereas its color is tuned towards blue region of spectrum. What is more, in Yb3+/Tm3+/Eu3+ system, 1G4 → 3H6 and 1G4 → 3F4 emissions result from twophoton excitation in terms of cooperative sensitization where two nearby Yb3+ ions are involved in IR excitation absorption process. Also, varied luminescence color under different sources of excitation qualified GdVO4: 20% Yb3+, 0.5% Tm3+, 5% Eu3+ phosphor for anticounterfeiting application. In applied cellulose medium, luminescence color and intensity remained unchanged in comparison to the phosphor in the powder state.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Shie, N. C., Hsieh, W. F. & Shy, J. T. Single frequency 1070 nm Nd:GdVO4 laser using a volume Bragg grating. Opt. Express. 19, 21109–21115 (2011).
Yi, S. S. et al. Photoluminescence behaviors of Eu-doped GdVO4 thin film phosphors grown by pulsed laser ablation. J. Alloys Compd. 408–412, 890–893 (2006).
Huang, S. J. et al. Luminescent GdVO4:Eu3+ functionalized mesoporous silica nanoparticles for magnetic resonance imaging and drug delivery. Dalt. Trans. 42, 6523–6530 (2013).
Przybylska, D., Grzyb, T., Erdman, A., Olejnik, K. & Szczeszak, A. Anti-counterfeiting system based on luminescent varnish enriched by NIR- excited nanoparticles for paper security. Sci. Rep. 12, 19388 (2022).
Katumo, N., Li, K., Richards, B. S. & Howard, I. A. Dual-color dynamic anti-counterfeiting labels with persistent emission after visible excitation allowing smartphone authentication. Sci. Rep. 12, 2100 (2022).
Moretti, E. et al. Luminescent Eu-doped GdVO4 nanocrystals as optical markers for anti-counterfeiting purposes. Chem. Pap. 71, 149–159 (2017).
Andresen, E. et al. Assessing the reproducibility and up-scaling of the synthesis of Er, Yb-doped NaYF4-based upconverting nanoparticles and control of size, morphology, and optical properties. Sci. Rep. 13, 2288 (2023).
Erdman, A., Kulpinski, P., Grzyb, T. & Lis, S. Preparation of multicolor luminescent cellulose fibers containing lanthanide doped inorganic nanomaterials. J. Lumin. 169, 520–527 (2016).
Skwierczyńska, M., Runowski, M., Kulpiński, P. & Lis, S. Modification of cellulose fibers with inorganic luminescent nanoparticles based on lanthanide(III) ions. Carbohydr. Polym. 206, 742–748 (2019).
Skwierczyńska, M. et al. Luminescent-magnetic cellulose fibers, modified with lanthanide-doped core/shell nanostructures. ACS Omega 3, 10383–10390 (2018).
Tymiński, A., Śmiechowicz, E., Martín, I. R. & Grzyb, T. Ultraviolet- and near-infrared-excitable LaPO4:Yb3+/Tm3+/Ln3+(Ln = Eu, Tb) nanoparticles for luminescent fibers and optical thermometers. ACS Appl. Nano Mater. 3, 6541–6551 (2020).
Shen, X., Hu, Q., Jin, Y. & Ge, M. Long-lived luminescence and photochromic cellulose acetate-based fiber: Preparation, characterization, and potential applications. Cellulose 30, 2181–2195 (2023).
Yan, Y. et al. Effect of SDS on morphology tailoring of GdVO4:Eu3+ powders under hydrothermal conditions in a wide pH range. J. Alloys Compd. 597, 282–290 (2014).
Guo, J., Wang, W., Lin, H. & Liang, X. High-repetition-rate and high-power picosecond regenerative amplifier based on a single bulk Nd:GdVO4 crystal. High Power Laser Sci. Eng. 7, 1–7 (2019).
Gavrilovic´, T. V., Jovanovic´, D. J., Lojpur, V. & Dramic´anin, M. D. Multifunctional Eu3+- and Er3+/Yb3+-doped GdVO4 nanoparticles synthesized by reverse micelle method. Sci. Rep. 4, 4209 (2014).
Tian, Y. Development of phosphors with high thermal stability and efficiency for phosphor-converted LEDs. J. Solid State Light. 1, 1–15 (2014).
Wang, D. & Kodama, N. Visible quantum cutting through downconversion in GdPO4:Tb3+ and Sr3Gd(PO4)3:Tb3+. J. Solid State Chem. 182, 2219–2224 (2009).
Han, Q. et al. Tunable multicolor emission based on dual-mode luminescence Y2O3:Eu@SiO2/Y2O3:Er(Tm/Yb) composite nanomaterials. J. Lumin. 241, 118541 (2022).
Cui, S. et al. Tunable concentration-dependent upconversion and downconversion luminescence in NaYF4: Yb3+, Er3+@ NaYF4: Yb3+, Nd3+ core-shell nanocrystals for a dual-mode anti-counterfeiting imaging application. Opt. Lett. 47, 2814–28171 (2022).
Wanas, W., Abd El-Kaream, S. A., Ebrahim, S., Soliman, M. & Karim, M. Cancer bioimaging using dual mode luminescence of graphene/FA-ZnO nanocomposite based on novel green technique. Sci. Rep. 13, 27 (2023).
Ayachi, F. et al. Dual-mode luminescence of Er3+/Yb3+ codoped LnP0.5V0.5O4 (Ln=Y, Gd, La) for highly sensitive optical nanothermometry. Mater. Today Chem. 27, 101352 (2023).
Marciniak, L., Bednarkiewicz, A. & Strek, W. Tuning of the up-conversion emission and sensitivity of luminescent thermometer in LiLaP4O12:Tm, Yb nanocrystals via Eu3+ dopants. J. Lumin. 184, 179–184 (2017).
Jin, C. & Zhang, J. Upconversion luminescence of Ca2Gd8(SiO4)6O2:Yb3+-Tm3+-Tb3+/Eu3+ phosphors for optical temperature sensing. Opt. Laser Technol. 115, 487–492 (2019).
Tymiński, A., Grzyb, T. & Lis, S. REVO4-based nanomaterials (RE = Y, La, Gd, and Lu) as Hosts for Yb3+/Ho3+, Yb3+/Er3+, and Yb3+/Tm3+ Ions: structural and up-conversion luminescence studies. J. Am. Ceram. Soc. 99, 3300–3308 (2016).
Li, Y. et al. Growth phase diagram and upconversion luminescence properties of NaLuF4:Yb3+/Tm3+/Gd3+ nanocrystals. RSC Adv. 7, 44531–44536 (2017).
Liu, J. et al. A highly sensitive and selective nanosensor for near-infrared potassium imaging. Sci. Adv. 6, 1–11 (2020).
Szczeszak, A. et al. Structural, spectroscopic, and magnetic properties of Eu3+-doped GdVO4 nanocrystals synthesized by a hydrothermal method. Inorg. Chem. 53, 12243–12252 (2014).
Alammar, T., Cybinska, J., Campbell, P. S. & Mudring, A. V. Sonochemical synthesis of highly luminescent Ln2O3:Eu3+ (Y, La, Gd) nanocrystals. J. Lumin. 169, 587–593 (2016).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A. 32, 751–767 (1976).
Xin, H., Lin, L. X., Wu, J. H. & Yan, B. Hydrothermal synthesis and multi-color photoluminescence of GdVO4:Ln3+ (Ln = Sm, Dy, Er) sub-micrometer phosphors. J. Mater. Sci. Mater. Electron. 22, 1330–1334 (2011).
Thakur, H. et al. Synthesis, structural analysis, upconversion luminescence and magnetic properties of Ho3+/Yb3+ co-doped GdVO4 nanophosphor. Mater. Chem. Phys. 253, 123333 (2020).
Jovanović, D. J. et al. Synthesis, structure and spectroscopic properties of luminescent GdVO4:Dy3+ and DyVO4 particles. Opt. Mater. (Amst) 76, 308–316 (2018).
Grzyb, T., Wecławiak, M. & Lis, S. Influence of nanocrystals size on the structural and luminescent properties of GdOF:Eu3+. J. Alloys Compd. 539, 82–89 (2012).
Zhao, J., Guo, C., Yu, J. & Yu, R. Spectroscopy properties of Eu3+ doped Ca 9R(VO4)7 (R=Bi, La, Gd and Y) phosphors by sol-gel method. Opt. Laser Technol. 45, 62–68 (2013).
Behrh, G. K., Gautier, R., Latouche, C., Jobic, S. & Serier-Brault, H. Synthesis and photoluminescence properties of Ca2Ga2SiO7:Eu3+ red phosphors with an intense 5D0 → 7F4 transition. Inorg. Chem. 55, 9144–9146 (2016).
Choi, Y. I., Yoon, Y., Kang, J. G. & Sohn, Y. Photoluminescence imaging of Eu(III) and Tb(III)-embedded SiO2 nanostructures. J. Lumin. 158, 27–31 (2015).
Han, S. et al. Luminescence behavior of Eu3+ in silica glass containing GdVO4: Eu nanocrystals. J. Non. Cryst. Solids. 532, 119894 (2020).
Szczeszak, A. et al. Structural, spectroscopic, and magnetic properties of Eu(3+)-doped GdVO4 nanocrystals synthesized by a hydrothermal method. Inorg. Chem. 53, 12243–12252 (2014).
Zhang, W. J. et al. Downshifting by energy transfer in Eu3+/Yb3+ codoped Ba4La6(SiO4)6O glass ceramics. Phys. B Condens. Matter. 508, 22–26 (2017).
Quintanilla, M., Núñez, N. O., Cantelar, E., Ocaña, M. & Cussó, F. Tuning from blue to magenta the up-converted emissions of YF3:Tm3+/Yb3+ nanocrystals. Nanoscale 3, 1046–1052 (2011).
Gea, W., Xua, M., Shia, J., Zhua, J. & Li, Y. Highly temperature-sensitive and blue upconversion luminescence properties of Bi2Ti2O7:Tm3+/Yb3+ nanofibers by electrospinning. J. Chem. Eng. 391, 123546 (2020).
Liao, M. et al. Mechanisms of Yb3+ sensitization to Tm3+ for blue upconversion luminescence in fluorophosphate glass. Mater. Lett. 61, 70–472 (2007).
Szczeszak, A. et al. P.438197 (2021).
Szczeszak, A. et al. P.438196 (2021).
Acknowledgements
This research was funded by scientific Grant LIDER (Grant No. 39/0141/L-9/17/NCBR/2018), The National Centre for Research and Development, Poland. We acknowledge Adrianna Nowak for her work in laboratory by phosphors’ syntheses.
Author information
Authors and Affiliations
Contributions
N.J.—Methodology, Investigation, Writing – Original Draft, Visualization ; J.Cz.—Writing – Review & Editing, Visualization; A.Sz.—Writing – Review & Editing, Supervision, Validation, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jaroch, N., Czajka, J. & Szczeszak, A. Luminescent materials with dual-mode excitation and tunable emission color for anti-counterfeiting applications. Sci Rep 13, 10773 (2023). https://doi.org/10.1038/s41598-023-37608-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37608-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.