Abstract
Prostaglandin E-major urinary metabolite (PGE-MUM) is a urinary biomarker reflecting ulcerative colitis (UC) activity. This prospective observational study aimed to evaluate the usefulness of PGE-MUM via rapid chemiluminescent enzyme immunoassay in detecting endoscopic remission (ER) and histologic remission (HR) in pediatric UC (6–16 years) in comparison with fecal calprotectin (FCP). ER and HR were defined as Mayo endoscopic score (MES) of 0 and Matts’ histological grades (Matts) of 1 or 2, respectively. A total of 104 UC and 39 functional gastrointestinal disorder (FGID) were analyzed. PGE-MUM levels were significantly higher in the UC group than in the FGID group (P < 0.001). FCP levels were significantly elevated in the group without ER and HR than in the group with ER and HR (P < 0.001 and P = 0.001), whereas PGE-MUM levels were significantly higher in the group without ER compared to the group with ER (P < 0.001). No significant differences were noted in the AUCs for PGE-MUM and FCP in detecting ER and HR. Although PGE-MUM was inferior to FCP for the detection of HR, it might have the potential for application as a biomarker of endoscopic activity in pediatric UC owing to its noninvasive and rapid method.
Similar content being viewed by others
Introduction
In order to improve the prognosis of ulcerative colitis (UC), endoscopic mucosal healing has been proposed as the ultimate goal of UC treatment1,2. However, recent studies have highlighted the importance of histologic healing as a novel treatment target3,4. Colonoscopy coupled with biopsy is considered the gold standard for the monitoring endoscopic and histologic activities in patients with UC, regardless of age. Nonetheless, bowel preparation for colonoscopy has been perceived as the most difficult part of the procedure by both pediatric patients and their caregivers5,6. Additionally, in pediatric patients, sedation or general anesthesia is often necessary during colonoscopy, and this procedure is generally considered more invasive compared to adult patients7. Under these circumstances, several biomarker tests have been proposed as alternatives for colonoscopy. One such test is the fecal calprotectin (FCP) test, which is commonly used in UC to quantify calprotectin, a protein released from leukocytes, and its value reflects the degree of intestinal mucosal inflammation8,9. Blood-based biomarkers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and leucine-rich α2-glycoprotein have been previously utilized to confirm healing in UC10,11,12. Although fecal- and blood-based biomarkers are useful, acquiring fecal or blood specimens in the pediatric filed may pose difficulties due to various concerns of patients or their guardians, such as pain, discomfort, timing, and messiness13. In recent years, the prostaglandin E-major urinary metabolite (PGE-MUM), that can be measured in urine, has been proposed as a biomarker14. PGE-MUM is a stable metabolite of prostaglandin E2 (an important chemical mediator of inflammation) and an indicator of large intestine inflammation in adult UC15,16. Moreover, we have also reported that PGE-MUM reflects endoscopic disease activity in pediatric UC13. Traditionally, PGE-MUM was measured using the radioimmunoassay (RIA) method; however, rapid measurement of PGE-MUM has recently become possible due to the development of fully automated chemiluminescent enzyme immunoassay (CLEIA)17.
Herein, we designed a multicenter prospective study to compare biomarkers, including PGE-MUM (measured via CLEIA) and FCP, in predicting endoscopic and histologic activity in pediatric UC.
Results
Patient characteristics
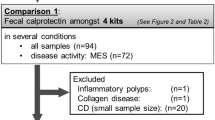
A total of 143 samples, including 104 UC patients and 39 patients with functional gastrointestinal disorders (FGIDs) as controls, were enrolled in this study from 14 pediatric centers in Japan between November 2018 and November 2020 (Fig. 1). After applying the inclusion and exclusion criteria, the final histological evaluation was performed in 76 UC patients because the remaining 28 tissue samples slides could not be sent due to changes in institutional regulations. The baseline characteristics of the patients with UC and controls are shown in Table 1. The PGE-MUM levels measured by the CLEIA in UC were significantly higher than those in controls (median 28.5 vs 18.6, P < 0.001).
Correlation between PGE-MUM measured by CLEIA and RIA methods
In the analysis of the correlation between PGE-MUM levels measured using CLEIA and RIA, a strong correlation was found between PGE-MUM levels measured by both methods (Spearman’s rank correlation coefficient 0.915, P < 0.0001) (Fig. 2). The median values of PGE-MUM by CLEIA were statistically higher than the median values of PGE-MUM by RIA (Table 1).
Correlations between biomarkers and clinical parameters (PUCAI, MES, Matts)
Analyses of the correlations between biomarkers (PGE-MUM, FCP) and clinical parameters (PUCAI, MES, and Matts) showed that PGE-MUM and FCP levels correlated well with each parameter compared to the other biomarkers (Fig. 3).
Comparison between PGE-MUM and FCP levels by endoscopic activities (mMES and sMES)
Correlations between the biomarkers and endoscopic findings (mMES) in the entire colon were analyzed. Both PGE-MUM and FCP levels were significantly elevated in patients with endoscopically active disease compared with the patients showing ER (Fig. 4a,b). As for endoscopic activities of the entire colon (sMES), both biomarker levels were significantly elevated in patients with sMES ≥ 6 compared with those who had sMES ≤ 5 (Fig. 4c,d).
Comparisons of PGE-MUM and FCP levels for endoscopic disease activity (mMES and sMES) in UC patients. (a) The PGE-MUM, and (b) FCP values were significantly lower in the ER group (mMES 0) than the non-ER group (mMES 1–3); (c) PGE-MUM, and (d) FCP values were significantly lower in the group with sMES ≤ 5 than the group with sMES ≥ 6.
Comparison of PGE-MUM and FCP levels by histologic activities (mMatts and sMatts)
Correlation analyses between biomarkers and histologic activities (mMatts) in the entire colon revealed that only the FCP levels were significantly elevated in patients with histologically active disease compared with those at HR (Fig. 5a,b).
Comparisons of PGE-MUM and FCP levels for histologic activity (mMatts and sMatts) in UC patients. (a) PGE-MUM and (b) FCP values were significantly lower in the HR group (mMatts 1–2) than the non-HR group (mMatts 3–5); (c) PGE-MUM, and (d) FCP values were significantly lower in the group with sMatts between 5 and 10 than those in the group with sMatts ≥ 11.
As for histologic activities of the entire colon (sMatts), both biomarker levels were significantly elevated in patients with sMatts ≥ 11 compared with patients who had sMatts ≤ 10 (Fig. 5c,d).
Predicting ER (MES 0 vs 1–3)
ROC curve analysis showed that PGE-MUM and FCP had good predictive values for identifying patients with ER (AUC 0.80 and 0.89, respectively) (Fig. 6a). Although FCP showed a relatively high AUC, the difference was not statistically significant (P > 0.05). The cut-off value of PGE-MUM for predicting ER (mMES = 0) was 17.4 μg/g creatinine. Levels of PGE-MUM < 17.4 μg/g creatinine could discriminate ER from the absence of ER with a sensitivity of 0.77 and specificity of 0.70, whereas levels of FCP < 250 mg/kg could discriminate ER from the absence of ER with a sensitivity of 0.82 and specificity of 0.70 (Fig. 6a).
Receiver operating characteristic curve for PGE-MUM, FCP in relation to endoscopic (MES), and histologic activities (Matts) in UC patients. (a) mMES and (b) mMatts. Comparison of the area under the receiver operating characteristic (ROC) curves, optimal cutoff values, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy (ACC) of PGE-MUM and FCP for the determination of endoscopic (a) and histologic (b) remission. The area under the ROC curves did not differ significantly.
Predicting pER (MES 0–1 vs 2–3)
ROC curve analysis showed that FCP had better predictive values for identifying patients with pER compared to PGE-MUM (AUC 0.87 and 0.74, respectively, P < 0.05) (Supplementary Fig. S1). The cut-off value of PGE-MUM for predicting ER (mMES = 0) was 20.4 μg/g creatinine. Levels of PGE-MUM < 20.4 μg/g creatinine could discriminate ER from the absence of pER with a sensitivity of 86.4 and specificity of 65.8, whereas levels of FCP < 250 mg/kg could discriminate pER from the absence of pER with a sensitivity of 95.3 and specificity of 51.3 (supplementary Fig. S1).
Predicting HR
ROC curve analysis showed that FCP had a superior predictive value for identifying patients with HR (AUC 0.79) compared to PGE-MUM (AUC 0.67) (Fig. 6b). Although FCP showed a relatively high AUC, the difference was not statistically significant (P > 0.05). The cutoff value of PGE-MUM for predicting HR (mMatts = 1.2) was 14.7 μg/g creatinine. The cutoff value of PGE-MUM could discriminate HR from the absence of HR with a sensitivity of 0.88 and specificity of 0.25, whereas levels of FCP < 100 mg/kg could discriminate HR from the absence of HR with a sensitivity of 0.94 and specificity of 0.33 (Fig. 6b).
Discussion
The present study is the first to report on the utility of rapidly measured PGE-MUM via CLEIA as a biomarker in pediatric UC. It is well-accepted that PGE2 is a crucial chemical mediator of inflammatory lesions, but the measurement of PGE2 is technically difficult because of its rapid metabolism18. PGE-MUM, a stable product of PGE2 metabolism, can be conveniently measured in urine samples. The stimulation of inflammatory cytokines, such as tumor necrosis factor-α, leads to the upregulation of cyclooxygenase-2, further leading to PGE2 secretion in inflamed colonic mucosal tissues of UC. In the blood, PGE2 is immediately metabolized to 15-keto-PGE2 by 15-hydroxyprostaglandin dehydrogenase (present in the lungs and colon). In the liver and kidney, 15-keto-PGE2 is converted into 13,14-dihydro-15-keto PGE2 by the action of prostaglandin Δ13-reductase, followed by β- and ω-oxidation, and is finally converted to PGE-MUM (7α-hydroxy-5,11-diketotetranor-prosta-1,16-dioic acid), which is excreted in the urine19. If the colon is highly inflamed, greater levels of PGE2 are secreted in the inflamed colonic mucosal tissue and released into the bloodstream, resulting in increased PGE-MUM levels in the urine. Arai et al. reported for the first time that PGE-MUM measured by RIA reflects the endoscopic (MES) and histologic (Matts grading) activity of UC in adult patients15. PGE-MUM is considered an ideal biomarker in pediatric UC patients because it can be measured in urine, which can be collected easily without causing pain. Furthermore, the technique of CLEIA has made it possible to measure PGE-MUM in a short time (less than 1 h)17.
In a previous study, we reported that PGE-MUM measured by RIA correlated with endoscopic and histologic activity in pediatric UC13. In this study, a good correlation was observed between PGE-MUM evaluated by CLEIA and the endoscopic and histologic activities of the entire colon in pediatric UC. Moreover, PGE-MUM had a diagnostic performance comparable to that of FCP for endoscopic activity; however, FCP had a superior diagnostic performance for detecting histologic activity.
Previous reports have proposed a multitude of threshold values for prognosticating ER and HR15,16. Sakurai et al. have suggested the employment of cutoff values of 14.5 and 14.2 µg/g・Cre to predict ER (mMES 0) and HR (mMatts 1–2), respectively16. Although the PGE-MUM values in their report were measured by RIA method, the pediatric and adult thresholds for PGE-MUM to predict ER may exhibit differences.
To the best of our knowledge, the present novel study is the largest prospective multicenter research on biomarkers in pediatric UC to date. Furthermore, the diagnostic performance of PGE-MUM in predicting ER (MES 0) was similar to that of FCP, which is accepted by Japanese health insurance, indicating that PGE-MUM can be sufficiently used as a biomarker for pediatric UC in routine practice.
In their study, Gupta et al. showed an association between higher relapse rates and persistent histologic activity in UC patients with ER20. Although there have been no reports on the usefulness of HR in pediatric UC, the achievement of HR in addition to ER might become an ultimate treatment goal in the future, as it can reduce clinical relapses in adult patients in whom HR has been achieved3,4,20. Biomarker studies on HR in UC are limited to the pediatric population. There have been some reports showing a strong correlation between FCP with histologic activity in pediatric UC, but the evidence has not been established because of the small sample sizes of these studies21,22,23. In our study, PGE-MUM was found to be inferior to FCP in terms of its diagnostic ability, slightly for ER but significantly for HR. Nonetheless, the ease of testing may outweigh the slightly inferior diagnostic utility, and more studies are needed in the future to better understand the role of PGE-MUM in pediatric UC.
Our study had several limitations. First, the target population for this study was 6–16 years old, so we could not evaluate whether PGE-MUM reflects disease activity in patients younger than six years, whose numbers have been increasing recently due to the emergence of early onset inflammatory bowel disease. Our unpublished data suggest that PGE-MUM levels are higher in infants; therefore, the normal value of PGE-MUM needs to be clarified, especially in infants. Furthermore, we did not use the Geboes score to assess histologic activity in our study, even though it has been validated as an accurate method of histologic assessment in UC patients24,25. In this study, FCP had better PPV regarding detecting HH. One of the reasons may be that the Matts grading weights inflammation cell infiltrations more heavily compared to the Geboes score. Future studies utilizing the Geboes scores in addition to Matts’ grading are needed to further clarify the relationship between various biomarkers and histologic disease activity in UC.
Overall, this study revealed that PGE-MUM was correlated with endoscopic activity in pediatric UC. Although it was somewhat inferior to FCP for HR prediction, it was almost identical to FCP in reflecting ER. PGE-MUM can be rapidly measured in urine by CLEIA, which is easier in terms of sample collection in comparison to stool or blood, and this is a clear advantage over FCP. Further studies are required before PGE-MUM can be used in clinical practice, including external validation as well as assessing reliability/agreement and responsiveness to changes after treatment.
Methods
Subjects and study design
Patients aged 6–16 years, who were evaluated and treated for UC at 14 participating Japanese pediatric centers between November 2018 and November 2020, were prospectively enrolled in this study. Additionally, controls diagnosed with FGIDs based on Rome IV by pediatric gastroenterologists were recruited from the Osaka Women’s and Children’s Hospital26. As the normal values for children remain elusive, FGID group was adopted to serve as a reference point. All controls underwent endoscopy including colonoscopy or FCP testing for diagnosis of FGIDs. UC patients aged between 6 and 16 years, who were diagnosed on the basis of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Revised Porto Criteria, were included in this study27. The exclusion criteria were as follows: (1) presence of proteinuria; (2) history of diabetes; (3) current use of stimulant laxatives (sennoside)28, nonsteroidal anti-inflammatory drugs (NSAIDs)29; (4) history of colorectal surgery and (5) pulmonary disorders including chronic fibrosing interstitial pneumonia30. Samples (urine, stool, and blood) were collected within 2 weeks prior to colonoscopy because the laxatives used in colonoscopy preparation could influence the PGE-MUM levels28.
Assessment of clinical, endoscopic, and histologic activity of UC
Clinical activity
The Pediatric Ulcerative Colitis Activity Index (PUCAI) was used to score the clinical activity of UC in each participant31. PUCAI was determined on the same day the samples were collected.
Endoscopic activity
The colonoscopic images taken in each institution were sent to Osaka Women’s and Children’s hospital. The assessment of endoscopic activity was determined using a blinded central review by three pediatric gastroenterologists. Any differences between these experts were resolved by consensus. Endoscopic activities were determined using Mayo endoscopic scoring (MES) for UC32. The colon was divided into five segments: cecum and ascending; transverse, descending, and sigmoid colon; and rectum. The highest endoscopic activity in the five segments was scored using the maximum MES (mMES). We defined the mMES of 0 as endoscopic remission (ER) and the MES 0–1 as partial endoscopic remission (pER). The sum of the maximum Mayo scores as modified Mayo scores (sMES) was calculated from the individual scores of the five segments, reflecting the endoscopic activity of the entire colon13,33,34.
Histologic activity
The histologic activity was determined using Matts’ histologic grading system35. Biopsy samples were collected from the most active inflammatory tissue in each segment of the colon under colonoscopic observation13. The biopsy samples were analyzed blindly by two pathologists. Any differences between two experts were resolved by consensus. Although ER was observed in the colon, mucosal specimens were obtained randomly from each segment. The most severe instance of inflammation in each patient was determined using the Matts score (mMatts). We defined mMatts of 1 and 2 as histologic remission (HR). The sum of the Matts scores as modified Matts scores (sMatts) was calculated from the individual scores of the five segments, reflecting the histologic activity of the entire colon13,16.
Measurement of biomarkers
PGE-MUM analysis
Spot urine samples were obtained before the day of the colonoscopy and centrifuged at 1000×g for 10 min. The derived supernatants were then stored at − 30 °C until analysis. All urine samples were sent to Fujirebio (Tokyo, Japan), where they were measured using both CLEIA and RIA methods, as previously described13,14,16,17,19. In CLEIA methods, PGE-MUM levels in urine samples were measured using a one-step immunoassay method based on CLEIA technology on the LUMIPULSE System (Fujirebio Inc., Tokyo, Japan). Prior to the immunoassay, the urine samples were treated with an alkaline solution to transform the PGE-MUM in the samples into bicyclic PGE-MUM, which is the form detected by the immunoassay. The bicyclic PGE-MUM in specimens and on particles competitively react with alkaline phosphatase-labeled anti-bicyclic PGE-MUM monoclonal antibodies, and antigen–antibody immunocomplexes are formed. After washing, a luminescent substrate solution is added, and the luminescent signal is measured. The PGE-MUM concentration of a specimen is calculated from the calibration curve17. In RIA methods, PGE-MUM levels in urine samples were measured using a radioimmunoassay kit (Institute of Isotopes Co., Ltd, Budapest, Hungary)19. Alkaline treatment was conducted by adding 100 μL of 1-mol NaOH to 50 μL of the urine sample. Thereafter, the samples were stored at room temperature for 30 min. With this treatment, the PGE-MUM in the sample was converted to bicyclic PGE-MUM. After neutralization by adding 100 μL of 1-mol hydrochloric acid, the treated urine sample was further diluted fivefold with 1000 μL of assay buffer (50-mM phosphate buffer, pH 7.4, containing 0.1% gelatin and 0.1% sodium azide). A sample or standard (100 μL) was dispensed into a reaction tube, and 100 μL of 125 I-bicyclic PGE-MUM (approximately 680 Becquerel) and 100 μL of rabbit antiserum to bicyclic PGE-MUM were added. After overnight incubation at 2–8 °C, 250 μL of the separating agent containing paramagnetic particles coated with antirabbit immunoglobulin was added to each tube and was incubated for 15 min at room temperature. The bound fraction was separated by centrifugation, and the radioactivity of each tube was measured16. The measured PGE-MUM values (CLEIA and RIA) were corrected for urinary creatinine levels.
FCP analysis
Fecal samples for measuring FCP concentration were collected within two weeks before the day of the colonoscopy. The samples were stored at − 20 °C for subsequent measurements. FCP levels were determined using the EliA Calprotectin 2 kit (Thermo Fisher Scientific, Tokyo, Japan).
Statistical analysis
For data analysis, continuous variables were expressed as the median and interquartile range (IQR). The Spearman’s rank correlation was used to investigate the relationships between the studied biomarkers. The Wilcoxon rank-sum test was applied to compare the groups. A receiver operating characteristic (ROC) curve was used to determine the cutoff value of each biomarker to predict ER or HR in patients with UC, and the corresponding area under the ROC curve (AUC) was also calculated. Further, to evaluate the cutoff points of PGE-MUM, we used the Youden index36. The cutoffs for ER and HR, as determined by FCP analysis (< 250 mg/kg and < 100 mg/kg, respectively), were adapted as the standard cutoffs37,38. Thereafter, we examined the sensitivity, specificity, predictive values, and accuracy of all evaluated biomarkers for ER and HR. All statistical analyses were performed using STATA 17.0 (StataCorp, College Station, TX, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). P values of < 0.05 were considered statistically significant.
Ethical statement
The study protocol complied with the ethical guidelines stated in the Declaration of Helsinki (2013 revision) and was approved by the Ethics Committee of Osaka Women’s and Children’s Hospital (registration number 1157-8) and its counterparts at other participating centers. Written informed consent was obtained from all enrolled patients and/or their parents.
Data availability
The data underlying this article will be shared by the corresponding author on reasonable request.
References
Frøslie, K. F., Jahnsen, J., Moum, B. A., Vatn, M. H., IBSEN Group. Mucosal healing in inflammatory bowel disease: Results from a Norwegian population-based cohort. Gastroenterology 133, 412–422 (2007).
Lichtenstein, G. R. & Rutgeerts, P. Importance of mucosal healing in ulcerative colitis. Inflamm. Bowel Dis. 16, 338–346 (2010).
Bryant, R. V. et al. Beyond endoscopic mucosal healing in UC: Histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 65, 408–414 (2016).
Ozaki, R. et al. Histological risk factors to predict clinical relapse in ulcerative colitis with endoscopically normal mucosa. J. Crohns. Colitis 12, 1288–1294 (2018).
Hagiwara, S.-I. et al. Pediatric patient and parental anxiety and impressions related to initial gastrointestinal endoscopy: A Japanese Multicenter Questionnaire Study. Scientifica 2015, 797564 (2015).
Vejzovic, V. Bowel cleansing prior to pediatric colonoscopy: The children’s and parent’s perspective. ARC J. Nurs. Healthc. 3, 18–23 (2017).
ASGE Standards of Practice Committee et al. Modifications in endoscopic practice for pediatric patients. Gastrointest. Endosc. 79, 699–710 (2014).
Mosli, M. H. et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis. Am. J. Gastroenterol. 110, 802–819 (2015) (quiz 820).
Takashima, S. et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am. J. Gastroenterol. 110, 873–880 (2015).
Chambers, R. E., Stross, P., Barry, R. E. & Whicher, J. T. Serum amyloid A protein compared with C-reactive protein, alpha 1-antichymotrypsin and alpha 1-acid glycoprotein as a monitor of inflammatory bowel disease. Eur. J. Clin. Investig. 17, 460–467 (1987).
Turner, D. et al. C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) or both? A systematic evaluation in pediatric ulcerative colitis. J. Crohns. Colitis 5, 423–429 (2011).
Shinzaki, S. et al. Leucine-rich alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J. Crohns. Colitis 11, 84–91 (2017).
Hagiwara, S.-I. et al. Prostaglandin E-major urinary metabolite as a biomarker for pediatric ulcerative colitis activity. J. Pediatr. Gastroenterol. Nutr. 64, 955–961 (2017).
Inagawa, T. et al. Simplified immunoassays of prostaglandin E main metabolite in human urine. Adv. Prostaglandin Thromboxane Leukot. Res. 11, 191–196 (1983).
Arai, Y. et al. Prostaglandin E-major urinary metabolite as a reliable surrogate marker for mucosal inflammation in ulcerative colitis. Inflamm. Bowel Dis. 20, 1208–1216 (2014).
Sakurai, T. et al. Prostaglandin E-major urinary metabolite diagnoses mucosal healing in patients with ulcerative colitis in remission phase. J. Gastroenterol. Hepatol. 37, 847–854 (2022).
Katagiri, N., Wakabayashi, M. & Arihiro, S. Development of fully automated chemiluminescent enzyme immunoassay system for measurement of prostaglandin E major urinary metabolites (PGE-MUM). Jpn. J. Clin. Lab. Autom. 42, 584–590 (2017).
Goodwin, J. S. & Ceuppens, J. Regulation of the immune response by prostaglandins. J. Clin. Immunol. 3, 295–315 (1983).
Okayasu, I. et al. Significant increase of prostaglandin E-major urinary metabolite in male smokers: A screening study of age and gender differences using a simple radioimmunoassay. J. Clin. Lab. Anal. 28, 32–41 (2014).
Gupta, A., Yu, A., Peyrin-Biroulet, L. & Ananthakrishnan, A. N. Treat to target: The role of histologic healing in inflammatory bowel diseases: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 19, 1800-1813.e4 (2021).
Canani, R. B. et al. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig. Liver Dis. 40, 547–553 (2008).
Dolinšek, J., Rižnik, P., Sabath, L. & Mičetić-Turk, D. Fecal calprotectin as a marker of the severity of mucosal inflammation in children with inflammatory bowel disease. Wien. Klin. Wochenschr. 128, 253–259 (2016).
Crawford, E. et al. Association of fecal calprotectin with endoscopic and histologic activity in pediatric inflammatory bowel disease. JPGN Rep. 2, e129 (2021).
Geboes, K. et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 47, 404–409 (2000).
Turner, D. et al. STRIDE-II: An update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 160, 1570–1583 (2021).
Hyams, J. S. et al. Functional disorders: Children and adolescents. Gastroenterology 150, 1456-1468.e2 (2016).
Levine, A. et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 58, 795–806 (2014).
Fujiwara, M. et al. Significant increase in prostaglandin E-main urinary metabolite by laxative administration: comparison with ulcerative colitis. Digestion 61, 201–206 (2000).
Arai, Y. et al. Prostaglandin E-major urinary metabolite as a biomarker for inflammation in ulcerative colitis: Prostaglandins revisited. Digestion 93, 32–39 (2016).
Horikiri, T. et al. Increased levels of prostaglandin E-major urinary metabolite (PGE-MUM) in chronic fibrosing interstitial pneumonia. Respir. Med. 122, 43–50 (2017).
Turner, D. et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: A prospective multicenter study. Gastroenterology 133, 423–432 (2007).
Schroeder, K. W., Tremaine, W. J. & Ilstrup, D. M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 317, 1625–1629 (1987).
Shimizu, H. et al. Both fecal calprotectin and fecal immunochemical tests are useful in children with inflammatory bowel disease. J. Gastroenterol. 57, 344–356 (2022).
Lobatón, T. et al. The Modified Mayo Endoscopic Score (MMES): A new index for the assessment of extension and severity of endoscopic activity in ulcerative colitis patients. J. Crohns. Colitis 9, 846–852 (2015).
Matts, S. G. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q. J. Med. 30, 393–407 (1961).
Youden, W. J. Index for rating diagnostic tests. Cancer 3, 32–35 (1950).
D’Haens, G. et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 18, 2218–2224 (2012).
Zittan, E. et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn’s disease. Inflamm. Bowel Dis. 22, 623–630 (2016).
Acknowledgements
The authors greatly appreciate Prof. Mutsunori Fujiwara, MD, PhD, for his valuable advice on this study. The authors also thank all participating patients, their families, and physicians for collaborating in data collection. We would like to thank Editage (www.editage.com) for English language editating.
Funding
This work was supported by the Uehara Memorial Foundation Research Grant (2018-181 to S-IH), Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics Grant (30-Y17 to S-IH), and Grant-in-Aid from the Japanese Society of Pediatric Inflammatory Bowel Disease (2019-4 to S-IH). This work was also partially funded by Fujirebio Inc., which had no role in the study design; collection, analysis, or interpretation of the data; writing of the manuscript; or the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
S.-I.H. designed the study. Data were collected by S.-I.H., N.A., K.H., T.H., T.I., H.S., T.Mi., T.K., R.K., R.M., H.K., F.K., Y.N., T.K., T.Ma., and H.H. S.-I.H. and D.H. analyzed and interpreted the data. S.-I.H., T.Ma., and H.H. made endoscopic diagnoses. T.Y. and I.O. made a histopathological diagnosis. S.-I.H. wrote the manuscript. M.S. and Y.E. critically revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Tatsuki Mizuochi reports personal fees from AbbVie GK and EA Pharma Co., Ltd. Masayuki Saruta reports personal fees from Abbvie GK, Janssen Pharma K.K., Mitsubishi Tanabe Pharma, Takeda Pharmaceutical Co., Ltd., EA Pharma Co., Ltd, Gilead Sciences K.K.; grant for commissioned/joint research from EPS Corporation: Scholarship grants from Zeria Pharmaceutical Co., Ltd, Mochida Pharmaceutical Co., Ltd, EA Pharma Co., Ltd, Kissei Pharmaceutical Co., Ltd. Reiko Kunisaki reports research grant and speaker’s and/or consultancy fees from AbbVie GK, EA Pharma Co., Ltd, Eli Lilly, Janssen Pharmaceutical, JIMRO Co., Ltd., Kissei Pharmaceutical Co., Ltd, Kyorin Pharmaceutical Co. Ltd., Nippon Kayaku Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Inc, Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., outside the submitted work. Other authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hagiwara, Si., Abe, N., Hosoi, K. et al. Utility of a rapid assay for prostaglandin E-major urinary metabolite as a biomarker in pediatric ulcerative colitis. Sci Rep 13, 9898 (2023). https://doi.org/10.1038/s41598-023-37145-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37145-6
This article is cited by
-
First case report of dichorionic diamniotic twins with chronic enteropathy associated with the SLCO2A1 gene
Clinical Journal of Gastroenterology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.