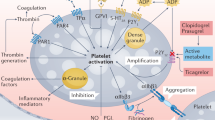
Abstract
Cangrelor, the first intravenous P2Y12 inhibitor (P2Y12-I), has been approved on the basis of three large RCTs from the CHAMPION program which nevertheless have been criticized for the low bleeding risk of the enrolled patients, the large quote of chronic coronary syndromes, and the use of Clopidogrel as control arm even in the setting of acute coronary syndromes (ACS). We sought to investigate, in the setting of ACS, the comparative performance of Cangrelor in terms of in-hospital ischemic and haemorrhagic outcomes compared with the current gold-standard of oral P2Y12-I. The study retrospectively enrolled 686 consecutive patients admitted to the Divisions of Cardiology of Policlinico of Bari and L. Bonomo Hospital of Andria for ACS and treated with percutaneous coronary intervention. The study population was divided according to the P2Y12-I treatment strategy in two groups: patients given an oral P2Y12-I and patients receiving Cangrelor in the cath lab followed by an oral P2Y12-I. Clinical endpoints included death, ischemic and bleeding events occurring during hospital stay. Cangrelor treated patients presented higher clinical risk profile at presentation and faced higher death rate. However, after PS matching, in-hospital mortality resulted comparable between the groups and Cangrelor use was associated with reduced in-hospital definite stent thrombosis (p = 0.03). Data from our real-world registry highlight that, in the setting of ACS, Cangrelor is prevalently used in patients with very challenging clinical presentations. The adjusted analysis provides for the first time promising data on stent thrombosis reduction associated with Cangrelor use.
Similar content being viewed by others
Introduction
Percutaneous coronary intervention (PCI) with drug eluting stent (DES) implantation has lately become the revascularization of choice for most patients with acute coronary syndromes (ACS)1,2. Despite the constant evolution of devices and techniques, stent thrombosis (ST) remains the clinically most relevant short-term complication after PCI, especially in the setting of ACS3. Full platelet inhibition is, indeed, required during percutaneous revascularizations and is achieved through dual antiplatelet therapy (DAPT): the association of acetylsalicylic acid and an inhibitor of the platelet P2Y12 receptor for adenosine 5’-diphosphate (P2Y12-I). Nevertheless, in time dependent clinical scenarios, effectiveness of DAPT is potentially hindered by the delayed effect derived by the oral administration of most P2Y12-I4. In fact, limitations of Clopidogrel, Prasugrel, and Ticagrelor (all oral P2Y12-I) are the slow onset and offset of action and the impossibility to be administrated or to be fully effective in patients with orotracheal intubation, vomit, and impaired intestinal absorption 5. In detail, the extremely short time gap between first medical contact (FMC) and primary PCI jeopardizes the effectiveness of the administration of oral P2Y12-I in ST elevation myocardial infarction (STEMI) patients, while in the setting of non-ST elevation (NSTE) ACS the administration of an oral P2Y12-I prior to coronary angiography (pretreatment strategy) is discouraged by the current ESC guidelines1,6.
In this scenario the potential role of Cangrelor, the first intravenous P2Y12-I approved by the European Medicines Agency (EMA) in 2017 based on the three large randomized clinical trials of the CHAMPION (Cangrelor versus standard therapy to achieve optimal management of platelet inhibition) program, is noteworthy7,8,9. However, these randomized trials have raised some criticisms such as the low bleeding risk of the enrolled cohorts, the large quote of chronic coronary syndromes (CCS), and mainly the use of Clopidogrel as control arm even in the setting of ACS. As Clopidogrel has not been the P2Y12-I of choice in ACS since 201210, the latter limitation seems the most crucial and represents a confounder for the interpretation of data on both ST and bleeding. Aim of our study was to evaluate the real-world performance of Cangrelor in ACS patients in terms of in-hospital ischemic and hemorrhagic outcomes compared with the current gold-standard of oral P2Y12-I.
Methods
The study, which was designed and written in accordance to the STROBE checklist, retrospectively enrolled all consecutive patients who accessed the Cardiology Divisions of the Azienda Ospedaliero Universitaria Consorziale—Policlinico of Bari and L. Bonomo Hospital of Andria with the diagnosis of ACS and underwent PCI. Enrollment started from the date of the first availability of Cangrelor in each center and ended in January 2021; the first patient treated with Cangrelor was in September 2019. The Independent Ethical Committee of the Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari approved the study. Informed consent was obtained according to the study protocol. PCI procedures were performed per standard of care and at the discretion of the treating physicians. All treatments were carried out in accordance with current guidelines and regulations. The use of Cangrelor, which was administered only to P2Y12-I naïve patients, was decided by the interventional cardiologists on an individual basis, taking into consideration both clinical and procedural features. In all cases the time-point of Cangrelor administration was after coronary angiography and immediately before PCI with a 30 microg/kg bolus followed by a 4 microg/kg/min infusion as per label recommendations. The adjunctive pharmacological therapy was at physicians' discretion and largely based on contemporary best practice according to the national and European scientific societies' guidelines. Taking part to the study did not modify in any way patients’ diagnostic and therapeutic workup. The registry was broadly inclusive; the only exclusion criteria were age younger than 18 years and enrollment in other clinical trials. Information on demographics, baseline clinical characteristics, processes of care, and in-hospital outcomes were collected.
Due to the observatory nature of the study no preliminary hypotheses were generated. Clinical endpoints were evaluated during hospital stay and included death, ischemic and bleeding events. Bleeding was defined according to the Bleeding Academic Research Consortium (BARC), Global Use of Strategies to Open occluded coronary arteries (GUSTO), Thrombolysis in Myocardial Infarction (TIMI), and International Society on Thrombosis and Haemostasis (ISHT) definitions11,12,13,14, acute myocardial infarction (AMI) on the basis of its fourth universal definition15 and periprocedural myocardial infarction according to the CHAMPION PHOENIX definition16. Patients at high bleeding risk (HBR) were identified according to the Academic Research Consortium (ARC) definition17. The hemorrhagic risk was also calculated based on the PRECISE DAPT score18. Definite or probable ST was assessed according to the definition of the Academic Research Consortium19; in detail, definite ST was defined as symptoms suggestive of an acute coronary syndrome and angiographic or pathologic confirmation of stent thrombosis, while probable ST as an unexplained death within 30 days or target vessel myocardial infarction without angiographic confirmation of stent thrombosis. Complex PCI was defined as a procedure with at least one of the following angiographic characteristics: 3 vessels treated, ≥ 3 stents implanted, ≥ 3 lesions treated, bifurcation with deployment of 2 stents, total stent length > 60 mm, and chronic total occlusion20,21. High-risk clinical profile was defined as cardiogenic shock (CS) and/or treatment with inotropic drugs and/or cardiocirculatory arrest (CCA) and/or orotracheal intubation (OTI) at presentation. CS was defined as systolic blood pressure ≤ 90 mmHg (without inotropic drugs or intra-aortic balloon support) that is unresponsive to intravenous fluid administration, secondary to cardiac dysfunction, and associated with signs of hypoperfusion (cold extremities, impaired mental status, or urine output ≤ 30 ml/h)22.
The study population was divided according to the P2Y12-I treatment strategy in two groups: patients given an oral P2Y12-I and those who received Cangrelor in the cath lab followed by an oral P2Y12-I (non-Cangrelor and Cangrelor group respectively). Baseline characteristics, procedural features, and follow-up data of the overall population and per group are presented. All endpoints were assessed at the time of discharge or afterward and mean hospitalization time was 7.65 ± 5.50 and 7.07 ± 4.24 days for the Cangrelor and non-Cangrelor group respectively (p = 0.167).
The database was built up by Excel software (Microsoft Corporation, Redmond, Washington, USA); statistical analysis was performed using SPSS version 26 software (IBM, Inc., Armonk, NY). Continuous variables are presented as means ± standard deviations and compared using paired Student’s t-tests. Categorical variables are shown as numbers with percentages and analyzed using the chi-square analysis and Fisher’s exact test for counts < 5. The relationship between Cangrelor use and both baseline characteristics and procedural features was examined using univariate logistic regression analysis with odds ratio (OR) and 95% confidence intervals (CIs). Statistically significant (p < 0.05) predictors of Cangrelor use were entered into multivariable logistic regression models. The data underlying this article will be shared on reasonable request to the corresponding author.
For in-hospital mortality, the association with baseline characteristics, procedural features, and in-hospital adverse events has been tested with an univariate logistic regression analysis; ORs were calculated with 95% CIs. Each of the statistically significant (p < 0.05) predictor of outcome was entered into multivariable logistic regression models.
A propensity score (PS) analysis was also used to adjust for differences in patients’ baseline and procedural characteristics; the following parameters were selected: age, gender, diabetes mellitus (DM), STEMI diagnosis, chronic kidney disease (CKD), high-risk clinical profile, HBR profile, left ventricle ejection fraction (LVEF) < 30%, and femoral access. These covariates were chosen among those significantly different within our population between the Cangrelor and non-Cangrelor group and/or significantly associated with mortality in the multivariate logistic regression model and/or well-known predictors of adverse events from the literature. The 1:1 nearest neighbor matching without replacement method was used (standard deviation and caliper value were 0.11 and 0.2 respectively) and performed by PScore module from Statistics for Data Analysis powered by SPSS. Standardized differences and c-statistic were used to confirm negligible differences in the mean or prevalence of selected covariates between treatment groups. For all tests significance was set for a 2-tailed value of p < 0.05.
Results
Cangrelor group and non-Cangrelor group included 198 and 488 patients, respectively. Mean age of the whole population was 67.4 ± 11.7 years; baseline clinical characteristics of patients as a whole and by group are depicted in Table 1. Patients in the non-Cangrelor group showed higher prevalence of DM and of prior AMI, PCI, and myocardial revascularization. Conversely, Cangrelor group presented higher-risk clinical profile confirmed by greater prevalence of LVEF < 30%, inotropic drug infusion, CCA, CS, and previous haemorrhages. Supplementary Table 1 shows oral P2Y12-I treatment in the overall population and by group: clopidogrel use was more prevalent in the Cangrelor group.
In the univariate and multivariate logistic regression analysis, predictors of Cangrelor use resulted prior bleeding and LVEF < 30%; high-risk clinical profile reached threshold for significance in the univariate while only approached significance in the multivariate analysis (Table 2).
Procedural features and in hospital follow-up data are described in Tables 3 and 4 respectively. Cangrelor group showed higher rate of femoral access and higher stent number and total stent length, despite a lower quote of multivessel coronary artery disease (CAD). In terms of clinical outcomes, Cangrelor treated patients faced higher occurrence of all-cause death. In the univariate logistic regression analysis, age, female sex, STEMI presentation, DM, CKD, LVEF < 30%, high-risk clinical profile, non-invasive ventilation (NIV), complex PCI, multivessel PCI, left-main (LM) PCI, femoral access, HBR profile, Cangrelor use, and in-hospital bleeding were associated with in-hospital all-cause death. The multivariate analysis proved that only age, STEMI, high-risk clinical profile, femoral access, and in-hospital bleeding were associated with in-hospital mortality (Table 5).
After PS-matching a population of 356 patients was selected; baseline clinical characteristics are shown in Supplementary Table 2. C-statistic, used as post-matching diagnostic, and standardized differences confirmed negligible differences in the mean or prevalence of the selected covariates (age, gender, DM, STEMI, CKD, high-risk clinical profile, HBR-ARC, LVEF < 30%, and femoral access) between treatment groups (Supplementary Fig. 1). Table 6 summarizes the in-hospital follow-up data of the PS-matched population: noteworthy, no statistically significant difference between the two groups was found in terms of all-cause death. Nonetheless, in divergence with the results of the unmatched population, Cangrelor use was associated with reduced in-hospital definite stent thrombosis (p = 0.03) (Fig. 1).
ACS Acute coronary syndrome, PS Propensity score, ASA Acetylsalicylic acid; P2Y12-I, P2Y12 inhibitor; MI Myocardial infarction, BARC Bleeding Academic Research Consortium, GUSTO Global use of strategies to open occluded coronary arteries, TIMI Thrombolysis in myocardial infarction, ISTH International Society on Thrombosis and Haemostasis.
Discussion
The main findings of our paper are the following: 1. Cangrelor was mainly used in ACS patients with high-risk clinical features and tendency to high bleeding risk; 2. the Cangrelor group underwent more extensive and complex coronary revascularization; 3. the Cangrelor group faced higher in-hospital mortality, which turned to be comparable between the two groups after PS adjustment for baseline clinical risk profile; 4. in the adjusted analysis Cangrelor use was associated with reduced in-hospital definite stent thrombosis in the absence of increased bleeding complications.
The present study explored the use of Cangrelor in the clinical scenario of ACS patients treated with PCI. To the best of authors’ knowledge this is the first real world investigation which analyzed in a comparative fashion Cangrelor performance. Our data confirmed that Cangrelor is more often used in clinically unstable patients such as those with CS and/or treated with inotropic drugs and/or with CCA at presentation and/or intubated and, concordantly, in those with a severely reduced LVEF. This is in line with previous evidence23,24 and can be partly explained by the impracticability of the oral route or the uncertainty of intestinal absorption in patients with high-risk clinical presentation, both limitations easily overcome by the intravenous administration of Cangrelor. Moreover, our analysis suggests the possible preference towards Cangrelor in patients with higher risk of bleeding as indicated by the higher rate of patients with history of previous bleeding and the higher (despite at the limit for significance) PRECISE DAPT score in the Cangrelor group. The higher bleeding risk in the Cangrelor group is further indirectly supported by the wider use of Clopidogrel in this group, which cannot be explained by the need for triple antithrombotic therapy being the prevalence of oral anticoagulation comparable between the two groups. This therapeutic choice could be hypothesized to be founded upon the rapid pharmacokinetic, in this case the fast offset of action, of Cangrelor which is likely perceived by the interventional cardiologists to be safer and more manageable than oral P2Y12-I.
In addition, procedural data highlight that, despite a greater quote of patients with multivessel CAD in the non-Cangrelor group, the patients treated with Cangrelor underwent more complex percutaneous interventions with a higher number of implanted stents per patient and a higher total stent length. Given the observational nature of the study, it can be only assumed that interventional cardiologists feel more confident in performing more extensive revascularizations when a full and rapid antiaggregation is guaranteed by the use of this intravenous antiplatelet agent.
Prerogative of Cangrelor, as mentioned above, is the rapidity of both onset and offset of action. Pharmacokinetic studies have proved indeed that platelet function is completely restored within 60 min after the stop of drug infusion, and Cangrelor is accordingly considered a periprocedural drug. Based on this assumption, and in line with the CHAMPION studies, the rationale for a clinical follow-up exceeding the hospital stay is lacking. Our outcome data suggest a trend toward better ischemic outcomes (lower rates of ischemic cerebro-cardiovascular complications, periprocedural AMI, definite ST) and slightly worse hemorrhagic complications. Despite the sample size does allow only hypotheses, these results appear in line with the registration trials of the CHAMPION program7,8,9. Notwithstanding the randomized nature, the CHAMPION studies present some limitations which have been widely recognized over time. Firstly the CHAMPION population was at relatively low ischemic risk since more than 30% of patients were addressed to PCI because of CCS25, which does not reflect the prevalent clinical setting in which the drug has been used, so far, in the real-world as suggested by several recent registries23,24,26. The second and probably the main point of criticism against the CHAMPION studies is the use of Clopidogrel in the control arm, despite more than two third of patients had ACS. In this subpopulation the reliability of the comparative evaluation of Cangrelor performance in terms of both ischemic and hemorrhagic events could result jeopardized. In opposition, in our study almost 80% of patients in the non-Cangrelor group received either Ticagrelor or Prasugrel (74.6% and 5.1% respectively) in line with the contemporary guidelines’ recommendations1,2.
On the other hand, our data must be interpreted with caution because of the non randomized nature of the enrollment. The Cangrelor group faced a significantly higher mortality because of the propensity to use this “new therapeutic weapon”, which allows to avoid bowel absorption and provides roughly instantaneous antiplatelet effect, in the most critical clinical scenarios. When we searched for the determinants of in-hospital death, age, high-risk clinical profile, in-hospital bleeding, and STEMI presentation resulted indeed predictors of outcome, while Cangrelor use did not.
Purposively, discrepancies in baseline clinical risk profile have been overcome with the propensity score matching. The adjusted analysis highlighted the absence of significant differences between groups in terms of mortality, which confirms our previous assumption. Even more remarkable, we found a significant reduction of in-hospital definite ST in the Cangrelor group, which was the key secondary endpoint of the Champion Phoenix trial. To the best of authors' knowledge, this finding represents the first report of reduced ST with Cangrelor in comparison to a group prevalently treated with the most potent oral P2Y12-I Ticagrelor and Prasugrel. Noteworthy, at variance with the registration trials, our endpoints were evaluated during hospital stay and not at 48 h from PCI; as a consequence we cannot exclude the influence of the oral P2Y12-I the Cangrelor patients have been switched into after infusion. Nevertheless, the Cangrelor group showed a higher percentage of patients treated with Clopidogrel than the non-Cangrelor group and this evidence further substantiates Cangrelor efficacy in preventing ST. In the matched population the use of Cangrelor did not conversely result to be associated to higher rate of bleeding events.
The present study should be interpreted in the light of some limitations. First, this was a nonrandomized study resulting in cohorts with differences in baseline, angiographic, and procedural characteristics. Although we sought to reduce potential confounding using PS-matching analysis, we were not able to correct for the unmeasured variables. Second, the use of Cangrelor and the entire procedural strategy were at the discretion of the physician. Third, sample size is small. As a consequence, our findings should be regarded as only hypotheses generating and would require further confirmation from a large, pragmatic, and randomized trial. Nevertheless, it is authors’ opinion that randomized trials on ACS patients treated with Cangrelor are not expected.
Conclusion
Data from our real-world registry highlight that in the ACS context Cangrelor is prevalently used in patients with very challenging clinical presentations. This bias justifies the higher mortality rate in the Cangrelor group at the unadjusted analysis. On the other hand, the adjusted analysis suggests the potential replicability in the real world of the beneficial effect of Cangrelor in terms of definite ST suggested by the randomized trials, what’s more in a population treated according to the current gold-standard of antithrombotic therapy.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Collet, J. P. et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 42, 1289–1367. https://doi.org/10.1093/eurheartj/ehaa575 (2021).
Ibanez, B. et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39, 119–177. https://doi.org/10.1093/eurheartj/ehx393 (2018).
Pepe, M. et al. Comparative effectiveness and safety of polymer-free biolimus-eluting stent and durable polymer everolimus-eluting stent in all-comer patients who underwent percutaneous coronary interventions. Am. J. Cardiol. 124, 195–204. https://doi.org/10.1016/j.amjcard.2019.04.015 (2019).
Valgimigli, M. et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 39, 213–260. https://doi.org/10.1093/eurheartj/ehx419 (2018).
Alexopoulos, D., Pappas, C., Sfantou, D. & Lekakis, J. Cangrelor in percutaneous coronary intervention: Current status and perspectives. J. Cardiovasc. Pharmacol. Ther. 23, 13–22. https://doi.org/10.1177/1074248417715004 (2018).
Pepe, M. et al. Time-dependent benefits of pre-treatment with new oral P2Y(12) -inhibitors in patients addressed to primary PCI for acute ST-elevation myocardial infarction. Catheter. Cardiovasc. Interv. 93, 592–601. https://doi.org/10.1002/ccd.27863 (2019).
Bhatt, D. L. et al. Intravenous platelet blockade with Cangrelor during PCI. N. Engl. J. Med. 361, 2330–2341. https://doi.org/10.1056/NEJMoa0908629 (2009).
Bhatt, D. L. et al. Effect of platelet inhibition with Cangrelor during PCI on ischemic events. N. Engl. J. Med. 368, 1303–1313. https://doi.org/10.1056/NEJMoa1300815 (2013).
Abtan, J. et al. Efficacy and safety of Cangrelor in preventing periprocedural complications in patients with stable angina and acute coronary syndromes undergoing percutaneous coronary intervention: The CHAMPION PHOENIX trial. JACC Cardiovasc. Interv. 9, 1905–1913. https://doi.org/10.1016/j.jcin.2016.06.046 (2016).
Members, A. T. F. et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur. Heart J. 33, 2569–2619. https://doi.org/10.1093/eurheartj/ehs215 (2012).
Gusto Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N. Engl. J. Med. 329, 673–682. https://doi.org/10.1056/nejm199309023291001 (1993).
Mega, J. L. et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): A randomised, double-blind, phase II trial. Lancet 374, 29–38. https://doi.org/10.1016/s0140-6736(09)60738-8 (2009).
Schulman, S. & Kearon, C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 3, 692–694. https://doi.org/10.1111/j.1538-7836.2005.01204.x (2005).
Mehran, R. et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 123, 2736–2747. https://doi.org/10.1161/circulationaha.110.009449 (2011).
Thygesen, K. et al. Fourth universal definition of myocardial infarction (2018). Circulation 138, e618–e651. https://doi.org/10.1161/cir.0000000000000617 (2018).
Leonardi, S. et al. Rationale and design of the Cangrelor versus standard therapy to acHieve optimal management of platelet InhibitiON PHOENIX trial. Am. Heart J. 163, 768-776.e762. https://doi.org/10.1016/j.ahj.2012.02.018 (2012).
Urban, P. et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for high bleeding risk. Eur. Heart J. 40, 2632–2653. https://doi.org/10.1093/eurheartj/ehz372 (2019).
Costa, F. et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 389, 1025–1034. https://doi.org/10.1016/s0140-6736(17)30397-5 (2017).
Mauri, L. et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N. Engl. J. Med. 356, 1020–1029. https://doi.org/10.1056/NEJMoa067731 (2007).
Giustino, G. et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J. Am. Coll. Cardiol. 68, 1851–1864. https://doi.org/10.1016/j.jacc.2016.07.760 (2016).
Pepe, M. et al. Impact of insulin-treated and noninsulin-treated diabetes mellitus in all-comer patients undergoing percutaneous coronary interventions with polymer-free biolimus-eluting stent (from the RUDI-FREE registry). Am. J. Cardiol. 124, 1518–1527. https://doi.org/10.1016/j.amjcard.2019.08.015 (2019).
Pepe, M. et al. Role of plasma glucose level on myocardial perfusion in ST-segment elevation myocardial infarction patients. J. Diabet. Compl. 32, 764–769. https://doi.org/10.1016/j.jdiacomp.2018.05.015 (2018).
Pepe, M. et al. Clinical use of cangrelor: A real-world multicenter experience from South Italy. Panminerva Med. 64, 9–16. https://doi.org/10.23736/s0031-0808.21.04437-2 (2022).
Grimfjärd, P., Lagerqvist, B., Erlinge, D., Varenhorst, C. & James, S. Clinical use of Cangrelor: Nationwide experience from the Swedish coronary angiography and angioplasty registry (SCAAR). Eur. Heart J. Cardiovasc. Pharmacother. 5, 151–157. https://doi.org/10.1093/ehjcvp/pvz002 (2019).
Steg, P. G. et al. Effect of Cangrelor on periprocedural outcomes in percutaneous coronary interventions: A pooled analysis of patient-level data. Lancet 382, 1981–1992. https://doi.org/10.1016/s0140-6736(13)61615-3 (2013).
De Luca, L. et al. Use of Cangrelor in patients with acute coronary syndromes undergoing percutaneous coronary intervention: Study design and interim analysis of the ARCANGELO study. Clin. Cardiol. 45, 913–920. https://doi.org/10.1002/clc.23878 (2022).
Author information
Authors and Affiliations
Contributions
E.C., M.P. and P.L.N. wrote the main manuscript text; M.C.C. and R.T. prepared tables; G.N. and P.L.N. did statistical analysis; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Giuseppe Biondi-Zoccai has consulted for Amarin, Balmed, Cardionovum, Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Microport, Opsens Medical, Terumo, and Translumina. All other authors report no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pepe, M., Carulli, E., Larosa, C. et al. Comparative effectiveness of Cangrelor in patients with acute coronary syndrome undergoing percutaneous coronary intervention: an observational investigation from the M.O.Ca. registry. Sci Rep 13, 10685 (2023). https://doi.org/10.1038/s41598-023-37084-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37084-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.