Abstract
Human epidermal growth factor receptor 2 (HER2) gene amplification helps identify breast cancer patients who may respond to targeted anti-HER2 therapy. This study aims to develop an automated method for quantifying HER2 fluorescence in situ hybridization (FISH) signals and improve the working efficiency of pathologists. An Aitrox artificial intelligence (AI) model based on deep learning was constructed, and a comparison between the AI model and traditional manual counting was performed. In total, 918 FISH images from 320 consecutive invasive breast cancers were analysed and automatically classified into 5 groups according to the 2018 ASCO/CAP guidelines. The overall classification accuracy was 85.33% (157/184) with a mean average precision of 0.735. In Group 5, the most common group, the consistency was as high as 95.90% (117/122), while the consistency was low in the other groups due to the limited number of cases. The causes of this inconsistency, including clustered HER2 signals, coarse CEP17 signals and some section quality problems, were analysed. The developed AI model is a reliable tool for evaluating HER2 amplification statuses, especially for breast cancer in Group 5; additional cases from multiple centres could further improve the accuracy achieved for other groups.
Similar content being viewed by others
Introduction
The human epidermal growth factor receptor 2 (HER2) oncogene is located on chromosome 17q121 and encodes a transmembrane tyrosine kinase receptor. Amplification of the HER2 gene occurs in 15–20% of breast cancer patients2 and is related to poor disease outcomes3. The introduction of the monoclonal antibody trastuzumab has greatly improved the prognoses of patients with HER2-positive breast cancer4. In addition, combined with the oestrogen receptor (ER), the progesterone receptor (PR), Ki-67, CK5/6, and the epidermal growth factor receptor (EGFR), HER2 plays an important role in the molecular classification of breast cancer 5. Different molecular subtypes might predict different outcomes6and may be associated with preferred treatment regimens5. Therefore, an accurate HER2 status assessment method is essential for breast cancer patients.
In clinical practice, immunohistochemistry (IHC) is the most widely used method. According to the 2018 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines, the immunostaining results of HER2 are reported as follows: 0, negative; 1+, negative; 2+, equivocal; and 3+, positive7. If an IHC result is 2+, a further HER2 assessment must be carried out by fluorescence in situ hybridization (FISH) with the same specimen or by a new test with another new specimen7. For FISH, HER2 amplification statuses are divided into five groups based on the HER2/CEP17 ratio and the average HER2 copy number7. Although IHC is efficient and economical, FISH is more objective and quantitative. In addition, FISH is superior in terms of predicting the clinical outcomes of breast cancer8. More importantly, HER2 gene amplification can also be found in HER2 IHC 0 or 1+ cases by FISH testing9,10. FISH is thus indispensable for HER2 assessment. However, it is undeniable that manually counting HER2 signals is time-consuming and that interobserver variability does exist. A series of automated image analysis methods for HER2 FISH interpretation have emerged over the past two decades, including automated image analysers11,12, automated scanning-based imaging approaches (Metasystems13 and the Ariol system14), the Micrometastasis Detection System (MDS)15, and automated signal enumeration systems (CW4000 CytoFISH software16 and Vysis AutoVysion17). These automated techniques are greatly helpful for HER2 FISH assessments and can provide results that are consistent with manual counting18. However, poorly segmented and incorrectly selected cells need to be manually removed11, and the automated scoring results produced by HER2 FISH on the Ariol system did not show excellent concordance with pathologists14. In addition, automated image analysis requires a large amount of storage power for recording FISH images, potentially resulting in clinical use limitations.
In recent years, deep learning and artificial intelligence (AI) have been greatly developed and utilized in a wide range of fields19. Based on a deep learning approach, a previous study established an algorithm for automatically predicting the molecular subtypes and detecting the intratumoral heterogeneity of breast cancer20. Additionally, an AI model can predict breast cancer grades, histologic subtypes, ER statuses21 and HER2 IHC scores22 from haematoxylin and eosin (HE)-stained images. However, studies assessing the HER2 gene amplification statuses of FISH images based on deep learning and AI are scarce23.
In the present study, a deep learning-based AI method was established for automated HER2 FISH evaluation. To evaluate the algorithm, breast cancers belonging to five HER2 FISH groups in a testing set were diagnosed by the Aitrox AI model and the gold standard, and their diagnosis results were compared.
Materials and methods
Sample selection and HER2 FISH image acquisition
Three hundred and twenty invasive breast cancers determined with both HER2 IHC and FISH detection were collected consecutively from the archives of the Department of Pathology, Fudan University Shanghai Cancer Center (FUSCC). All 320 cases included primary breast cancers (293 cases) and metastatic breast cancer tissues in lymph nodes (17 cases), the lung (3 cases), the liver (3 cases), the chest wall (3 cases), and bone (1 case). All samples were included in the study with approval from the independent ethical committee/institutional review board, and all participants signed informed consent forms. All methods were performed in accordance with the relevant guidelines and regulations. All study activities were conducted in accordance with the Declaration of Helsinki and relevant guidelines and regulations.
Representative 4-μm-thick formalin-fixed paraffin-embedded (FFPE) tumour sections were assessed by two pathologists (TX and QMB) to determine the carcinoma areas using HE-stained sections. Afterwards, the PathVysion HER2 DNA Probe Kit (Abbott Molecular, Abbott Park, Illinois) was used for HER2 FISH according to the manufacturer’s instructions. The specific experimental method for FISH was as follows. The sections were baked at 65 °C for 30 min and dewaxed 3 times in xylene at room temperature for 10 min each time, followed by immersion in 100% ethanol for 5 min. The sections were sequentially placed in 100% ethanol for 2 min, 85% ethanol for 2 min and 70% ethanol for 2 min, and the sections were immersed in pure water at room temperature for 3 min. The sections were treated with pure water at 88–92 °C for 30 min. The pepsin solution was prepared as follows: pepsin powder (activity 1:3000, Solarbio) (75 mg) was dissolved in 150 ml 0.01 HCL with pH ≈ 2.0. After cooling the sections to below 60 °C, they were immersed in pepsin solution and incubated for 15–20 min at 54 °C. The sections were then rinsed once in pure water. The sections were dehydrated in 70% ethanol for 1 min at room temperature. The slides were then dried. The probe working solution was added dropwise to the target hybridization areas of the sections, a coverslip was immediately applied, and the periphery of the coverslip was sealed with rubber cement. The slides were placed in a hybridization apparatus, and a hybridization procedure was set. Denaturation was performed at 80 °C for 8 min, and this was followed by hybridization at 39 °C for 16–18 h. Then, the slide sealant was carefully removed in a dark environment, and the slides were immersed in 0.4× saline sodium citrate solution for approximately 10 min. The slides were placed in a 0.3% NP-40/0.4× saline sodium citrate solution (preheated to 68–72 °C, pH ≈ 7.0), shaken for 1–3 s, and rinsed for 2 min. The slides were incubated at room temperature in 0.1% NP-40/2× saline sodium citrate (pH ≈ 7.0), shaken for 1–3 s, and rinsed for 1 min. The slides were then placed in 70% ethanol at room temperature and rinsed for 1 min. The slides were removed and dried. Approximately 20 µL of DAPI was added dropwise to each slide, followed by a coverslip.
The FISH results obtained for HER2 were independently evaluated by two pathologists (TX and QMB) according to the criteria described in the 2018 ASCO/CAP guidelines7. The HER2 FISH results were designated into five groups. The FISH images of tumours were manually selected for scanning. High-quality FISH images were captured using the Microscope Image Information System (MIIS) from Shanghai Aitrox Technology Corporation Limited (Shanghai, China) and saved in JPEG file format.
Aitrox AI model
Based on a deep convolutional neural network (DCNN), our method, named the Aitrox AI model, consists of two steps for automatically detecting HER2 amplification statuses in FISH images with a tumour cell nucleus detector and a signal detector. In the first step, tumour cell nuclei are localized in a whole FISH image by the tumour cell nucleus detector. The tumour cell nucleus detector has an architecture based on You Only Look Once version 3 (YOLOv3)24. YOLOv3 is a state-of-the-art DCNN that is widely used in object detection cases. YOLOv3 consists of two modules: a feature extractor and a predictor. The feature extractor uses a network named Darknet-53 with successive 3 × 3 and 1 × 1 convolutional layers and shortcut connections, and it performs on par with state-of-the-art classifiers and while converging more quickly24. The predictor predicts bounding boxes using dimension clusters as anchor boxes at three different scales, which integrates rich feature maps from low to high levels and the classes that the bounding boxes may contain25. A whole FISH image goes through the tumour cell nucleus detector, during which bounding boxes with tumour cell nucleus probabilities are predicted. The bounding boxes with higher probabilities (above a specified threshold) are taken as detected tumour cell nuclei.
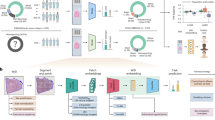
In the second step, the numbers of HER2 and CEP17 signals are counted by the signal detector in one detected tumour cell nucleus derived from the tumour cell nucleus detector. ResNet 1826, a DCNN with good feature extraction performance, is adopted as the signal detector. The signal detector takes one detected tumour cell nucleus as input and outputs its HER2 and CEP17 gene signal quantities, which is an obvious regression task. Subsequently, to convert the localization and counting results acquired from the tumour cell nucleus detector and the signal detector into image-level predictions, the numbers of HER2 gene signals and CEP17 gene signals in one FISH image are obtained, from which the average HER2 gene copy number is obtained and the HER2/CEP17 ratio is calculated. The HER2 gene copy number equals the ratio of the number of tumour cell nuclei to the number of HER2 gene signals in one FISH image. The HER2/CEP17 ratio is defined as the ratio of the number of CEP17 gene signals to the number of HER2 gene signals within a FISH image. The whole workflow of the proposed deep learning framework is shown in Fig. 1.
Pipeline of the Aitrox AI model for the automatic detection of HER2 amplification statuses in FISH images. First, a whole FISH image goes through the tumour cell nucleus detector with a feature extractor and predictor to localize the tumour cell nuclei. The detected tumour cell nuclei are cropped from the original image as patches. Second, the signal detector takes patches as input and outputs their HER2 and CEP17 gene signal quantities. HER2 amplification statuses are classified into 5 groups according to the ASCO/CAP guidelines.
Preprocessing and training procedure
The FISH images were divided into three groups: training, validation and test groups. To train the tumour cell nucleus detector and the signal detector, the FISH images were manually annotated by two pathologists (TX and MR) with two steps corresponding to the two DCNNs. First, bounding box annotations were provided to train the tumour cell nucleus detector. The bounding boxes were comprehensively annotated according to their tumour cell characteristics, such as their nucleus sizes and tissue structures. These bounding boxes had four coordinates for their positions and ranges in images and one single label named the tumour cell nucleus for their class. Second, the numbers of HER2 gene signals and CEP17 gene signals for each bounding box were annotated not only to train the signal detector but also to calculate the HER2 gene copy number and HER2/CEP17 ratio. Within one FISH image, the number of tumour cell nuclei equalled the number of bounding boxes, which could be easily obtained after detecting the tumour cell nuclei with the tumour cell nucleus detector.
During the training procedure, a loss function and optimization were employed to improve the performance of the DCNNs. To better train the tumour cell nucleus detector, the loss function combined the generalized intersection over union (GIoU) loss, objectness loss and classification loss. Since the IoU loss only considers the ratio of the areas of intersection and merging between the real box and the prediction box, when the prediction box and the target box do not intersect, the gradient disappears, and the loss cannot accurately reflect the degree of overlap between the two boxes. We used the GIoU loss to add a penalty term to the IoU loss to alleviate the above problems and make the prediction results more accurate. We used adaptive moment estimation (Adam) optimization for training the tumour cell nucleus detector with a fixed learning rate of 1e−3. Adam combines the advantages of Adagrad, which is good at dealing with sparse gradients, and root-mean-square propagation (RMSprop), which is good at handling nonstationary targets. The parameters of each iteration were adjusted within a certain range so that these parameters remained relatively stable. For the signal detector, we took the mean square error loss as the loss function and Adam as the optimization function with a fixed learning rate of 1e−3. In the pretraining phase, pretrained weights were loaded during the training procedures of both DCNNs. The pretrained weights of the tumour cell nucleus detector were obtained through pretraining on the COCO dataset27, while the pretrained weights of the signal detector were obtained via pretraining on the ImageNet dataset28. We used rotation, translation, etc., to enhance each input FISH image. Moreover, both DCNNs were trained on one NVIDIA GeForce 1080Ti. The validation set was used to select the best-performing model during the training procedure.
Ethics approval
This study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Results
A total of 918 FISH images scanned from 320 patients were obtained from FUSCC. The FISH images were divided into three sets: training (551/918, 60.02%), validation (183/918, 19.94%) and test sets (184/918, 20.04%). Each patient's FISH images only appeared within a single dataset. For the automated analysis, 4969 and 1275 bounding boxes with tumour cell nucleus labels were obtained in the training set and validation set, respectively. Each set was classified into five groups based on the ASCO/CAP guidelines. Detailed information on the distributions of the 918 FISH images contained in the three sets is shown in Fig. 2. No significant differences were observed among the three sets with respect to clinicopathologic characteristics, especially between the training and test sets (Table 1).
The case-based classifier’s performance was tested on 184 FISH images, and it achieved 85.33% (157/184) accuracy. The mean average precision (mAP) curve of the test dataset was obtained through the tumour cell nucleus detector network, and the mAP for the test dataset was 0.735 (Fig. 3). A comparison between the classification results obtained by the pathologists and the Aitrox AI model on the test set is shown in Fig. 4. In Group 1, 67.86% (38/56) of the samples were correctly classified through the Aitrox AI model, while the remaining 32.14% (18/56) were equally classified into Group 2, Group 4 and Group 5. In Group 2, both samples (2/2) were misclassified into Group 5. Group 3 only contained one sample, which was misclassified into Group 5. In Group 4, 66.67% (2/3) of the samples were correctly classified. In Group 5, 95.90% (117/122) of the samples were correctly classified, while the remaining 1.64% (2/122) and 2.46% (3/122) were classified into Group 2 and Group 4, respectively.
Representative samples from the five groups that were correctly predicted by the Aitrox AI model are shown in Fig. 5. Figure 5a shows the original image, the original image with labelled bounding boxes, and the original image with the AI-predicted bounding boxes for Group 1. The pathologist-labelled and AI-predicted average HER2 copies, CEP17 copies and HER2/CEP17 ratios were 13.80 and 8.00, 2.60 and 2.60, and 5.31 and 3.08, respectively. Figure 5b shows the original image, the original image with labelled bounding boxes, and the original image with the AI-predicted bounding boxes for Group 4. The pathologist-labelled and AI-predicted average HER2 copies, CEP17 copies and HER2/CEP17 ratios were 4.08 and 4.22, 2.75 and 3.28, and 1.49 and 1.29, respectively. Figure 5c shows the original image, the original image with labelled bounding boxes, and the original image with the AI-predicted bounding boxes for Group 5. The pathologist-labelled and AI-predicted average HER2 copies, CEP17 copies and HER2/CEP17 ratios were 3.20 and 2.86, 2.21 and 2.24, and 1.33 and 1.29, respectively.
Selected and representative samples from the five groups that were correctly predicted by the Aitrox AI model. (a) Group 1. (b) Group 4. (c) Group 5. The first column shows the original images of the five groups. The second column shows the original images labelled by pathologists, which were used as the gold standard in the test dataset. The third column shows the original images predicted by our Aitrox AI model. The HER2/CEP17 ratio was calculated for both the gold standard and our Aitrox AI model as the ratio of the number of CEP17 gene signals to the number of HER2 gene signals in one image, and details can be found in the last column.
Figure 6 shows selected and representative samples from the five groups that were incorrectly predicted by the Aitrox AI model. Figure 6a–c show the original image, the original image with labelled bounding boxes, and the original image with the AI-predicted bounding boxes for Group 1. However, the AI model incorrectly classified this sample into Group 2, Group 4 and Group 5. Figure 6d–f show the original image, the original image with labelled bounding boxes, and the original image with the AI-predicted bounding boxes for Groups 2, 3 and 4, respectively. However, the AI model incorrectly classified these samples into Group 5. Figure 6g,h show the original image, the original image with labelled bounding boxes, and the original image with the AI-predicted bounding boxes for Group 5. However, the AI model incorrectly classified these samples into Group 2 and Group 4. Detailed information is provided in Fig. 6.
Selected and representative samples from the five groups that were incorrectly predicted by the Aitrox AI model. (a–c) Group 1. (d) Group 2. (e) Group 3. (f) Group 4. (g,h) Group 5. The first column shows the original images of the five groups. The second column shows the original images labelled by pathologists, which were used as the gold standard in the test dataset. The third column shows the original images predicted by the Aitrox AI model. The HER2/CEP17 ratio was calculated for both the gold standard and our Aitrox AI model as the ratio of the number of CEP17 gene signals to the number of HER2 gene signals in one image, and details can be found in the last column.
Discussion
HER2 protein overexpression and gene amplification statuses are crucial markers for evaluating prognoses and making treatment decisions for breast cancer patients. It is promising that the HER2 IHC score could be predicted using automated image analysis29, and the experimental results exhibited good concordance with the FISH results30. For HER2 FISH interpretation, a computer-aided image analysis method has been developed. The overall accuracy of automated spot counting and manual scoring ranged from 82 to 100%15,16,17,31,32. In addition, studies have demonstrated that image analysis can be performed a significantly shorter evaluation time17,33. However, other studies have suggested that image analysis underestimates the HER2 and CEP17 data of FISH images compared with the results of the conventional method34, and manual intervention is always required during the image analysis process17. In this study, an automated detection method (the Aitrox AI model) for FISH images was constructed through a two-step deep learning pipeline. First, the nuclei of tumours in FISH images were localized and classified by the tumour cell nucleus detector network. Then, the numbers of HER2 and CEP17 gene signals were calculated by the signal detector network, and a final HER2/CEP17 ratio was obtained. This model correctly classified the majority of samples into different HER2 amplification status subgroups, especially for Group 5.
Among the incorrectly classified Group 1 cases, HER2 signals formed clusters in 12 cases, and AI obviously underestimated the HER2 count. In accordance with the presented results, previous studies have demonstrated that clusters are not suitable for regression-based methods; they instead recommended density-based methods35. However, undefined results are obtained through density-based methods when no CEP17 signals are available because signal quantification is completed on a single fluorescence channel35. In fact, it is easy to identify cases with clustered HER2 signals and to manually draw conclusions in clinical practice. In addition, the numbers of CEP17 signals determined by the AI model were higher than the manual counts in eight misclassified Group 1 cases and in two misclassified Group 2 cases, presumably because the CEP17 signals were slightly coarse. One case each in Group 3 and Group 4 was mistakenly classified into Group 5. In the misclassified images, the HER2 signals in some cells were gathered, and the intensity levels of HER2 signals in some cells were inconsistent, which may have been the reason for the low HER2 signal count produced by the AI method. Among the incorrectly classified Group 5 cases, two cases were mistakenly classified into Group 2. In one case, the weak CEP17 signals may not have been detected by the signal detector, and the reason for the other case was that a few cells were selected by the tumour cell nucleus detector. In the other three cases mistakenly assigned to Group 4, a few clustered nuclei were counted as one nucleus by AI in the two cases, leading to an increased number of HER2 signals. The remaining case may have been caused by counting bias. In histological samples, nuclei often overlap, so their signals cannot be counted exactly within one tumour cell nucleus. A new method was suggested, which counted all dots in representative tumour areas and assessed HER2/CEP17 ratios without considering the nucleus boundaries18. However, the HER2 copy number is also important, and HER2/CEP17 ratios cannot be considered alone when evaluating HER2 gene statuses. Therefore, AI may not be suitable for cases containing overlapping nuclei.
The present study has several limitations. In the tumour cell nucleus detector, the tile sampling method is used; however, the size of the tile does not always correspond to the size of a tumour cell nucleus, particularly when nuclei overlap. Nevertheless, tile-sampling analysis still performed well in previous research17 as well as in our study. A tumour cell nucleus sampling classifier should be considered in our future work. Furthermore, only a few FISH images were contained in Group 2, Group 3, and Group 4. Our data might be representative of real-world data since our cases were collected consecutively from FUSCC. Nevertheless, we included 918 FISH images in our study, making it the largest dataset in this field to our knowledge. To achieve improved AI classification accuracy for these three groups, further studies should include more cases from multiple centres.
Heterogeneity was also not assessed in our study. Heterogeneity can manifest in multiple patterns. HER2-amplified cells and nonamplified cells can be completely separated or intermingled36. Several studies have focused on automatically evaluating HER2 heterogeneity. Nguyen et al.37 developed a high-content quantitative analysis method based on microfluidic experimentation and image processing to detect HER2 intratumoral heterogeneity at the cellular level. Radziuviene et al.34 reported that an automated high-capacity nonselective tumour cell assay could generate evidence-based HER2 intratumor heterogeneity. However, the complicated experimental procedure in the abovementioned method may limit its clinical application.
Based on these limitations, we propose a method for combining pathologists with the Aitrox AI model, since scanning an entire FISH slice is time-consuming and requires significant storage space, and manual counting is insufficient for accurately counting tumour cells. Pathologists review the FISH sections under a fluorescence microscope (400x) to determine whether the HER2 signals are heterogeneous. If no heterogeneity is observed, several representative tumour regions are manually selected and then submitted to the Aitrox AI model for interpretation. If heterogeneity exists, several representative tumour regions in different groups will be manually selected and then submitted to AI for interpretation. In addition, pathologists could determine whether the HER2 signals form clusters, the signal intensities, and whether the nuclei overlapped during scanning whole-slide images to decide whether the slide is suitable to submit for Aitrox AI model interpretation. More importantly, HER2 amplification statuses could be preliminarily determined during this process. The artificial selection of regions can ensure the inclusion of tumour components. Pathologists can evaluate the AI interpretation result and manually modify it if the result does not meet expectations. Combining the advantages of manual quality control and AI counting, this method can not only ensure the accuracy of pathological reports but also improve the efficiency of pathologists, demonstrating great clinical practicability.
In conclusion, the Aitrox AI model is a reliable tool for automatically evaluating HER2 amplification statuses, especially for breast cancers in Group 5. Further studies involving more cases in other groups from multiple centres are still needed.
Data availability
The training, validation and test datasets utilized for the development of the deep-learning model cannot be publicly available due to the need to protect the privacy and health information of the patients involved. However, the datasets can be provided upon request if the Institutional Review Board of the ethics committee of Fudan University Shanghai Cancer Center (FUSCC) grants approval. The corresponding author (QMB) should be contacted if someone wants to request the data from this study.
References
Popescu, N. C., King, C. R. & Kraus, M. H. Localization of the human erbB-2 gene on normal and rearranged chromosomes 17 to bands q12–21.32. Genomics 4, 362–366 (1989).
Ross, J. S. et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14, 320–368 (2009).
Andrulis, I. L. et al. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J. Clin. Oncol. 16, 1340–1349 (1998).
Gianni, L. et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375, 377–384 (2010).
Schnitt, S. J. Classification and prognosis of invasive breast cancer: From morphology to molecular taxonomy. Mod. Pathol. 23, S60–S64 (2010).
Sørlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98, 10869–10874 (2001).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Pauletti, G. et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: A direct comparison of fluorescence in situ hybridization and immunohistochemistry. J. Clin. Oncol. 18, 3651–3664 (2000).
Owens, M. A., Horten, B. C. & Da Silva, M. M. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin. Breast Cancer 5, 63–69 (2004).
Yamashita, H. et al. HER2 gene amplification in ER-positive HER2 immunohistochemistry 0 or 1+ breast cancer with early recurrence. Anticancer Res. 40, 645–652 (2020).
Klijanienko, J. et al. Detection and quantitation by fluorescence in situ hybridization (FISH) and image analysis of HER-2/neu gene amplification in breast cancer fine-needle samples. Cancer 87, 312–318 (1999).
Prins, M. J., Ruurda, J. P., van Diest, P. J., van Hillegersberg, R. & ten Kate, F. J. Evaluation of the HER2 amplification status in oesophageal adenocarcinoma by conventional and automated FISH: A tissue microarray study. J. Clin. Pathol. 67, 26–32 (2014).
Hicks, D. G. & Tubbs, R. R. Assessment of the HER2 status in breast cancer by fluorescence in situ hybridization: A technical review with interpretive guidelines. Hum. Pathol. 36, 250–261 (2005).
Turashvili, G. et al. Inter-observer reproducibility of HER2 immunohistochemical assessment and concordance with fluorescent in situ hybridization (FISH): Pathologist assessment compared to quantitative image analysis. BMC Cancer 9, 165 (2009).
Ellis, C. M., Dyson, M. J., Stephenson, T. J. & Maltby, E. L. HER2 amplification status in breast cancer: A comparison between immunohistochemical staining and fluorescence in situ hybridisation using manual and automated quantitative image analysis scoring techniques. J. Clin. Pathol. 58, 710–714 (2005).
Moerland, E., van Hezik, R. L., van der Aa, T. C., van Beek, M. W. & van den Brule, A. J. Detection of HER2 amplification in breast carcinomas: Comparison of Multiplex Ligation-dependent Probe Amplification (MLPA) and Fluorescence In Situ Hybridization (FISH) combined with automated spot counting. Cell Oncol. 28, 151–159 (2006).
Stevens, R. et al. Analysis of HER2 gene amplification using an automated fluorescence in situ hybridization signal enumeration system. J. Mol. Diagn. 9, 144–150 (2007).
López, C. et al. Is it necessary to evaluate nuclei in HER2 FISH evaluation?. Am. J. Clin. Pathol. 139, 47–54 (2013).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Jaber, M. I. et al. A deep learning image-based intrinsic molecular subtype classifier of breast tumors reveals tumor heterogeneity that may affect survival. Breast Cancer Res. 22, 12 (2020).
Couture, H. D. et al. Image analysis with deep learning to predict breast cancer grade, ER status, histologic subtype, and intrinsic subtype. NPJ Breast Cancer 4, 30 (2018).
Anand, D. et al. Deep learning to estimate human epidermal growth factor receptor 2 status from hematoxylin and eosin-stained breast tissue images. J. Pathol. Inform. 11, 19 (2020).
Zakrzewski, F. et al. Automated detection of the HER2 gene amplification status in Fluorescence in situ hybridization images for the diagnostics of cancer tissues. Sci. Rep. 9, 8231 (2019).
Redmon, J. & Farhadi, A. YOLOv3: An incremental improvement. arXiv:1804.02767 (2018)
Pan, H., Chen, G. & Jiang, J. Adaptively dense feature pyramid network for object detection. IEEE Access 7, 81132–81144 (2019).
He, K., Zhang, X., Ren, S. & Sun, J. Deep residual learning for image recognition. In 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). Las Vegas, NV, USA: IEEE, 770–778 (2016).
Lin, T. Y. et al. Microsoft COCO: Common objects in context. In Computer Vision—ECCV 2014 (eds Fleet, D. et al.) 740–755 (Springer, 2014).
Deng, J., Dong, W., Socher, R., Li, L.J., Kai, L. & Li, F.F. ImageNet: A large-scale hierarchical image database. In 2009 IEEE Conference on Computer Vision and Pattern Recognition. Miami, FL, USA: IEEE, 248–255 (2009).
Holten-Rossing, H., Møller Talman, M. L., Kristensson, M. & Vainer, B. Optimizing HER2 assessment in breast cancer: Application of automated image analysis. Breast Cancer Res. Treat. 152, 367–375 (2015).
Dennis, J. et al. Quantification of human epidermal growth factor receptor 2 immunohistochemistry using the Ventana Image Analysis System: Correlation with gene amplification by fluorescence in situ hybridization: The importance of instrument validation for achieving high (> 95%) concordance rate. Am. J. Surg. Pathol. 39, 624–631 (2015).
Konsti, J. et al. A public-domain image processing tool for automated quantification of fluorescence in situ hybridisation signals. J. Clin. Pathol. 61, 278–282 (2008).
Furrer, D. et al. Validation of a new classifier for the automated analysis of the human epidermal growth factor receptor 2 (HER2) gene amplification in breast cancer specimens. Diagn. Pathol. 8, 17 (2013).
Theodosiou, Z. et al. Evaluation of FISH image analysis system on assessing HER2 amplification in breast carcinoma cases. Breast 17, 80–84 (2008).
Radziuviene, G. et al. Automated image analysis of HER2 fluorescence in situ hybridization to refine definitions of genetic heterogeneity in breast cancer tissue. Biomed. Res. Int. 2017, 2321916 (2017).
Höfener, H. et al. Automated density-based counting of FISH amplification signals for HER2 status assessment. Comput. Methods Programs Biomed. 173, 77–85 (2019).
Hanna, W. M. et al. HER2 in situ hybridization in breast cancer: Clinical implications of polysomy 17 and genetic heterogeneity. Mod. Pathol. 27, 4–18 (2014).
Nguyen, H. T., Migliozzi, D., Bisig, B., de Leval, L. & Gijs, M. A. M. High-content, cell-by-cell assessment of HER2 overexpression and amplification: A tool for intratumoral heterogeneity detection in breast cancer. Lab. Invest. 99, 722–732 (2019).
Funding
This work was supported by the Science and Technology Commission of Shanghai Municipality (Grant number 19441904900); the Shanghai Science and technology development fund (Grant number 19MC1911000); the National Key Research and Development Program of China (Grant number YFC20170110100); the Shanghai Municipal Key Clinical Specialty (Grant number shslczdzk01301); the Innovation Program of Shanghai Science and technology committee (Grant number 20Z11900300); and the Innovation Group Project of Shanghai Municipal Health Commission Grant (Grant number 2019CXJQ03).
Author information
Authors and Affiliations
Contributions
Q.M.B., X.Y.Z. and C.H. designed the study. Q.F., L.C.T. and B.Y.W. implemented the AI algorithm. H.C. performed the HER2 FISH experiments. T.X., M.R. and H.C.W. collected the clinical data and annotated the FISH images. X.L.Z. and Y.Y. summarized the clinicopathologic features. C.C.F. and L.L. conducted the AI data analysis. T.X., B.Y.W. and L.L. wrote the manuscript. Q.M.B. and X.Y.Z. reviewed the manuscript. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, T., Chang, H., Ren, M. et al. Deep learning to automatically evaluate HER2 gene amplification status from fluorescence in situ hybridization images. Sci Rep 13, 9746 (2023). https://doi.org/10.1038/s41598-023-36811-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36811-z
This article is cited by
-
Current status and prospects of artificial intelligence in breast cancer pathology: convolutional neural networks to prospective Vision Transformers
International Journal of Clinical Oncology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.