Abstract
Previous research on the effects of body mass on the pelvic girdle focused mostly on adult females and males. Because the ontogenetic plasticity level in the pelvis remains largely unknown, this study investigated how the association between body mass index (BMI) and pelvic shape changes during development. It also assessed how the large variation in pelvic shape could be explained by the number of live births in females. Data included CT scans of 308 humans from infancy to late adulthood with known age, sex, body mass, body stature, and the number of live births (for adult females). 3D reconstruction and geometric morphometrics was used to analyze pelvic shape. Multivariate regression showed a significant association between BMI and pelvic shape in young females and old males. The association between the number of live births and pelvic shape in females was not significant. Less plasticity in pelvic shape in adult females than during puberty, perhaps reflects adaptation to support the abdominopelvic organs and the fetus during pregnancy. Non-significant susceptibility to BMI in young males may reflect bone maturation accelerated by excessive body mass. Hormonal secretion and biomechanical loading associated with pregnancy may not have a long-term effect on the pelvic morphology of females.
Similar content being viewed by others
Introduction
Shape variation in the human pelvis was analyzed in the context of sexual dimorphism1, age2, thermoregulation3, past population history4, mechanical constraints5,6,7, stature8, evolution, and birth canal variation3,9,10. Much attention was also paid to pelvic morphology in females, which reflects competing functional demands (e.g. locomotor, childbirth, abdominopelvic organ support)11,12,13. Huseynoy et al.11 showed that the female pelvis is characterized by great plasticity in the context of obstetrical dimensions during ontogeny. Developmental trajectories of females and males separate in early infancy and change substantially with increasing obstetrical dimensions in females until age 40–45 years when the female pelvis begins to change in parallel with the male trajectory11. The greatest differences in pelvic shape between the sexes are achieved at the time of maximum fertility in females11. The amount and duration of hormone secretion (i.e. estrogen and androgen) are likely the main drivers of these differences in developmental trajectories between sexes by inducing pelvic bone remodeling14,15. The pattern of human pelvic dimorphism is conservative compared to the highly variable magnitude of the sex differences15, which may depend partly on environmental factors such as mechanical loadings. However, the potential influence of bone-loading conditions such as body mass on the developmental trajectories of pelvises in females and males remains relatively unexplored.
While various studies have investigated the effects of body mass and posture on the human pelvis, they focused on mature females16 or males17. One longitudinal study18 showed that adolescents with higher body fat tended to have a larger pelvic width, possibly due to adipose estrogenization. However, a higher biomechanical load could not be excluded as a causal factor18. Nevertheless, the association between body mass and pelvic shape during growth has yet to be explored. The human pelvis is one of the skeleton’s most important weight-bearing bone structures due to its bipedal locomotion. Moreover, it provides attachments for muscles, supports and protects the abdominal and reproductive organs, stabilizes the spine, and in females, enables childbirth19,20,21,22. Therefore, it can be assumed that mechanical loading, such as body mass, is one of the factors inducing the pelvic girdle’s developmental trajectories through adaptive bone remodeling. Moreover, considering the obstetrical requirements of females and variable ontogenetic plasticity of the pelvis11, the morphological response of pelvic shape can differ in magnitude between sexes depending on the ontogenetic phase.
Another question is whether the number of live births can shape the functional plasticity of the pelvic girdle in females. For example, a mouse model showed that females with the largest number of offspring had the most divergent pelvic shape23. However, previous human studies found no differences in pelvic shape between parous and nonparous females11,24. Despite that, each pregnancy and parturition increases pelvic organ (urethral and bladder) mobility25 and is associated with prolonged hormonal secretion (e.g. high levels of estrogen, progesterone, and relaxin) that stimulates bone remodeling and weakens pelvic ligaments26,27, which can lead to pelvic floor dysfunction28,29,30. Therefore, does prolonged hormonal stimulation and biomechanical stress associated with each subsequent pregnancy increase adaptation in obstetrical dimensions in females? Consequently, the potential relationship between the number of live births and pelvic shape in females at the age of the greatest fertility requires reassessment.
In summary, this study: (1) assesses the relationship between pelvic shape and body mass category of females and males during ontogeny; (2) examines the relationship between the number of live births and the pelvic shape in females at the age of greatest fertility (i.e. 25–45 years)11,31. It is expected that female pelvis shape be more influenced by body mass than the male pelvis, as females exhibit higher ontogenetic plasticity (Hypothesis 1). Secondly, the pelvic shape is expected to change with the number of live births in females of reproductive age, given that the obstetrical dimensions of the female pelvis are induced by prolonged hormonal stimulation during pregnancy (Hypothesis 2). To test these two hypotheses, a three-dimensional (3D) reconstruction of the pelvic girdle was integrated with geometric morphometrics to assess pelvic plasticity from infancy to late adulthood in a large forensic sample of 308 individuals.
Results
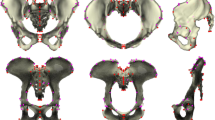
PCA was performed on the multivariate regression residuals to visualize the variation of the complete data set without ontogenetic allometry. Females and males from both groups (young and old) are characterized by relatively similar shape variation, which minimizes the likelihood of a negative impact on further regression results. In addition, Fig. 1 shows that the age groups of both sexes are not differentiated along PC1 and PC2. PC1 and PC2 explained 27.35% and 17.26% of the total variation in the data set, respectively. Both sexes diverged along the PC1 axis representing the ilium’s bending and widening and the size of the subpubic angle (Movies 1S, 2S). PC2 represents the width of the pelvic inlet and bending of the ischium (Fig. 1, Movies 3S, 4S). The multivariate regression of pelvic shape and BMI categories was significant in females and males from the young (0–25 years) and old (> 25 years) groups (Table 1; Figs. 2, 3). The pelvic girdle of young females with underweight and healthy BMIs (N = 30) showed relatively more upright ilium and prominent ischial spines and relatively higher everted sacrum than those with overweight and obese BMIs (N = 20; Fig. 2; for additional visualization in three orientations, see Movies 5S, 6S and 7S). However, the relative anteroposterior and transverse dimensions of the pelvic outlet and inlet did not seem to differ regarding BMI categories in young females (Fig. 2; Movies 5S, 6S and 7S). In males with underweight and healthy BMIs (N = 51) in the old group (> 25 years), the pelvic girdle had a relatively: more upright ilium, a higher sacrum base, a larger subpubic angle, a more forward acetabulum, more backward spina iliaca anterior-inferior, and anterior–superior and less triangular pelvic outlet than those with overweight and obese BMIs (N = 50; Fig. 3; Movies 8S, 9S and 10S). The association between the pelvic shape and the number of live births in 43 females aged 25–45 was insignificant (predicted 1.72% of shape variation; p = 0.715; Fig. 3S).
Discussion
This is the first study investigating the association between body mass and pelvic shape changes during development. The geometric morphometric analysis showed a significant relationship between pelvic shape and BMI category only in young females (0–25 years) and old males (> 25 years). Therefore, these results indicate that before the end of maturation (i.e. 0–25 years), the female pelvis shows greater plasticity and susceptibility to body mass than the pelvic girdle of similarly aged males. However, is this greater plasticity during female development an adaptation to future obstetrical challenges?
Comparing the obtained pattern in pelvis shape variability during growth to other studies is difficult since they focused mainly on adults16,17,32,33,34. Nevertheless, the observed differences in pelvic shape between BMI categories result from a highly integrated system in which functional and anatomical components interact35. However, a previous study showed that not all pelvic components exhibit the same magnitude of interaction2. The linear dimensions and correlation matrices showed that the ilium was less constrained than the pubis in both sexes and than the pelvis outlet and inlet in females2. These findings may explain why this geometric morphometric analysis showed more pronounced changes in ilium orientation than in pelvic canal shape in young females. The birth canal shape in young females likely shows a higher canalization and is under stronger evolutionary constraints than non-obstetrical traits that respond independently. Therefore, the results suggest that body mass does not determine pelvis inlet and outlet shape during development, although it may influence variability in canal dimensions in adult females32. In addition, the widening of the ilium in young females may be a biomechanical response to the mechanical loading of excess body mass.
The BMI categories were equally numerous in both sexes. In addition, both sexes in the young and old groups are characterized by relatively similar shape variation. Therefore, the absence of pelvic plasticity with BMI in young males is not due to sampling bias or differences in the variation between groups. These results might reflect differences in the ontogenetic pelvic shape trajectories between sexes36 and sex-specific hormone susceptibility. Obese boys and girls show accelerated bone maturation and earlier pubertal events partially due to increased leptin and irisin concentration37,38. Being overweight or obese might accelerate the already faster shape maturation rate of the pelvic girdle, especially the ilium, in young males, making it less prone to shape changes. Sex hormones that play a role in leptin synthesis may enhance this effect in young boys38. However, it must be emphasized that the complex relationship between BMI and sex hormones leptin and irisin in subadults remains largely unknown. Therefore, further studies are needed to confirm this hypothesis.
Kurki39 and Huseynov et al.11 found that the pelvic dimensions of adult females and males are equally plastic. Shorter and lower-weight females have relatively large inter-landmark birth canal distances16. Similarly, body mass is the main factor explaining pelvic shape variability in modern and medieval males17. These patterns contrast with the results of this study, where the relationship between BMI category and pelvic shape was significant only for males in the old group (> 25 years). The geometric morphometric analysis showed changes in the ilium, subpubic angle size, and acetabular orientation of the male pelvis. One potential explanation for these discrepancies is that the pelvis of modern adult females is less plastic and differs from that of Ricklan et al.16. Because the human pelvis has a neutral variance pattern34, discrepancies among studies can reflect differences in population histories of their investigated groups. Another potential cause is differences in their methodological approaches. Ricklan et al.16 examined pelvic dimensions, body mass, and height, while this study explored BMI and shape using geometric morphometrics. Nevertheless, BMI categories did not explain shape variation in the female pelvis, even though the pelvic girdle is susceptible to changes after puberty11. The absence of shape changes due to excess body mass is potentially an advantage in heavier adult females since the pelvis supports the abdominopelvic organs and fetus during pregnancy19.
The geometric morphometric analysis found that the number of live births could not explain shape variation in the female pelvis. Previous studies on females with known maternal status provided similar results since the pelvis of parous and nonporous females did not differ significantly11. Therefore, increasing mobility of the pubic symphysis and sacroiliac joints during pregnancy40,41 may not majorly affect pelvic morphology. Nevertheless, this finding is important in the context of the secular trend in maternal obesity, especially in low- and middle-income countries42. Wells42 found that overweight and obese mothers had higher risks of macrosomia in offspring and obstructed labor. The absence of changes in the pelvic shape, particularly obstetrical traits, in overweight and obese females, resulting in a birth canal ill-suited to large infants, can explain the disproportional cephalopelvic risk.
The relationship between pelvic capacity and body size is population-specific due to variation in body proportions43,44. Moreover, there are interpopulation differences in postnatal ontogeny trajectories of pelvic shape36. Because this study examined a relatively homogenous group born in the United States (details on ethnicity in Table 2S), albeit from different states, the pelvic plasticity patterns observed cannot be considered universal. Studies on other populations are needed to clarify whether body mass determines the developmental processes leading to the mature pelvis in females and males. Other aspects can also advance understanding of the functional plasticity of the human pelvis. For example, since the secular trend of pubertal timing is observed worldwide45, it is important to study whether earlier maturation influences the functional plasticity of the pelvic girdle, which could be investigated by menarcheal age since it is the most accurate pubertal stage in females46. Demographic and medical data of modern populations would be invaluable in this regard. Other studies16,17 have provided invaluable information on pelvic morphological variation. However, their findings cannot be used as a general pattern since they separately focused on mature males and females. Therefore, studies should focus on both sexes at different ontogenetic phases since this study showed different plasticities in males and females during development. While most studies on the human pelvis are observational47, it is impossible to investigate cause-effect relationships. Therefore, large-scale forensic data with demographic and medical information may be useful in future experimental studies and animal models.
Material and methods
The material consisted of high-resolution full-body CT scans of 308 humans (157 females and 151 males) from infancy to late adulthood (Tables 1S and 2S) with information on sex, age in months at death, living weight, and height. The number of live births of 43 females aged 25–45 was also collected. The volumetric, demographic, and medical data were acquired from the New Mexico Decedent Image Database48 (details in the Supplement). Next, body mass index (BMI) was calculated for each individual using the equation: BMI = body mass (kg)/body height (m2). In individuals over 20 years of age, BMI was classified based on the following ranges: underweight (≤ 18.5), healthy weight (18.5–24.9), overweight (25.0–29.9), and obese (≥ 30.0). Individuals under 20 years of age were classified using sex-specific growth charts as underweight (< 10th percentile), healthy weight (10–85th), overweight (85–95th), and obese (> 95th).
Whole body CT scans were acquired using a standard protocol with 0.5 mm slice thickness. 3D reconstruction and mesh cleaning of the pelvic girdle was performed using free software such as 3D Slicer (v.4.11)49 and Gom Inspect (v. Hotfix 4)50, respectively. One pelvis on which 61 landmarks (LM) were manually digitized was selected as a template (Table 3S). Next, 476 surface semilandmarks (SLM) were automatically generated from the template mesh. Then, SLM sliding was performed based on the manually digitized 61 LM on each pelvic girdle using the minimum bending energy criterion of the SlicerMorph extension in 3D Slicer (details in the Supplement). The LM set contained 10 LM pairs that fuse during pelvic development. Therefore, in individuals with completed fusion, the mean position was calculated for each of these 10 LM pairs (Figs. 1S, 2S).
The raw coordinate data were divided into two groups, young (individuals aged 0–25) and old (individuals aged > 25), to separately analyze changes in the pelvic shape during stages of intense and complete skeletal development, respectively. A Generalized Procrustes Analysis was performed to remove the differences in location, orientation, and scale of the raw coordinates and to obtain the Procrustes coordinates (details in the Supplement). Multivariate regression with the permutation test (10,000 rounds) was performed between the Procrustes coordinates and the centroid size in young and old groups to test for ontogenetic allometry. The results were statistically significant in both groups (Table 4S). Therefore, all further geometric morphometric analysis was performed on the multivariate regression residuals to remove the ontogenetic allometry effect. This approach was because size can be a crucial component of the shape variation during growth51, and the study analyses individuals at different developmental stages. Therefore, removing the allometry effect ensures that the detected variation in the pelvic shape was not due to differences in the geometric size. This was especially important in the young group, which, due to the rapid pace of development, is characterized by high variation in size.
Principal component analysis (PCA) on the multivariate regression residuals was used to visualize the variation pattern of the pelvic shape. The PCA was carried out without allometric effect to explore whether both groups (young and old) within sex show relatively similar shape variation, which is important for the potential impact on the results of further geometric morphometric analysis (i.e. the multivariate regression).
Two further geometric morphometric analyses were also carried out without an allometric effect. The association between pelvic shape (i.e., the residuals of the allometric regression) and BMI categories by sex was performed using the multivariate regression separately in the young and old groups. The multivariate regression was also used to investigate the association between pelvic shape (i.e. the residuals of the allometric regression) and the number of live births in 43 females aged 25–45 (Table 6S). This geometric morphometric analysis focused on females in a narrow age range since the female pelvis shows relevant obstetrical dimensions during this period11. Statistical analyses were performed using MorphoJ (v.1.07a). In turn, the R software (v.4.2.0) with the package ggplot252 was used to create the figures. All results with p < 0.05 were considered statistically significant.
The author confirms that all stages of the research (collection, CT scanning, and analysis) were performed in accordance with the fundamental ethical principles and regulations on the analysis of human remains. All analysed CT scans were derived from the New Mexico Decedent Image Database and no new patients were scanned for this study. The acquisition of the medical image series and biological data of deceased persons was done retrospectively in compliance with the Declaration of Helsinki for the protection of data privacy. CT scans of decedents are not regarded as human subjects under U.S. federal laws pertaining to research on human subjects. Further, Health Insurance Portability and Accountability Act (HIPAA) protections do not apply to data obtained in the investigation of a person's death. Moreover, the scans do not comprise any personal identifiable information, and because the next of kin interviewed were not subjects of research, no review was required by the Institutional Review Board (IRB) of the New Mexico Decedent Image Database. However, the project descriptions and research design were reviewed by the College of Arts and Sciences, Department of Anthropology who considered the research to collect the derivatives was exempt from IRB review.
Data availability
The data that support the findings of this study are available from the New Mexico Decedent Image Database but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the New Mexico Decedent Image Database. To request the data, please visit the website: https://nmdid.unm.edu/ and read the information for researchers in the tab “How to use” or contact directly with the New Mexico Decedent Image Database via the project email address (NMDID@unm.edu). 3D raw coordinates with biological variables, R code used to create figures, PCA coefficients and morphoj file are available on an external repository (Mendeley Data: https://doi.org/10.17632/k85nv2j2p5.1)53.
References
Betti, L. Sexual dimorphism in the size and shape of the os coxae and the effects of microevolutionary processes. Am. J. Phys. Anthropol. 153, 167–177 (2014).
Mallard, A. M., Savell, K. R. R. & Auerbach, B. M. Morphological integration of the human pelvis with respect to age and sex. Anat. Rec. 300, 666–674 (2017).
Gruss, L. T. & Schmitt, D. The evolution of the human pelvis: Changing adaptations to bipedalism, obstetrics and thermoregulation. Philos. Trans. R. Soc. B Biol. Sci. 370, 20140063 (2015).
Betti, L. Human variation in pelvic shape and the effects of climate and past population history. Anat. Rec. 300, 687–697 (2017).
Mohr, M., Pieper, R., Löffler, S., Schmidt, A. R. & Federolf, P. A. Sex-specific hip movement is correlated with pelvis and upper body rotation during running. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2021.657357 (2021).
Ruff, C. Mechanical constraints on the hominin pelvis and the ‘obstetrical dilemma’. Anat. Rec. 955, 946–955 (2017).
Warrener, A. G., Lewton, K. L., Pontzer, H. & Lieberman, D. E. A wider pelvis does not increase locomotor cost in humans, with implications for the evolution of childbirth. PLoS ONE 10, e0118903 (2015).
Fischer, B. & Mitteroecker, P. Covariation between human pelvis shape, stature, and head size alleviates the obstetric dilemma. Proc. Natl. Acad. Sci. 112, 5655–5660 (2015).
Betti, L. & Manica, A. Human variation in the shape of the birth canal is significant and geographically structured. Proc. R. Soc. B Biol. Sci. 285, 20181807 (2018).
Weaver, T. D. & Hublin, J.-J. Neandertal birth canal shape and the evolution of human childbirth. Proc. Natl. Acad. Sci. U. S. A. 106, 8151–8156 (2009).
Huseynov, A. et al. Developmental evidence for obstetric adaptation of the human female pelvis. Proc. Natl. Acad. Sci. 113, 5227–5232 (2016).
Wells, J. C. K., DeSilva, J. M. & Stock, J. T. The obstetric dilemma: An ancient game of russian roulette, or a variable dilemma sensitive to ecology?. Yearb. Phys. Anthropol. 149, 40–71 (2012).
Whitcome, K. K., Miller, E. E. & Burns, J. L. Pelvic Rotation Effect on Human Stride Length: Releasing the Constraint of Obstetric Selection. 763, 752–763 (2017).
Steinetz, B. G. et al. Transmission of relaxin and estrogens to suckling canine pups via milk and possible association with hip joint laxity. Am. J. Vet. Res. 69, 59–67 (2008).
Fischer, B., Grunstra, N. D. S., Zaffarini, E. & Mitteroecker, P. Sex differences in the pelvis did not evolve de novo in modern humans. Nat. Ecol. Evol. 5, 625–630 (2021).
Ricklan, S. J., Decrausaz, S. L., Wells, J. C. K. & Stock, J. T. Obstetric dimensions of the female pelvis are less integrated than locomotor dimensions and show protective scaling patterns: Implications for the obstetrical dilemma. Am. J. Hum. Biol. 33, 1–20 (2021).
Musielak, B. et al. Variation in pelvic shape and size in Eastern European males: A computed tomography comparative study. PeerJ 2019, e6433 (2019).
Novak, J. M. et al. The relationship between adolescent obesity and pelvis dimensions in adulthood: A retrospective longitudinal study. PeerJ 2020, 1–19 (2020).
Pavličev, M., Romero, R. & Mitteroecker, P. Evolution of the human pelvis and obstructed labor: New explanations of an old obstetrical dilemma. Am. J. Obstet. Gynecol. 222, 3–16 (2020).
Wall-Scheffler, C. M., Kurki, H. K. & Auerbach, B. M. Pelves of the hominin lineage. In The Evolutionary Biology of the Human Pelvis 46–98 (Cambridge University Press, 2020). https://doi.org/10.1017/9781108185738.005.
Lewis, C. L., Laudicina, N. M., Khuu, A. & Loverro, K. L. The human pelvis: Variation in structure and function during gait. Anat. Rec. 300, 633–642 (2017).
DeSilva, J. M. & Rosenberg, K. R. Anatomy, development, and function of the human pelvis. Anat. Rec. 300, 628–632 (2017).
Schutz, H., Donovan, E. R. & Hayes, J. P. Effects of parity on pelvic size and shape dimorphism in Mus. J. Morphol. 270, 834–842 (2009).
Reitter, A. et al. Does pregnancy and/or shifting positions create more room in a woman’s pelvis?. Am. J. Obstet. Gynecol. 211, 662.e1-662.e9 (2014).
Dietz, H. P., Eldridge, A., Grace, M. & Clarke, B. Does pregnancy affect pelvic organ mobility?. Aust. New Zeal. J. Obstet. Gynaecol. 44, 517–520 (2004).
Heckman, J. & Sassard, R. Musculoskeletal considerations in pregnancy. J. Bone Jt. Surg. 76, 1720–1730 (1994).
Dehghan, F. et al. The effect of relaxin on the musculoskeletal system. Scand. J. Med. Sci. Sport https://doi.org/10.1111/sms.12149 (2014).
Harvey, M. A., Johnston, S. L. & Davies, G. A. L. Mid-trimester serum relaxin concentrations and post-partum pelvic floor dysfunction. Acta Obstet. Gynecol. Scand. 87, 1315–1321 (2008).
Schimpf, M. & Tulikangas, P. Evolution of the female pelvis and relationships to pelvic organ prolapse. Int. Urogynecol. J. 16, 315–320 (2005).
Ashton-Miller, J. A. & DeLancey, J. O. L. Functional anatomy of the female pelvic floor. Ann. N. Y. Acad. Sci. 1101, 266–296 (2007).
Martin, J., Hamilton, B. E., Osterman, M. J. K. & Driscoll, A. K. Births: Final Data for 2019. https://www.researchgate.net/publication/350680527_Births_Final_Data_for_2019 (2019).
Kurki, H. K. Skeletal variability in the pelvis and limb skeleton of humans: Does stabilizing selection limit female pelvic variation?. Am. J. Hum. Biol. 25, 795–802 (2013).
Jagesur, S., Wiid, A., Pretorius, S., Bosman, M. C. & Oettlé, A. C. Assessment of the variability in the dimensions of the intact pelvic canal in South Africans: A pilot study. HOMO- J. Comp. Hum. Biol. 68, 30–37 (2017).
Betti, L., von Cramon-Taubadel, N., Manica, A. & Lycett, S. J. Global geometric morphometric analyses of the human pelvis reveal substantial neutral population history effects, Even across sexes. PLoS One 8, e55909 (2013).
Wagner, G. & Schwenk, K. Evolutionarily stable configurations: Functional integration and the evolution of phenotypic stability. In Evolutionary Biology Vol. 31 (eds Hecht, M. et al.) 155–218 (Springer Science + Business Media, 2000).
Wilson, L. A. B., Ives, R., Cardoso, H. F. V. & Humphrey, L. T. Shape, size, and maturity trajectories of the human ilium. Am. J. Phys. Anthropol. 156, 19–34 (2015).
Klein, K. O. et al. Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J. Clin. Endocrinol. Metab. 83, 3469–3475 (1998).
Zhang, L. et al. What is the relationship between body mass index, sex hormones, leptin, and irisin in children and adolescents? A Path Analysis. Front. Pediatr. 10, 1–8 (2022).
Kurki, H. K. Bilateral asymmetry in the human pelvis. Anat. Rec. 300, 653–665 (2017).
Dehghan, F. et al. The effect of relaxin on the musculoskeletal system. Scand. J. Med. Sci. Sport. 24, 220–229 (2014).
Becker, I., Woodley, S. J. & Stringer, M. D. The adult human pubic symphysis: A systematic review. J. Anat. 217, 475–487 (2010).
Wells, J. C. K. The new “Obstetrical Dilemma”: Stunting, obesity and the risk of obstructed labour. Anat. Rec. 300, 716–731 (2017).
Rosenberg, K. R. et al. The functional significance of neandertal pubic length [and comments and reply]. Curr. Anthropol. 29, 595–617 (1988).
Kurki, H. K. Protection of obstetric dimensions in a small-bodied human sample. Am. J. Phys. Anthropol. 133, 1152–1165 (2007).
Eckert-Lind, C. et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: A systematic review and meta-analysis. JAMA Pediatr. 174, 1–11 (2020).
Cole, T. J. The secular trend in human physical growth: A biological view. Econ. Hum. Biol. 1, 161–168 (2003).
Verbruggen, S. W. & Nowlan, N. C. Ontogeny of the human pelvis. Anat. Rec. 300, 643–652 (2017).
Edgar, H. et al. New Mexico Decedent Image Database (University of New Mexico, 2020). https://doi.org/10.25827/5s8c-n515.
Fedorov, A. et al. 3D Slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging 30, 1323–1341 (2012).
GOM Metrology. https://www.gom.com/.
Outomuro, D. & Johansson, F. A potential pitfall in studies of biological shape: Does size matter?. J. Anim. Ecol. 86, 1447–1457 (2017).
Wickham, H. ggpolt2: Elegant Graphics for Data Analysis (Springer International Publishing, 2016).
Kubicka, A. M. Dataset—Changes in plasticity of the pelvic girdle from infancy to late adulthood in Homo sapiens. Mendeley Data V1 https://data.mendeley.com/datasets/k85nv2j2p5 (2023) doi:https://doi.org/10.17632/k85nv2j2p5.1.
Acknowledgements
The author thanks the New Mexico Decedent Image Database for enabling the use of the volumetric and demographic data for this study. The author is also grateful to the two reviewers (especially Dr. Nicole Torres-Tamayo from the University of Roehampton, London) for their very helpful comments. “Publication was co-financed within the framework of the Polish Ministry of Science and Higher Education’s program: “Regional Excellence Initiative” in the years 2019–2023 (No. 005/RID/2018/19)”, financing amount 12 000 000,00 PLN.
Funding
Polish National Agency for Academic Exchange: PPN/BEK/2018/1/00390/U/00001.
Author information
Authors and Affiliations
Contributions
A.M.K.: Conceptualization, methodology, investigation, visualization, writing.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Supplementary Video 3.
Supplementary Video 4.
Supplementary Video 5.
Supplementary Video 6.
Supplementary Video 7.
Supplementary Video 8.
Supplementary Video 9.
Supplementary Video 10.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubicka, A. Changes in plasticity of the pelvic girdle from infancy to late adulthood in Homo sapiens. Sci Rep 13, 9698 (2023). https://doi.org/10.1038/s41598-023-36703-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36703-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.