Abstract
It is commonly assumed that the strong sexual dimorphism of the human pelvis evolved for delivering the relatively large human foetuses. Here we compare pelvic sex differences across modern humans and chimpanzees using a comprehensive geometric morphometric approach. Even though the magnitude of sex differences in pelvis shape was two times larger in humans than in chimpanzees, we found that the pattern is almost identical in the two species. We conclude that this pattern of pelvic sex differences did not evolve de novo in modern humans and must have been present in the common ancestor of humans and chimpanzees, and thus also in the extinct Homo species. We further suggest that this shared pattern was already present in early mammals and propose a hypothesis of facilitated variation as an explanation: the conserved mammalian endocrine system strongly constrains the evolution of the pattern of pelvic differences but enables rapid evolutionary change of the magnitude of sexual dimorphism, which in turn facilitated the rapid increase in hominin brain size.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are openly available in an OSF repository80, https://osf.io/bd4gw/.
Code availability
The code written to analyse the data is openly available in an OSF repository80, https://osf.io/bd4gw/.
References
Fischer, B. & Mitteroecker, P. Covariation between human pelvis shape, stature, and head size alleviates the obstetric dilemma. Proc. Natl Acad. Sci. USA 112, 5655–5660 (2015).
Fischer, B. & Mitteroecker, P. Allometry and sexual dimorphism in the human pelvis. Anat. Rec. 300, 698–705 (2017).
Huseynov, A. et al. Developmental evidence for obstetric adaptation of the human female pelvis. Proc. Natl Acad. Sci. USA 113, 5227–5232 (2016).
Schultz, A. H. Sex differences in the pelves of primates. Am. J. Phys. Anthropol. 7, 401–424 (1949).
Tague, R. G. Sexual dimorphism in the human bony pelvis, with a consideration of the Neandertal pelvis from Kebara cave, Israel. Am. J. Phys. Anthropol. 88, 1–21 (1992).
Washburn, S. L. Sex differences in the pubic bone. Am. J. Phys. Anthropol. 6, 199–208 (1948).
Rogers, T. & Saunders, S. Accuracy of sex determination using morphological traits of the human pelvis. J. Forensic Sci. 39, 1047–1056 (1994).
Hager, L. D. Sex differences in the sciatic notch of great apes and modern humans. Am. J. Phys. Anthropol. 99, 287–300 (1996).
Durić, M., Rakocević, Z. & Donić, D. The reliability of sex determination of skeletons from forensic context in the Balkans. Forensic Sci. Int. 147, 159–164 (2005).
Dunsworth, H. M. Expanding the evolutionary explanations for sex differences in the human skeleton. Evol. Anthropol. 29, 108–116 (2020).
Grunstra, N. D. S. et al. Humans as inverted bats: a comparative approach to the obstetric conundrum. Am. J. Hum. Biol. 31, e23227 (2019).
Leutenegger, W. Functional aspects of pelvic morphology in simian primates. J. Hum. Evol. 3, 207–222 (1974).
Pavličev, M., Romero, R. & Mitteroecker, P. Evolution of the human pelvis and obstructed labor: new explanations of an old obstetrical dilemma. Am. J. Obstet. Gynecol. https://doi.org/10.1016/j.ajog.2019.06.043 (2019).
Rosenberg, K. R. The evolution of modern human childbirth. Am. J. Phys. Anthropol. 35, 89–124 (1992).
Wood, B. A. & Chamberlain, A. T. The primate pelvis: allometry or sexual dimorphism? J. Hum. Evol. 15, 257–263 (1986).
Betti, L. Sexual dimorphism in the size and shape of the os coxae and the effects of microevolutionary processes. Am. J. Phys. Anthropol. 153, 167–177 (2014).
Steudel, K. Sexual dimorphism and allometry in primate ossa coxae. Am. J. Phys. Anthropol. 55, 209–215 (1981).
Tague, R. G. Big-bodied males help us recognize that females have big pelves. Am. J. Phys. Anthropol. 127, 392–405 (2005).
Ridley, M. Pelvic sexual dimorphism and relative neonatal brain size really are related. Am. J. Phys. Anthropol. 97, 197–200 (1995).
Moffett, E. A. Dimorphism in the size and shape of the birth canal across anthropoid primates. Anat. Rec. 300, 870–889 (2017).
Tague, R. G. Pelvic sexual dimorphism in a metatherian, Didelphis virginiana: implications for eutherians. J. Mammal. 84, 1464–1473 (2003).
Lovejoy, C. O. Evolution of human walking. Sci. Am. 259, 118–125 (1988).
Gruss, L. T. & Schmitt, D. The evolution of the human pelvis: changing adaptations to bipedalism, obstetrics and thermoregulation. Philos. Trans R. Soc. Lond. B 370, 20140063 (2015).
Churchill, S. E. & Vansickle, C. Pelvic morphology in Homo erectus and early Homo. Anat. Rec. 300, 964–977 (2017).
Tague, R. G. & Lovejoy, C. O. The obstetric pelvis of A.L. 288-1 (Lucy). J. Hum. Evol. 15, 237–255 (1986).
Häusler, M. & Schmid, P. Comparison of the pelves of Sts 14 and AL 288-1: implications for birth and sexual dimorphism in australopithecines. J. Hum. Evol. 29, 363–383 (1995).
Tague, R. G. & Lovejoy, C. O. AL 288-1—Lucy or Lucifer: gender confusion in the Pliocene. J. Hum. Evol. 35, 75–94 (1998).
Arsuaga, J. L. & Carretero, J. M. Multivariate analysis of the sexual dimorphism of the hip bone in a modern human population and in early hominids. Am. J. Phys. Anthropol. 93, 241–257 (1994).
Kurki, H. K. Skeletal variability in the pelvis and limb skeleton of humans: does stabilizing selection limit female pelvic variation? Am. J. Hum. Biol. 25, 795–802 (2013).
Torres‐Tamayo, N. et al. The torso integration hypothesis revisited in Homo sapiens: contributions to the understanding of hominin body shape evolution. Am. J. Phys. Anthropol. 167, 777–790 (2018).
Torres‐Tamayo, N. et al. Assessing thoraco-pelvic covariation in Homo sapiens and Pan troglodytes: a 3D geometric morphometric approach. Am. J. Phys. Anthropol. 173, 514–534 (2020).
Hirata, S., Fuwa, K., Sugama, K., Kusunoki, K. & Takeshita, H. Mechanism of birth in chimpanzees: humans are not unique among primates. Biol. Lett. 7, 686–688 (2011).
Nissen, H. W. & Yerkes, R. M. Reproduction in the chimpanzee: report on forty-nine births. Anat. Rec. 86, 567–578 (1943).
Huseynov, A., Ponce de León, M. S. & Zollikofer, C. P. E. Development of modular organization in the chimpanzee pelvis. Anat. Rec. 300, 675–686 (2017).
Rosenberg, K. & Trevathan, W. Bipedalism and human birth: the obstetrical dilemma revisited. Evol. Anthropol. 4, 161–168 (1995).
Wells, J. C. K. Between Scylla and Charybdis: renegotiating resolution of the ‘obstetric dilemma’ in response to ecological change. Philos. Trans R. Soc. Lond. B 370, 20140067 (2015).
Mitteroecker, P. & Fischer, B. Adult pelvic shape change is an evolutionary side effect. Proc. Natl Acad. Sci. USA 113, E3596 (2016).
Mitteroecker, P. How human bodies are evolving in modern societies. Nat. Ecol. Evol. 3, 324–326 (2019).
Zollikofer, C. P. E., Scherrer, M. & Ponce de León, M. S. Development of pelvic sexual dimorphism in hylobatids: testing the obstetric constraints hypothesis. Anat. Rec. 300, 859–869 (2017).
Calder, W. A. III The kiwi and egg design: evolution as a package deal. BioScience 29, 461–467 (1979).
Congdon, J. D. & Gibbons, J. W. Morphological constraint on egg size: a challenge to optimal egg size theory? Proc. Natl Acad. Sci. USA 84, 4145–4147 (1987).
Cordero, G. A. Is the pelvis sexually dimorphic in turtles? Anat. Rec. 301, 1382–1389 (2018).
Lister, A. M. in The Proboscidea: Trends in Evolution and Paleoecology (eds Shoshani, J. & Tassy, P.) 254–259 (Oxford Univ. Press, 1996).
Schutz, H., Donovan, E. R. & Hayes, J. P. Effects of parity on pelvic size and shape dimorphism in Mus. J. Morphol. 270, 834–842 (2009).
Tague, R. G. Pelvic sexual dimorphism among species monomorphic in body size: relationship to relative newborn body mass. J. Mammal. 97, 503–517 (2016).
Crelin, E. S. & Newton, E. V. The pelvis of the free-tailed bat: sexual dimorphism and pregnancy changes. Anat. Rec. 164, 349–357 (1969).
Nwoha, P. U. Sex differences in the bony pelvis of the fruit-eating bat, Eidolon helvum. Folia Morphol. 59, 291–295 (2000).
Duetsch, J. & Peterson, R. Using pelvis morphology to identify sex in moose skeletal remains. Alces 48, 1–6 (2012).
Kaufmann, C. A., Álvarez, M. C., L’Heureux, L. G. & Gutiérrez, M. A. Dimorfismo sexual en la pelvis de Lama guanicoe (Artiodactyla, Camelidae): un caso de aplicación en el sitio Paso Otero 1, Buenos Aires, Argentina. Mastozool. Neotrop. 20, 47–59 (2013).
West, B. A tale of two innominates. Circaea 6, 107–114 (1990).
Berdnikovs, S. Evolution of sexual dimorphism in mustelids. PhD dissertation, Univ. Cincinnati (2005).
Schutz, H. Mammalian pelvic size and shape dimorphism: the effects of locomotion and parturition. PhD dissertation, Univ. Colorado at Boulder (2008).
Long, D. R. & Rose, F. L. Pelvic girdle size relationships in three turtle species. J. Herpetol. 23, 315–318 (1989).
Prieto-Marquez, A., Gignac, P. M. & Joshi, S. Neontological evaluation of pelvic skeletal attributes purported to reflect sex in extinct non-avian archosaurs. J. Vertebr. Paleontol. 27, 603–609 (2007).
Shatkovska, O. V., Ghazali, M., Mytiai, I. S. & Druz, N. Size and shape correlation of birds’ pelvis and egg: impact of developmental mode, habitat, and phylogeny. J. Morphol. 279, 1590–1602 (2018).
Iguchi, T., Irisawa, S., Fukazawa, Y., Uesugi, Y. & Takasugi, N. Morphometric analysis of the development of sexual dimorphism of the mouse pelvis. Anat. Rec. 224, 490–494 (1989).
Uesugi, Y., Taguchi, O., Noumura, T. & Iguchi, T. Effects of sex steroids on the development of sexual dimorphism in mouse innominate bone. Anat. Rec. 234, 541–548 (1992).
Steinetz, B. G. et al. Transmission of relaxin and estrogens to suckling canine pups via milk and possible association with hip joint laxity. Am. J. Vet. Res. 69, 59–67 (2008).
Dehghan, F. et al. The effect of relaxin on the musculoskeletal system. Scand. J. Med. Sci. Sports 24, e220–e229 (2014).
Middleton, E. R. Ecogeographic influences on trunk modularity in recent humans. PhD dissertation, New York Univ. (2015).
DeSilva, J. M. & Lesnik, J. J. Brain size at birth throughout human evolution: a new method for estimating neonatal brain size in hominins. J. Hum. Evol. 55, 1064–1074 (2008).
Hublin, J.-J., Neubauer, S. & Gunz, P. Brain ontogeny and life history in Pleistocene hominins. Philos. Trans R. Soc. Lond. B. 370, 20140062 (2015).
Gerhart, J. & Kirschner, M. The theory of facilitated variation. Proc. Natl Acad. Sci. USA 104, 8582–8589 (2007).
Pigliucci, M. & Muller, G. (eds) Evolution, the Extended Synthesis (MIT Press, 2010).
Laland, K. N. et al. The extended evolutionary synthesis: its structure, assumptions and predictions. Proc. R. Soc. Lond. B 282, 20151019 (2015).
Adams, J., Greenwood, P. & Naylor, C. Evolutionary aspects of environmental sex determination. Int. J. Invertebr. Reprod. Dev. 11, 123–135 (1987).
Geffroy, B. & Douhard, M. The adaptive sex in stressful environments. Trends Ecol. Evol. 34, 628–640 (2019).
Mittwoch, U. Sex-determining mechanisms in animals. Trends Ecol. Evol. 11, 63–67 (1996).
Bachtrog, D. et al. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899 (2014).
DelPrete, H. Similarities in pelvic dimorphisms across populations. Am. J. Hum. Biol. 31, e23282 (2019).
Gunz, P., Mitteroecker, P., Neubauer, S., Weber, G. W. & Bookstein, F. L. Principles for the virtual reconstruction of hominin crania. J. Hum. Evol. 57, 48–62 (2009).
Reynolds, H. M., Snow, C. C. & Young, J. W. Spatial Geometry of the Human Pelvis Memo. Report No. FAA-AM-B2-9 (Federal Aviation Administration, 1982); https://www.faa.gov/data_research/research/med_humanfacs/oamtechreports/1980s/media/AM82-09.pdf
Fischer, B. & Mitteroecker, P. Data from: Covariation between human pelvis shape, stature, and head size alleviates the obstetric dilemma. Dryad https://doi.org/10.5061/DRYAD.2D728 (2016).
Gower, J. C. Generalized Procrustes analysis. Psychometrika 40, 33–51 (1975).
Rohlf, F. J. & Slice, D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Biol. 39, 40–59 (1990).
Mitteroecker, P., Gunz, P., Windhager, S. & Schaefer, K. A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix It. J. Mammal. 24, 59–66 (2013).
Gunz, P., Mitteroecker, P. & Bookstein, F. L. in Modern Morphometrics in Physical Anthropology (ed. Slice, D.) 73–98 (Springer, 2005).
Gunz, P. & Mitteroecker, P. Semilandmarks: a method for quantifying curves and surfaces. Hystrix It. J. Mammal. 24, 103–109 (2013).
Bookstein, F. L. Morphometric Tools for Landmark Data: Geometry and Biology (Cambridge Univ. Press, 1991).
Fischer, B., Grunstra N. D. S., Zaffarini, E. & Mitteroecker P. Data and code for ‘Sex differences in the pelvis did not evolve de novo in modern humans’. OSF https://doi.org/10.17605/OSF.IO/BD4GW (2021).
Acknowledgements
We thank the Center for Academic Research and Training in Anthropology, the Primate Research Institute of the University of Kyoto, F. Zachos (Mammal Collection, Natural History Museum Vienna), M. Häusler, C. Fornai and V. Krenn (University of Zurich and University of Vienna) for access to the chimpanzee pelves. We thank H. Reynolds for sharing the human landmark data. B.F. and P.M. acknowledge support from the Austrian Science Fund FWF (Elise Richter grant no. V-826 to B.F., grant no. P29397 to P.M.). N.D.S.G. was funded by a postdoctoral fellowship from the Konrad Lorenz Institute. E.Z. was supported by an Erasmus+ student exchange fellowship from the European Union.
Author information
Authors and Affiliations
Contributions
B.F. designed the comparative study. N.D.S.G., E.Z. and B.F. developed the chimpanzee landmark scheme and E.Z. and N.D.S.G. acquired the chimpanzee data. N.D.S.G. and B.F. derived the homologous landmark scheme. B.F. acquired the human data. B.F. and P.M. cleaned and analysed the data. B.F., N.D.S.G. and P.M. wrote the paper. All authors commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Ecology & Evolution thanks Philipp Gunz, Nicole Torres and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
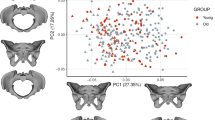
Extended Data Fig. 1 Principal component analysis (PCA) of pelvis shape, jointly for humans and chimpanzees.
The black arrows represent the allometry vectors, which were estimated here using a non-pelvic size measure for each species (femoral head diameter in chimpanzees and body height in humans, compare Extended Data Fig. 2) in contrast to Fig. 2, where they were estimated based on pelvic centroid size. For these non-pelvic size variables, z-scores were computed independently for each species. The allometry vectors were then estimated by regressing the shape coordinates on the z-scores for each species and projected into the PC spaces. As in Fig. 2, the allometry vectors are species-specific but not sex-specific. The vectors are shown twice, superimposed on the sex means for each species. The direction of allometry is distinct from the direction of sex differences in both species. The individual data shown are the same as in Fig. 2. Each small point corresponds to an individual (humans: males=blue, females=red; chimpanzees: males=light blue, females=light red). The larger points in the same colors correspond to the sex means for each species.
Extended Data Fig. 2 Pelvic centroid size vs. femoral head diameter in chimpanzees and body height in humans, respectively.
Correlations for these pairs of size measures were 0.62 in chimpanzees and 0.70 in humans. Femoral head diameter was available for 31 out of 34 chimpanzees and stature was available for all 99 human individuals.
Extended Data Fig. 3 Inlet area in mm2 in humans and chimpanzees for females (red) and males (blue).
Inlet area was calculated as the area of the polygon defined by the 2D inlet landmarks. Humans: female mean=11540 (sd=1248), male mean=10376 (sd=1047); Chimpanzees: female mean=9517 (sd=1107), male mean=8661 (sd=1263).
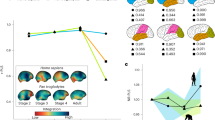
Extended Data Fig. 4 Reanalysis of data from DelPrete (2019).
These data comprise means for females and males of 26 pelvic variables (linear distances, curved distances, and circumferences) from 6 populations (skeletal collections): White individuals from Hamann-Todd collection (HTW, 60 males, 59 females); Black individuals from Hamann-Todd (HTB, 60 m., 60 f.); Whites from Terry collection (TEW, 52 m., 52 f.), Blacks from Terry collection (TEB, 52 m., 52 f.); Coimbra collection (CO, 84 m., 71 f.); Spitalfields collection (SP, 31 m., 35 f.). We conducted a principal component analysis of these data. Shown are the sex means of males (blue) and females (red) for each population within the first three principal components (accounting for 94% of the total variance). The sex difference vectors (lines connecting the sex means) of the six populations are close to parallel in the first three principal components (panels A and B), illustrating the similar pattern of sex differences in the pelvis between human populations, despite some variation in magnitude (panel C). The magnitude was calculated as the Euclidean length of the sex differences vector.
Supplementary information
Rights and permissions
About this article
Cite this article
Fischer, B., Grunstra, N.D.S., Zaffarini, E. et al. Sex differences in the pelvis did not evolve de novo in modern humans. Nat Ecol Evol 5, 625–630 (2021). https://doi.org/10.1038/s41559-021-01425-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-021-01425-z
This article is cited by
-
Changes in plasticity of the pelvic girdle from infancy to late adulthood in Homo sapiens
Scientific Reports (2023)
-
Mapping sexual dimorphism signal in the human cranium
Scientific Reports (2023)