Abstract
This study evaluated the efficacy and safety of cisplatin and nedaplatin in three-week doublet agent concurrent chemoradiotherapy (CCRT) for patients with locally advanced cervical cancer (LACC). We retrospectively enrolled patients with stage IIB-IIIC2 cervical cancer who received doublet agent CCRT from January 2015 to December 2020. Clinical outcomes were analyzed using the Kaplan–Meier method and a Cox proportional hazards model. Propensity score (PS) matching analysis was used to compare cisplatin plus docetaxel group and nedaplatin plus docetaxel group. A total of 295 patients were included. The 5-year overall survival rate (OS) and progression free survival rate (PFS) were 82.5% and 80.4%, respectively. After PS matching, there were 83 patients each in the nedaplatin group and cisplatin group. There were no significant differences in objective response rates (97.6% and 98.8%, p = 0.212), 5-year OS rate (96.5 vs 69.8, p = 0.066), PFS rate (90.8 vs 72.4, p = 0.166), and toxicity between the two groups. Doublet agent concurrent chemoradiotherapy is feasible, safe, and shows high efficacy in LACC patients. Here, cisplatin group has a trend of better prognosis, suggesting that cisplatin is preferred and nedaplatin can be considered for replacement when cisplatin is intolerant.
Similar content being viewed by others
Introduction
Cervical cancer, as the most common gynecologic malignancy, is the fourth female malignant tumor in the world in terms of female morbidity and mortality1, and its treatment remains a challenge. About 45% patients are diagnosed with locally advanced cervical cancer (LACC), including FIGO stage IIB-IVA, and the standard treatment was platinum-based concurrent radiotherapy and chemotherapy (CCRT)2,3. However, the use of platinum-based dual therapy or platinum single-dose therapy in CCRT is still controversial4,5. Multiple meta-analyses showed that compared with radiotherapy plus platinum single-dose therapy, radiotherapy plus platinum dual therapy could significantly prolong the prognosis of patients with LACC, but the incidence of adverse events would also increase6,7,8. At present, there are many platinum drug options, such as cisplatin, carboplatin, nedaplatin, etc. Nedaplatin is a second-generation platinum analogue, as a derivative of cisplatin, which was approved for the first time in Japan in 1983. Its solubility in water is about ten times that of cisplatin, and its digestive and renal toxicity is lower than that of cisplatin910]. A meta-analysis comparing single-agent CCRT showed that nedaplatin group had a better ORR and lower chemotherapy toxicity compared with cisplatin group11. Another phase III randomized, controlled trial showed the 3-year OS rate of nedaplatin group was similar with cisplatin group, and the gastrointestinal reaction was milder, and the hepatotoxicity was heavier compared with cisplatin group12. However, a study of propensity score matching has different results: the recurrence rate of nedaplatin group was significantly higher, and the 3-year PFS rate was lower compared with cisplatin group. The 3-year OS rate and grade 3 to 4 toxicity were similar between the nedaplatin and cisplatin groups13. The above studies are to explore the efficacy and safety of platinum single drug weekly therapy, and the results are controversial. In the field of platinum based dual reagent CCRT, there are fewer studies comparing the efficacy of different platinum drugs. This study aims to evaluate the efficacy and safety of cisplatin and nedaplatin in doublet agent CCRT.
Materials and methods
Patients
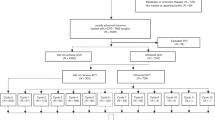
A cohort of patients diagnosed with cervical cancer were retrospectively examined at the Second Xiangya Hospital of Central South University and the Hunan Provincial People’s Hospital from January 2015 to December 2020. The following patients were included in the study: (1) those with a pathological diagnosis of cervical squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma, (2) those with stage IIB-IIIC2 cervical cancer (according to the FIGO 2018 staging system) who received platinum-based doublet agent CCRT, (3) those whose follow-up data were complete, and (4) those with IMRT. The following patients were excluded from the study: (1) those with other tumors, infectious diseases, hematological diseases, any mental disorder or somatic comorbidities, severe liver or renal dysfunction, or uncontrolled life-threatening illness, (2) those who received previous radiotherapy and/or chemotherapy; (3) those who had distant metastasis; or (4) those who were pregnancy or lactation. The last follow-up was conducted on August 31, 2022. All methods were performed in accordance with the relevant guidelines and regulations, and informed consent was obtained from all subjects and/or their legal guardian(s). The study was conducted in accordance with the Declaration of Hensinki and approved by the appropriate ethics review board of the Second Xiangya Hospital of Central South University, Changsha, China.
Treatment
All patients underwent CCRT, and some patients with good tolerance also received adjuvant chemotherapy after CCRT. Chemotherapy protocols were selected according to the patients' renal function and physical status. Patients received concurrent chemotherapy with docetaxel (75 mg/m2) combined with carboplatin (AUC = 4–5)/cisplatin (50–70 mg/m2)/nedaplatin (50–75 mg/m2), once every three weeks. So, patients were divided into cisplatin group, carboplatin group and nedaplatin group. The patient's whole blood cell count, renal function, and liver function were routinely monitored during treatment.
Intensity-modulated radiation therapy was used for external irradiation, which was planned with the Varian Eclipse Treatment Planning System version 11.0 (Varian Medical Systems, Palo Alto, CA, USA) and delivered with 6-MV X-rays using Varian 23EX (Varian Medical Systems).
Gross tumor volume (GTV) includes positive lymph nodes, which was confirmed using computed tomography (CT), magnetic resonance imaging (MRI), or positron-emission tomography (PET), and the clinical target volume (CTV) included the regional-nodal basin. The planning target volume was delineated by margins of 5–10 mm around the GTV and CTV. Cone-beam CT was performed weekly. The CTV dose was 45–50 Gy with 1.8 or 2 Gy administered daily, and the GTV dose was 60 Gy with 2.4 Gy administered daily. Patients received 2D intracavitary brachytherapy (iridium 192) with a dose of 30 Gy/5fx to point A (defined as 2 cm lateral to the central canal of the uterus and 2 cm above the cervical opening) once a week, without EBRT treatment on the day of intracavitary treatment. The entire RT course (including EBRT and brachytherapy) was completed within 8 weeks.
Data collection and follow-up
Clinical information, including that of age, initial stage, pathological pattern, tumor diameter, chemotherapy protocol, and imaging studies (CT, MRI, or positron emission tomography/CT) was collected. The imaging examination was carried out 2 months after the end of radiotherapy, and the treatment response was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Follow-up after the completion of CCRT includes every 3 months in the first 2 years, every 6 months in the next 3 years, and every year thereafter. At each follow-up visit, physical examination, imaging examination, hematology examination or cervical cytology examination are selected according to the patient's condition.
Statistical analysis
The SPSS software (version 26.0; SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Overall survival (OS) calculated from the date of diagnosis until the date of death or final follow‐up. Progression‐free survival (PFS) was defined as survival from the date of diagnosis until the date of (1) disease progression, (2) relapse, (3) mortality from any cause, or (4) final follow‐up.
Fisher’s exact test and χ2 test was used to determine whether there was a correlation between two variables. Univariate analysis was performed using the Kaplan–Meier method to assess the 5-year OS and PFS rates. Statistical differences between survival curves were evaluated using the log-rank test. Multivariate Cox proportional hazards models were created using the input selection technique of factors significant in the univariate analysis. Patients were classified into the cisplatin group and nedaplatin group. Treatment outcomes were compared between the two groups after 1:1 ratio propensity score (PS) matching with the nearest neighbor matching method using calipers of width equal to 0.02 of the SD of the logit of the PS, and adjusted for age, stage, BMI, pathological pattern, tumor diameter, differentiation, complication, and chemotherapy cycles14. In all tests, P-values < 0.05 indicated a significant difference. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated to assess the magnitude of risk. And I confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Institutional review board statement
The study was conducted in accordance with the Declaration of Hensinki and approved by the appropriate ethics review board of the Second Xiangya Hospital of Central South University, Changsha, China.
Results
Patient characteristics
A total of 295 patients enrolled. The median follow-up time was 53 months (20–92 months). The median age was 55 years (32–73 years). The median number of chemotherapy cycle is 4 (1–6 cycles). There were 44 patients received concurrent chemotherapy with median cycle of 2, and 251 patients received concurrent and adjuvant chemotherapy with median cycle of 4. There were 198 patients were treated with nedaplatin, 87 with cisplatin and 10 with carboplatin. In the nedaplatin group, 11 patients only received one cycle of chemotherapy because they could not tolerate chemotherapy. In the cisplatin group, 3 patients only received one cycle of chemotherapy. Patients stopped chemotherapy if they couldn't tolerate it, and no patients received reduced dose chemotherapy. Patients’ characteristics are summarized in Table 1. All patients completed external irradiation, while only 8 patients did not complete brachytherapy. Among them, 5 patients were unable to tolerate adverse reactions, and 3 patients were due to family and social reasons.
Prognostic analysis and propensity score analysis
All patients received doublet agent concurrent chemoradiotherapy, and the 5-year OS and PFS rates were 82.5% and 80.4%, respectively (Fig. 1A,B). In the univariate analysis, chemotherapy protocol, and therapy response significantly influenced the OS, and only therapy response significantly influenced the PFS rates (Table 2). The stage was divided into stage II, stage IIIA/B, and stage IIIC for stratified analysis. It was found that there was a significantly difference in OS between stage II and stage IIIc (OS: 87.9% vs. 70.1%, p = 0.045) (Supplemental Table S1). Patients with tumor diameter < 4 cm had better PFS trend than those who ≥ 4 cm (PFS: 86.2% vs. 75%, p = 0.054). The above variables with statistical significance in univariate analysis, such as stage, tumor diameter, and chemotherapy, were included in multivariate analysis, and no independent prognostic factors were found (Table 3).
Patients who received carboplatin had worser OS than those who received cisplatin and nedaplatin (OS: 64% vs. 95.2% vs. 82.1%, p = 0.017). Then comparing the prognosis difference between cisplatin and nedaplatin group. The clinical characteristics of the cisplatin group and nedaplatin group were compared. It was found that pathology and tumor diameter between the two groups were not well-balanced (Supplementary Table S2). After PS matching, there were 83 patients each in the cisplatin group and nedaplatin group. All background factors between the two groups were well-balanced (Supplementary Table S2). Responses and survival outcomes before and after matching are summarized in Table 4. There was no significant difference between cisplatin group and nedaplatin group after matching in terms of ORR (97.6% vs. 98.8%, p = 0.212), 5-year OS rate (96.5% vs. 69.8%, p = 0.066), and PFS rate (90.8% vs. 72.4%, p = 0.166), however in the survival curve, it can be found that cisplatin group has a better prognosis trend than nedaplatin group (Fig. 1C,D).
Toxicity
In total, 85.4% of patients experienced hematologic toxicity, of which 46.1% had grade 3–4 hematologic toxicity; 59.3% had nausea/vomiting, of which 10.5% had grade 3–4 nausea/vomiting; 47.8% had radiation proctitis, 28.5% had radiation cystitis, and no patients had grade 3–4 radiation proctitis or cystitis.
After PS matching, there was no significant difference between cisplatin group and nedaplatin group in terms of hematotoxicity (85.5% vs.79.5%, p = 0.307), grade 3–4 hematotoxicity (36.0% vs.46.9%, p = 0.209), grade 3–4 neutropenia (34.9% vs.44.6%, p = 0.268), thrombocytopenia (53% vs.51.8%, p = 0.488), grade 3–4 thrombocytopenia (21.7% vs.16.9%, p = 0.431), grade 3–4 anemia (7.2% vs. 1.2%, p = 0.053), nausea and vomiting (63.9% vs.54.2%, p = 0.207), grade 3–4 nausea and vomiting (13.3% vs.6.0%, p = 0.115), radiation proctitis (48.2% vs.45.9%, p = 0.756), and radiation cystitis (33.7% vs.22.9%, p = 0.121). However, in terms of neutropenia (78.3% vs.71.1%, p = 0.003) and anemia (83.1% vs.79.5%, p = 0.003), the cisplatin group was greater than the nedaplatin group (Table 5).
Discussion
Platinum-based CCRT is the standard treatment for patients with locally advanced cervical cancer. The main function of platinum-based chemotherapy is to increase the sensitivity of radiotherapy and eradicate micrometastasis15. At present, the most recommended regimen of concurrent chemotherapy is weekly cisplatin monotherapy16. However, the effect of cisplatin monotherapy is limited, and the 5-year OS rate in patients with IIB-IVA stage was approximately 60%2. Therefore, many studies have focused on platinum-based dual-agent CCRT with synergistic effects. A meta-analysis compared the efficacy and safety of RT with cisplatin monotherapy and RT with platinum-based doublet therapy in patients with locally advanced cervical cancer. The results showed that RT with platinum-based doublet therapy significantly prolonged the OS (HR 0.75, 95% CI 0.60–0.94, p = 0.01) and the PFS (HR 0.78, 95% CI 0.65–0.94, p = 0.01), but increased adverse reactions compared to the cisplatin monotherapy group6. Many other studies confirmed that platinum-based dual-agent CCRT has improved survival8. Our study evaluated the efficacy and safety of platinum-based dual chemotherapy, and the results showed that dual-agent CCRT had excellent long-term therapeutic effect, and the 5-year OS and PFS rates were 82.5% and 80.4%, respectively, which is better than cisplatin monotherapy and consistent with the current studies.
At present, many platinum-based dual-agent regimens of CCRT have been studied, A network meta-analysis enrolled 11 cohort studies on CCRT for LACC, including cisplatin monotherapy, paclitaxel monotherapy, and platinum combined chemotherapy. The results showed that cisplatin plus docetaxel might be the best choice of CCRT regimens in the treatment of LACC17. In addition, paclitaxel plus cisplatin18, gemcitabine plus cisplatin4, and 5-FU plus cisplatin19 in CCRT all showed good curative effect.
In this study, we analyzed the efficacy of carboplatin, cisplatin and nedaplatin based dual-agent concurrent chemoradiotherapy. The results showed that the prognosis of carboplatin-based CCRT is worse than that of cisplatin and nedaplatin-based CCRT. Due to the small number of patients receiving carboplatin treatment in this study, the result is controversial. A study showed that there were no statistically significant differences in recurrence and survival rates between the carboplatin CCRT group and the historical cisplatin CCRT group in 51 LACC patients with morbidity risks20. Another retrospective study also showed that carboplatin group have similar 3-years overall survival, progression free survival, overall response rate, and toxic effects when compared to cisplatin group21. However, a meta-analysis showed that carboplatin CCRT group showed a poorer tumor response and a trend toward inferior survival compared with cisplatin CCRT group. Although this is consistent with the results of this study, larger controlled studies are still needed to validate them.
Our study also showed that there is no significant difference between the cisplatin group and the nedaplatin group in terms of ORR, 5-year OS rate and PFS rate when all background factors were balanced among groups, but the cisplatin group has a better prognosis trend. Nedaplatin and cisplatin act in a similar way, both of which produce anti-tumor activity by inhibiting the replication of tumor cell DNA. However, there are still differences between these two drugs. For example, cisplatin is the substrates of membrane transporters such as hOCT1, hOCT2, and hMATE1. Nedaplatin are not transported by the transporters mentioned above. It indicates that the resistance modes of the two drugs are different, and they are not completely cross resistant. In terms of clinical application, cisplatin has a wider anti-tumor spectrum and more comprehensive data, making it the preferred drug for cervical cancer at present. An early phase 2 clinical trial showed that the CCRT regimen of paclitaxel and nedaplatin was well tolerated and effective for LACC. The 2-year PFS and OS were 82% and 93%, respectively, and 3 cases (9%) had grade 3 late complications, which suggested that concurrent chemoradiotherapy based on nedaplatin is effective and safe22. Another randomized phase III trial to evaluate the efficacy and safety of nedaplatin and cisplatin three-week monotherapy in CCRT showed that the 1-year PFS and OS in the nedaplatin and cisplatin groups were similar23. However, a meta-analysis showed nedaplatin group had higher ORR and lower toxicity than cisplatin group, which is better than other concurrent single-agent chemotherapy, such as docetaxel, paclitaxel, fluoropyrimidine, paclitaxel liposome, and irinotecan11. Therefore, when cisplatin is intolerant, it is feasible to use nedaplatin instead.
In terms of safety, a study showed that nedaplatin less frequently causes renal toxicity in comparison to cisplatin due to lower kidney accumulation24. However, there is still controversy regarding other toxicity. Our study shows that the hematological toxicity of cisplatin is slightly higher than that of nedaplatin, and there is no difference between nausea/vomiting, radiation cystitis and proctitis. However, a study showed that there were no significant differences in hematological toxicity between the nedaplatin and cisplatin for CCRT of cervical cancer, and vomiting, nausea and anorexia were more common in the cisplatin group whereas effects on liver function were more common in the nedaplatin group12. Another study comparing nedaplatin and cisplatin monotherapy CCRT showed that a higher frequency of grade 3–4 neutropenia, grade 1–2 anemia, thrombocytopenia in the nedaplatin group than the cisplatin group, Whereas nausea in the cisplatin group was higher than in the nedaplatin group23. The comparison of adverse events of nedaplatin and cisplatin in different studies has different results, which may be closely related to the enrolled population, specific treatment plans, etc., and needs to be verified by large prospective randomized clinical studies.
In terms of prognostic factor of CCRT, stage and tumor size were recognized to be closely related to the prognosis. A phase III randomized clinical trial of cisplatin monotherapy versus gemcitabine combined with cisplatin for CCRT of LACC showed that stage and large tumor size were associated with poor prognosis, regardless of treatment assigned25. Another study reestimated the published prognostic model of LACC and found that the reestimated prognostic factors (including FIGO stage and tumor size) in the validation sample were related to the reduction of OS and CSS26. Our study also showed that the stage and tumor size still affect the prognosis of patients with LACC in dual-agent CCRT. However, they are not independent prognostic factors. Whether the better effectiveness of dual-agent chemotherapy weakens their impact on prognosis needs further discussion.
Adjuvant chemotherapy after CCRT has always been controversial. A meta-analysis in 2008 and a study in 2011 suggested patients could benefit from adjuvant chemotherapy after CCRT5,27. In recent years, both of ACTLACC trial and OUTBACK trial evaluated the effect of adjuvant chemotherapy after CCRT, and the results showed that adjuvant chemotherapy after CCRT did not improve survival compared to CCRT alone, and the rate of grade 3–5 adverse events within one year was higher28,29. However, ACTLACC trial excluded patients with enlarged paraaortic lymph nodes, and OUTBACK trial did not conduct stratification analysis of clinical characteristics. Therefore, the results have limitations. A review analyzed the relevant studies of adjuvant chemotherapy and suggested that there is an absolute need for further research in order to optimally define the position of adjuvant chemotherapy in the treatment of LACC30. Our study showed 85.1% of patients received 4–6 cycles of chemotherapy, and the prognosis of patients receiving 1–3 cycles of chemotherapy is the same as that of patients receiving 4–6 cycles, which means that adjuvant chemotherapy did not increase the efficacy, which consistent with current mainstream research results.
There are many advances in the treatment of LACC. Immunotherapy has changed the first-line treatment mode of cervical cancer, and some studies have also been carried out in the CCRT of LACC, such as: adjuvant durvalumab with chemoradiotherapy31, toripalimab combined with platinum-based CCRT32, and pembrolizumab with chemoradiotherapy33 et al. The CCRT regimen of these studies is weekly cisplatin monotherapy, which are still being recruited. The study of dual-agent CCRT combined with immunotherapy has not been carried out.
This study is a double-center retrospective study. The baseline situation is complex. Although propensity score matching is used for adjustment, the population is small, which has a certain impact on the results. Especially in the prognosis analysis, the influence trend of cisplatin on prognosis was seen, but there was no statistical difference. It is necessary to further expand the sample size. In addition, there is no single-agent group for control. This study has certain limitations.
Conclusion
Our study suggests that doublet agent concurrent chemoradiotherapy is feasible, safe, and shows high efficacy in LACC patients. Here, the cisplatin group and nedaplatin group have similar prognosis and side effects. However, cisplatin group has the trend of better prognosis, suggesting that cisplatin is preferred and nedaplatin can be considered for replacement when cisplatin is intolerant.
Data availability
The authors agree to share anonymized data upon reasonable request by researchers. Someone wants to request the data from this study, please contact the corresponding author.
References
Siegel, R. L. et al. Cancer statistics, 2021. CA Cancer J. Clin. 71(1), 7–33 (2021).
Rose, P. G. et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 340(15), 1144–1153 (1999).
Wright, J. D. et al. Prognostic performance of the 2018 international federation of gynecology and obstetrics cervical cancer staging guidelines. Obstet. Gynecol. 134(1), 49–57 (2019).
Wang, C. C. et al. A randomized trial comparing concurrent chemoradiotherapy with single-agent cisplatin versus cisplatin plus gemcitabine in patients with advanced cervical cancer: An Asian Gynecologic Oncology Group study. Gynecol. Oncol. 137(3), 462–467 (2015).
Duenas-Gonzalez, A. et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J. Clin. Oncol. 29(13), 1678–1685 (2011).
Ma, S. et al. Platinum single-agent vs platinum-based doublet agent concurrent chemoradiotherapy for locally advanced cervical cancer: A meta-analysis of randomized controlled trials. Gynecol. Oncol. 154(1), 246–252 (2019).
Petrelli, F. et al. Radiotherapy with concurrent cisplatin-based doublet or weekly cisplatin for cervical cancer: A systematic review and meta-analysis. Gynecol. Oncol. 134(1), 166–171 (2014).
Deng, T. et al. Comparison of platinum monotherapy with concurrent chemoradiation therapy versus platinum-based dual drug therapy with concurrent chemoradiation therapy for locally advanced cervical cancer: A systematic review and meta-analysis. Infect. Agent Cancer 17(1), 18 (2022).
Shimada, M., Itamochi, H. & Kigawa, J. Nedaplatin: A cisplatin derivative in cancer chemotherapy. Cancer Manag. Res. 5, 67–76 (2013).
Kanzawa, F. et al. Antitumor activity of a new platinum compound (glycolate-o, o’) diammineplatinum (II) (254-S), against non-small cell lung carcinoma grown in a human tumor clonogenic assay system. Anticancer Res. 8(3), 323–327 (1988).
Zhang, Y. et al. Efficacy of concurrent single-agent chemotherapy using radiotherapy in patients with cervical cancer: A meta-analysis. Int. J. Clin. Exp. Med. 8(6), 8661–8673 (2015).
Yang, X. et al. A phase III randomized, controlled trial of nedaplatin versus cisplatin concurrent chemoradiotherapy in patients with cervical cancer. ESMO Open 7(5), 100565 (2022).
Li, P. et al. Comparison of nedaplatin- and cisplatin-based concurrent chemoradiotherapy in locally advanced cervical cancer patients: A propensity score analysis. Int. J. Gynecol. Cancer 28(5), 1029–1037 (2018).
Rubin, D. B. Estimating causal effects from large data sets using propensity scores. Ann. Intern. Med. 127(8 Pt 2), 757–763 (1997).
Chen, C. C. et al. The prognostic factors for locally advanced cervical cancer patients treated by intensity-modulated radiation therapy with concurrent chemotherapy. J. Formos. Med. Assoc. 114(3), 231–237 (2015).
Kinjyo, Y. et al. Concurrent weekly cisplatin versus triweekly cisplatin with radiotherapy for locally advanced squamous-cell carcinoma of the cervix: A retrospective analysis from a single institution. Br. J. Radiol. 90(1076), 20170241 (2017).
Fu, Z. Z. et al. Efficacy and toxicity of different concurrent chemoradiotherapy regimens in the treatment of advanced cervical cancer: A network meta-analysis. Medicine (Baltimore) 96(2), e5853 (2017).
Umayahara, K. et al. Phase II study of concurrent chemoradiotherapy with weekly cisplatin and paclitaxel in patients with locally advanced uterine cervical cancer: The JACCRO GY-01 trial. Gynecol. Oncol. 140(2), 253–258 (2016).
Nedovic, J. et al. Cisplatin monotherapy with concurrent radiotherapy versus combination of cisplatin and 5-fluorouracil chemotherapy with concurrent radiotherapy in patients with locoregionally advanced cervical carcinoma. J. BUON 17(4), 740–745 (2012).
Nam, E. J. et al. Comparison of carboplatin- and cisplatin-based concurrent chemoradiotherapy in locally advanced cervical cancer patients with morbidity risks. Oncologist 18(7), 843–849 (2013).
Sebastiao, A. M. et al. Carboplatin-based chemoradiotherapy in advanced cervical cancer: An alternative to cisplatin-based regimen?. Eur. J. Obstet. Gynecol. Reprod. Biol. 201, 161–165 (2016).
Zhang, M. Q., Liu, S. P. & Wang, X. E. Concurrent chemoradiotherapy with paclitaxel and nedaplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: Preliminary results of a phase II study. Int. J. Radiat. Oncol. Biol. Phys. 78(3), 821–827 (2010).
He, S. et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in patients with stage IIB-IVA cervical cancer: A randomized phase III trial. Front. Oncol. 11, 798617 (2021).
Zhou, J. et al. The drug-resistance mechanisms of five platinum-based antitumor agents. Front. Pharmacol. 11, 343 (2020).
Duenas-Gonzalez, A. et al. Efficacy in high burden locally advanced cervical cancer with concurrent gemcitabine and cisplatin chemoradiotherapy plus adjuvant gemcitabine and cisplatin: Prognostic and predictive factors and the impact of disease stage on outcomes from a prospective randomized phase III trial. Gynecol. Oncol. 126(3), 334–340 (2012).
Lora, D. et al. Prognostic models for locally advanced cervical cancer: External validation of the published models. J. Gynecol. Oncol. 28(5), e58 (2017).
Group MA. Medical Research Council Clinical Trials Unit: Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 26, 5802–5812 (2016).
Tangjitgamol, S. et al. A randomized controlled trial comparing concurrent chemoradiation versus concurrent chemoradiation followed by adjuvant chemotherapy in locally advanced cervical cancer patients: ACTLACC trial. J. Gynecol. Oncol. 30(4), e82 (2019).
Mileshkin, L. R. et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 24(5), 468–482 (2023).
Cerina, D. et al. Is there a place for adjuvant chemotherapy in the treatment of locally advanced cervical cancer?. Curr. Oncol. 29(8), 5223–5237 (2022).
Mayadev, J. et al. CALLA: Efficacy and safety of concurrent and adjuvant durvalumab with chemoradiotherapy versus chemoradiotherapy alone in women with locally advanced cervical cancer: A phase III, randomized, double-blind, multicenter study. Int. J. Gynecol. Cancer 30(7), 1065–1070 (2020).
Chen, J. et al. Toripalimab combined with concurrent platinum-based Chemoradiotherapy in patients with locally advanced cervical Cancer: An open-label, single-arm, phase II trial. BMC Cancer 22(1), 793 (2022).
Domenicalorusso, N. C. et al. ENGOT-cx11/KEYNOTE-A18: A phase III, randomized, double-blind study of pembrolizumab with chemoradiotherapy in patients with high-risk locally advanced cervical cancer. J. Clin. Oncol. 38(15), 6096 (2020).
Acknowledgements
The authors thank all the staff in the department of Oncology of the Second Xiangya Hospital of Central South University, Changsha, China.
Funding
This study was supported by Changsha Natural Science Foundation (grant no. kq2208339) in China.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection, M.S., W.Z., and Y.F.; data analysis, H.T.; writing-original draft preparation, Y.Z. and S.F.; writing-review and editing, J.W; supervision, X.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Fan, S., Shan, M. et al. Propensity score matching analysis to comparing cisplatin versus nedaplatin based doublet agent concurrent chemoradiotherapy for locally advanced cervical cancer. Sci Rep 13, 9352 (2023). https://doi.org/10.1038/s41598-023-36433-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36433-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.