Abstract
Background
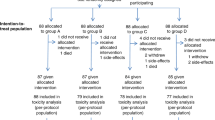
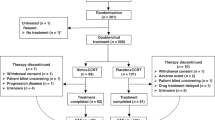
The optimal number of neoadjuvant chemotherapy (NAC) cycles remains to be established for treating oesophageal squamous cell carcinoma (ESCC). We compared two versus three courses of NAC for treating locally advanced ESCC in a multi-institutional, randomised, Phase II trial.
Methods
We randomly assigned 180 patients with locally advanced ESCC at 6 institutions to either two (N = 91) or three (N = 89) courses of DCF (docetaxel 70 mg/m2, cisplatin 70 mg/m2 i.v. on day 1, fluorouracil 700 mg/m2 continuous infusion for 5 days) every 3 weeks, prior to surgery. The primary endpoint was 2-year progression-free survival (PFS) with an intention-to-treat analysis.
Results
Patient background parameters were well-balanced. The R0 resection rates were 98.9 and 96.5% in the two- and three-course groups, respectively (P = 0.830). In resected cases, the two- and three-course groups had comparable pN0 rates (P = 0.225) and histological responses (P = 0.898). The 2-year PFS rate was also comparable between the two groups (71.4 vs. 71.1%, P = 0.669). Among subgroups based on baseline characteristics, only patients aged under 65 years old showed a tendency for better survival with the three-course treatment (hazard ratio = 2.612, 95% confidence interval: 1.012–7.517).
Conclusions
Two courses of a DCF regimen showed potential as an optional NAC treatment for locally advanced ESCC.
Clinical trial registration
University Hospital Medical Information Network Clinical Trials Registry of Japan (identification number UMIN 000015788).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Colvin H, Mizushima T, Eguchi H, Takiguchi S, Doki Y, Mori M. Gastroenterological surgery in Japan: the past, the present and the future. Ann Gastroenterological Surg. 2017;1:5–10.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Hofstetter W, Swisher SG, Correa AM, Hess K, Putnam JB Jr, Ajani JA, et al. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:376–84.
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl J Med. 2012;366:2074–84..
Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Yamasaki M, Yasuda T, Yano M, Hirao M, Kobayashi K, Fujitani K, et al. Multicenter randomized phase ii study of cisplatin and fluorouracil plus docetaxel (dcf) compared with cisplatin and fluorouracil plus adriamycin (acf) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (ogsg1003). Ann Oncol. 2017;28:116–20.
Hagi T, Makino T, Yamasaki M, Yamashita K, Tanaka K, Saito T, et al. Pathological regression of lymph nodes better predicts long-term survival in esophageal cancer patients undergoing neoadjuvant chemotherapy followed by surgery. Ann Surg. 2020; https://doi.org/10.1097/SLA.0000000000004238.
Makino T, Yamasaki M, Tanaka K, Masuike Y, Tatsumi M, Motoori M, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2019;270:1090–5.
Urakawa S, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, et al. Lymph node response to neoadjuvant chemotherapy as an independent prognostic factor in metastatic esophageal cancer. Ann Surg. 2021;273:1141–9.
Shiraishi O, Yamasaki M, Makino T, Motoori M, Miyata H, Shinkai M, et al. Feasibility of preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil versus adriamycin, cisplatin, and 5-fluorouracil for resectable advanced esophageal cancer. Oncology. 2021;92:101–8.
Makino T, Yamasaki M, Miyazaki Y, Wada N, Takahashi T, Kurokawa Y, et al. Utility of initial induction chemotherapy with 5-fluorouracil, cisplatin, and docetaxel (dcf) for t4 esophageal cancer: a propensity score-matched analysis. Dis Esophagus. 2018; https://doi.org/10.1093/dote/dox130.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl J Med. 2006;355:11–20.
Yoshikawa T, Morita S, Tanabe K, Nishikawa K, Ito Y, Matsui T, et al. Survival results of a randomised two-by-two factorial phase ii trial comparing neoadjuvant chemotherapy with two and four courses of s-1 plus cisplatin (sc) and paclitaxel plus cisplatin (pc) followed by d2 gastrectomy for resectable advanced gastric cancer. Eur J Cancer. 2016;62:103–11.
Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, et al. Three-arm phase iii trial comparing cisplatin plus 5-fu (cf) versus docetaxel, cisplatin plus 5-fu (dcf) versus radiotherapy with cf (cf-rt) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, next study). Jpn J Clin Oncol. 2013;43:752–5.
Shiraishi O, Makino T, Yamasaki M, Tanaka K, Yamashita K, Ishida T, et al. Two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for treating locally advanced esophageal cancer: short-term outcomes of a multicenter randomized phase II trial. Esophagus. 2021; https://doi.org/10.1007/s10388-021-00831-3.
Sobin LH GM, Wittekind C. TNM classification of malignant tumors. 7th edn. Oxford: Wiley-Blackwell; 2010.
Makino T, Yamasaki M, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, et al. Short- and long-term outcomes of larynx-preserving surgery for cervical esophageal cancer: analysis of 100 consecutive cases. Ann Surg Oncol. 2016;23:858–65.
Makino T, Yamasaki M, Tanaka K, Tatsumi M, Takiguchi S, Hatazawa J, et al. Importance of positron emission tomography for assessing the response of primary and metastatic lesions to induction treatments in t4 esophageal cancer. Surgery. 2017;162:836–45.
Makino T, Yamasaki M, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, et al. Solitary lymph node recurrence of esophageal squamous cell carcinoma: surgical failure or systemic disease? Ann Surg Oncol. 2016;23:2087–93.
Hagi T, Makino T, Yamasaki M, Tanaka K, Nishida N, Sakai D, et al. Dysphagia score as a predictor of adverse events due to triplet chemotherapy and oncological outcomes in 434 consecutive patients with esophageal cancer. Ann Surg Oncol. 2019;26:4754–64.
Yamamoto K, Makino T, Sato E, Noma T, Urakawa S, Takeoka T, et al. Tumor-infiltrating m2 macrophage in pretreatment biopsy sample predicts response to chemotherapy and survival in esophageal cancer. Cancer Sci. 2020;111:1103–12.
Makino T, Miyata H, Yamasaki M, Fujiwara Y, Takiguchi S, Nakajima K, et al. Utility of response evaluation to neo-adjuvant chemotherapy by (18)f-fluorodeoxyglucose-positron emission tomography in locally advanced esophageal squamous cell carcinoma. Surgery. 2010;148:908–18.
Yamashita K, Makino T, Miyata H, Miyazaki Y, Takahashi T, Kurokawa Y, et al. Postoperative infectious complications are associated with adverse oncologic outcomes in esophageal cancer patients undergoing preoperative chemotherapy. Ann Surg Oncol. 2016;23:2106–14.
Hashimoto T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, et al. The pattern of residual tumor after neoadjuvant chemotherapy for locally advanced esophageal cancer and its clinical significance. Ann Surg. 2020;271:875–84.
Sugase T, Makino T, Yamasaki M, Tanaka K, Hashimoto T, Miyazaki Y, et al. Histological changes of superficial esophageal squamous cell carcinoma after preoperative chemotherapy. Esophagus. 2018; https://doi.org/10.1007/s10388-018-0626-8.
Makino T, Yamasaki M, Miyata H, Yoshioka S, Takiguchi S, Fujiwara Y, et al. P53 mutation status predicts pathological response to chemoradiotherapy in locally advanced esophageal cancer. Ann Surg Oncol. 2010;17:804–11.
Makino T, Yamasaki M, Takeno A, Shirakawa M, Miyata H, Takiguchi S, et al. Cytokeratins 18 and 8 are poor prognostic markers in patients with squamous cell carcinoma of the oesophagus. Br J Cancer. 2009;101:1298–306.
Makino T, Yamasaki M, Takemasa I, Takeno A, Nakamura Y, Miyata H, et al. Dickkopf-1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:2058–64.
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl J Med. 2021;384:1191–203.
Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Cin Oncol. 2009;27:5062–7.
Zhang G, Zhang C, Sun N, Xue L, Yang Z, Fang L, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for the treatment of esophageal squamous cell carcinoma: a propensity score-matched study from the National Cancer Center in China. J Cancer Res Clin Oncol. 2021; https://doi.org/10.1007/s00432-021-03659-7.
Acknowledgements
The authors would like to thank the staff of all the centres that participated in the data collection process for this study.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Contributions
TM, SU and TI contributed to the design and analysis of the results and the writing of the manuscript. MY, KT, KY, OS, KS, HM, MM, KF, AT, MH, YK and TS contributed to the implementation of the research and analysis of the results. MY, HE, YD and TY contributed to the design and implementation of the research.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients provided written informed consent for trial participation. The study protocol was approved by the Institutional Review Board in each of six participating hospitals, before patient enrolment.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Makino, T., Yamasaki, M., Tanaka, K. et al. Multicenter randomised trial of two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for locally advanced oesophageal squamous cell carcinoma. Br J Cancer 126, 1555–1562 (2022). https://doi.org/10.1038/s41416-022-01726-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01726-5
This article is cited by
-
Thymol Enhances 5-Fluorouracil Cytotoxicity by Reducing Migration and Increasing Apoptosis and Cell Cycle Arrest in Esophageal Cancer Cells: An In-vitro Study
Indian Journal of Clinical Biochemistry (2024)
-
Risk stratification of oesophageal squamous cell carcinoma using change in total lesion glycolysis and number of PET-positive lymph nodes
British Journal of Cancer (2023)
-
Density and maturity of peritumoral tertiary lymphoid structures in oesophageal squamous cell carcinoma predicts patient survival and response to immune checkpoint inhibitors
British Journal of Cancer (2023)