Abstract
The combination of re-irradiation and bevacizumab has emerged as a potential therapeutic strategy for patients experiencing their first glioblastoma multiforme (GBM) recurrence. This study aims to assess the effectiveness of the re-irradiation and bevacizumab combination in treating second-progression GBM patients who are resistant to bevacizumab monotherapy. This retrospective study enrolled 64 patients who developed a second progression after single-agent bevacizumab therapy. The patients were divided into two groups: 35 underwent best supportive care (none-ReRT group), and 29 received bevacizumab and re-irradiation (ReRT group). The study measured the overall survival time after bevacizumab failure (OST-BF) and re-irradiation (OST-RT). Statistical tests were used to compare categorical variables, evaluate the difference in recurrence patterns between the two groups, and identify optimal cutoff points for re-irradiation volume. The results of the Kaplan–Meier survival analysis indicated that the re-irradiation (ReRT) group experienced a significantly higher survival rate and longer median survival time than the non-ReRT group. The median OST-BF and OST-RT were 14.5 months and 8.8 months, respectively, for the ReRT group, while the OST-BF for the none-ReRT group was 3.9 months (p < 0.001). The multivariable analysis identified the re-irradiation target volume as a significant factor for OST-RT. Moreover, the re-irradiation target volume exhibited excellent discriminatory ability in the area under the curve (AUC) analysis, with an optimal cutoff point of greater than 27.58 ml. These findings suggest that incorporating re-irradiation with bevacizumab therapy may be a promising treatment strategy for patients with recurrent GBM resistant to bevacizumab monotherapy. The re-irradiation target volume may serve as a valuable selection factor in determining which patients with recurrent GBM are likely to benefit from the combined re-irradiation and bevacizumab treatment modality.
Similar content being viewed by others
Introduction
Glioblastoma multiforme (GBM) is a highly aggressive primary brain tumor with a 5-year relative survival rate of only 6.9%1. Although bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor (VEGF), has received FDA approval for treating recurrent GBM, its effectiveness in progressive GBM after initial therapy remains limited2,3,4,5. While bevacizumab is a valuable second-line treatment for patients with GBM who have failed other therapies, such as radiotherapy, temozolomide, and lomustin6, there is still a pressing need for innovative therapeutic approaches for recurrent GBM following bevacizumab failure.
Re-irradiation in combination with bevacizumab has emerged as a promising treatment option for recurrent GBM. This is partly due to advances in radiation therapy techniques, which allow for better sparing of critical structures and reduced risk of neurologic toxicities7. Although potential late neurologic toxicities may be associated with re-irradiation, careful patient selection and treatment planning can help minimize these risks8,9. Notably, the recent phase II NRG Oncology/RTOG 1205 trial demonstrated a significant survival benefit in recurrent GBM when treated with re-irradiation and bevacizumab, accompanied by acceptable toxicity profiles10. Additionally, bevacizumab has been found to reduce the incidence of radionecrosis in high-grade glioma patients treated with re-irradiation11. These findings suggest that re-irradiation with bevacizumab may be a viable treatment option for patients with recurrent GBM after bevacizumab failure, addressing an unmet need in the field.
Despite the scarcity of treatment options for recurrent GBM following bevacizumab failure, the optimal approach relies on various factors12,13,14,15. Prior research has shown no survival benefit in continuing bevacizumab therapy for recurrent GBM beyond disease progression16,17. However, comprehensive studies comparing the introduction of re-irradiation during bevacizumab failure to the best supportive care are lacking. Consequently, this study seeks to evaluate the effectiveness of re-irradiation combined with bevacizumab in patients with second progression GBM resistant to bevacizumab monotherapy, and to explore factors that may affect survival outcomes. By examining the impact of re-irradiation, this research aims to provide valuable insights into optimizing treatment strategies for this challenging patient group.
Materials and methods
Data source and patient selection
This retrospective study included 78 consecutive patients with recurrent GBM who underwent surgery and postoperative chemoradiotherapy at a hospital between December 2009 and December 2019. Following the first recurrence, patients received bi-weekly 100 mg/m2 bevacizumab monotherapy. Progressive disease was confirmed through MRI scans, as defined by Macdonald and RANO criteria. Patients' MRIs were followed at three-month intervals until death. Regular MRI scans confirmed the second progression. Patients who did not receive bevacizumab therapy or treatments other than bevacizumab at the first progression were excluded (as shown in Supplementary 1). Of the remaining patients, those who developed a second progression after single-agent bevacizumab therapy were divided into two groups: 35 underwent best supportive care (non-ReRT group), and 29 received bevacizumab and re-irradiation (ReRT group). The selection of re-irradiation was based on patients' performance and preferences, as well as factors such as recurrence volume and location and the risk of radionecrosis. The Institutional Review Board (IRB) of Taichung Veterans General Hospital approved this retrospective study. Informed consent was obtained from all subjects, and all experiments were conducted in compliance with the relevant guidelines and regulations established by the IRB.
Data collection
A predefined protocol was used to collect patient characteristics and tumor/treatment-related parameters from medical records. The response to bevacizumab treatment was evaluated by comparing MRI scans before and after the initial treatment to determine the Objective Response Rate (ORR). Karnofsky Performance Scale (KPS) scores were obtained at the time of the second progression, indicating bevacizumab failure. The failure pattern of bevacizumab was classified as locoregional, leptomeningeal spread (LMS), or both. LMS was diagnosed based on the appearance on MRI as linear or nodular lesions with high signal intensity on T2-weighted images and low signal intensity on T1-weighted images that were enhanced with gadolinium contrast agent18. Other treatment-related parameters, such as patterns of bevacizumab failure, characteristics of targets for re-irradiation, the extent of resection (EOR), and re-irradiation target volume, were also collected. To evaluate the coverage of tumor sites and normal brain tissues, a Dose Volume Histogram (DVH) was plotted. The normal brain was defined by total brain volume, excluding the planning target volume (PTV) or gross tumor volume (GTV). Brain V50, V60, and V80, which refer to the percentage of the normal brain receiving at least 50, 60, and 80 Gy, respectively, were calculated based on the whole brain volume. 80 Gy, respectively, were calculated based on the whole brain volume.
Endpoints and statistical analyses
The study's primary endpoint was to investigate the survival benefit of adding re-irradiation to continuing bevacizumab in patients with recurrent GBM after bevacizumab failure. The secondary endpoint was to explore factors that may help clinicians choose treatment options. The study measured overall survival time after bevacizumab failure (OST-BF) and re-irradiation (OST-RT), censoring surviving patients on dates without follow-up. Statistical tests such as Fisher's exact test, independent t-test, Chi-square test, and Mann–Whitney U test were used to compare categorical variables on patient characteristics and evaluate the difference in recurrence patterns between the two groups. ROC analysis was used to identify the optimal cutoff points for re-irradiation volume to determine which patients may have better survival. Survival curves were estimated using the Kaplan–Meier method and log-rank tests to determine their significance. Univariate and multivariate analyses were performed using Cox proportional hazards models. All statistical tests were conducted using SPSS version 19 software, with p-values less than 0.05 considered statistically significant.
Results
The study assessed differences in patient characteristics and treatment-related parameters between the two groups, as shown in Table 1. No significant differences were observed in gender, age, tumor location, multifocal GBM, the extent of resection during the first surgery, IDH1-R132H mutation (available for 25 patients), V50, or V60 between the two groups. However, the ReRT group had a significantly higher proportion of patients with neurological symptoms at bevacizumab failure (82.8% vs. 60.0%, p = 0.047) and a higher proportion of patients who underwent re-surgery after bevacizumab failure (37.9% vs. 14.3%, p = 0.030). Moreover, the ReRT group demonstrated a significantly higher ORR to bevacizumab than the non-ReRT group (complete response, 67.9% vs. 14.3%, p = 0.004). Additionally, a significantly higher proportion of LMS before or after bevacizumab therapy was observed in the ReRT group compared to the non-ReRT group (69.0% vs. 40.0%, p = 0.021). The study found no significant differences in other variables between the two groups.
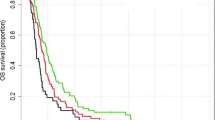
The Kaplan–Meier survival analysis revealed that the ReRT group had a significantly higher survival rate and longer median survival time than the none-ReRT group. The median overall survival time after bevacizumab failure (OST-BF) and after re-irradiation (OST-RT) was 14.5 months and 8.8 months, respectively, for the ReRT group. In comparison, the OST-BF for the none-ReRT group was 3.9 months (95% CI: 1.9–5.9, p < 0.001) (Fig. 1). The survival curve for the ReRT group remained higher than that of the none-ReRT group throughout the follow-up period, indicating a sustained survival benefit.
The analysis of overall survival time after bevacizumab failure (OST-BF) and a comparison of OST-BF and overall survival time after re-irradiation (OST-RT) between the two groups. (A) The Kaplan–Meier curves for OST-BF in none-ReRT and the ReRT group. The ReRT group exhibited significantly longer OST-BF than the none-ReRT group (median 13.5 vs. 3.9 months, p < 0.001). (B) The ReRT group exhibited significantly longer OST-RT than the OST-BT in none-ReRT group (median 8.8 vs. 3.9 months, p < 0.001).
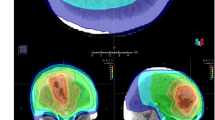
Cox univariate analysis for OST-BF in all patients identified KPS at bevacizumab failure, ORR, bevacizumab failure pattern, re-irradiation, and re-irradiation target volume as significant predictors of survival time after bevacizumab failure (Table 2). Further multivariable analysis showed that patients with a higher KPS score, good bevacizumab responder, and re-irradiation had longer survival times. Similarly, univariate analysis for OST-RT in the ReRT group revealed KPS at bevacizumab failure, bevacizumab ORR, re-surgery after bevacizumab failure, worse bevacizumab failure pattern, and re-irradiation target volume as significant predictors (Table 3). The multivariable analysis found only the re-irradiation target volume to be significant. In addition, the area under the curve (AUC) analysis showed that the re-irradiation target volume had an excellent discriminatory ability, with an optimal cutoff point of greater than 27.58 ml, a sensitivity of 74.07%, and a specificity of 100.00% (Supplementary 2). These results indicate that the re-irradiation target volume and survival time after re-irradiation may be applicable prognostic factors for these patients. Figure 2 depicts a long-time survivor with a high KPS in the ReRT group who had a small re-irradiation target and did not experience exacerbated cerebral edema after re-irradiation.
Discussion
Recurrent GBM presents a significant challenge for clinicians due to the limited effectiveness of current therapeutic options, such as lomustine and bevacizumab. In a phase III trial (EORTC 26101), the combination of bevacizumab and lomustine did not significantly improve overall survival compared to lomustine monotherapy, though progression-free survival did improve4. However, the combination therapy was associated with increased rates of adverse events and thrombocytopenia, which is a significant limitation of lomustine chemotherapy19. Other agents, including fotemustine, irinotecan, and regorafenib, have been investigated but lack high-certainty evidence of superiority over lomustine20. With its relatively minor adverse events, bevacizumab monotherapy is considered a salvage treatment to enhance the quality of life with limited survival benefits21. In some countries where lomustine is unavailable, bevacizumab monotherapy represents the only treatment option for recurrent GBM. The combination of re-irradiation and bevacizumab has emerged as a promising therapeutic approach for recurrent GBM patients who fail bevacizumab treatment, particularly in cases where lomustine is unavailable or ineffective.
Re-irradiation as a potential treatment for recurrent GBM has been limited due to concerns about potential neurologic toxicities. Radiation-induced brain necrosis is a known complication of re-irradiation and is associated with higher radiation doses and larger volumes of irradiated brain tissue. Previous studies have evaluated the risks of radiation necrosis in patients who received repeated stereotactic radiosurgery (SRS) or SRS after whole-brain irradiation22,23,24,25,26. A meta-analysis of fractionated radiation therapy found that the incidence of brain radionecrosis was 5% and 10% at biologically effective doses (BED) of 120 Gy and 150 Gy, respectively, for a fraction size less than 2.5 Gy27. In a study by McKay et al., the V40 Gy (median BED 306.67) was proposed to be predictive of radiation necrosis in patients treated with a second course of stereotactic radiosurgery (SRS) after local failure28. In our study, re-irradiation was primarily administered at a dose of 46 Gy in 20 fractions, resulting in an accumulated BED of 187.2 Gy to the planning target volume (PTV) and with a mean 9.4% brain V80 (BED 133.3 Gy). This treatment regimen appears to present an acceptable theoretical risk for radionecrosis. Furthermore, our study identified the re-irradiation target volume as an important prognostic factor for survival time after re-irradiation, with smaller target volumes associated with better survival outcomes and, theoretically, fewer neurotoxicities. This finding is consistent with previous research suggesting that the extent of disease and target volume may affect the outcome of re-irradiation treatment in patients with recurrent GBM29,30.
As recurrent GBM poses a significant challenge for patients, alternative treatment options include tumor treating fields therapy (TTFields)31, re-surgery32, or investigational agents33,34,35. Continued bevacizumab therapy beyond the second progression of GBM has also been studied as an alternative treatment option36. In this study, the addition of re-irradiation to bevacizumab treatment in patients who had failed bevacizumab monotherapy demonstrated a survival benefit. This combination therapy could provide an additional treatment option for patients, especially in settings where lomustine is unavailable or ineffective. The synergistic effects of re-irradiation and bevacizumab may improve local tumor control and maintain anti-angiogenic properties, ultimately leading to better outcomes. Future studies should focus on optimizing the dose and fractionation schemes for re-irradiation and identifying the ideal patient population that may benefit most from this combination therapy. Additionally, the integration of novel therapies, such as immune checkpoint inhibitors or targeted molecular therapies, could be explored in combination with re-irradiation and bevacizumab to potentially enhance the efficacy of treatment for recurrent GBM.
However, it is important to acknowledge the limitations of this study. One limitation is the small sample size, which may restrict the generalizability of the findings. Furthermore, our study did not include various important molecular factors such as IDH status, autophagy-related genes, hsa-miR-196a-5p, and transcription factors like CASZ1 as predictors for glioma prognosis37,38,39,40. Incorporating these factors into predictive models, such as a nomogram, would provide a more comprehensive and accurate tool for personalized treatment decisions and improved prognosis assessment in recurrent GBM patients. Future research should aim to address these limitations and incorporate a broader range of molecular factors to enhance the predictive models' clinical utility.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Ostrom, Q. T. et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro. Oncol. 24, v1–v95 (2022).
Friedman, H. S. et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 27, 4733–4740 (2009).
Nagane, M. et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn. J. Clin. Oncol. 42, 887–895 (2012).
Wick, W. et al. Lomustine and bevacizumab in progressive glioblastoma. N. Engl. J. Med. 377, 1954–1963 (2017).
Smolenschi, C. et al. Bevacizumab in real-life patients with recurrent glioblastoma: Benefit or futility?. J. Neurol. https://doi.org/10.1007/s00415-023-11600-w (2023).
Wenger, K. J. et al. Bevacizumab as a last-line treatment for glioblastoma following failure of radiotherapy, temozolomide and lomustine. Oncol. Lett. 14, 1141–1146 (2017).
Kazmi, F., Soon, Y. Y., Leong, Y. H., Koh, W. Y. & Vellayappan, B. Re-irradiation for recurrent glioblastoma (GBM): A systematic review and meta-analysis. J. Neurooncol. 142, 79–90 (2019).
Combs, S. E. et al. Re-irradiation of recurrent gliomas: Pooled analysis and validation of an established prognostic score-report of the Radiation Oncology Group (ROG) of the German Cancer Consortium (DKTK). Cancer Med. 7, 1742–1749 (2018).
Møller, S. et al. Toxicity and efficacy of re-irradiation of high-grade glioma in a phase I dose- and volume escalation trial. Radiother. Oncol. 125, 223–227 (2017).
Tsien, C. I. et al. NRG oncology/RTOG1205: A randomized phase II trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J. Clin. Oncol. 41, 1285–1295 (2023).
Fleischmann, D. F. et al. Bevacizumab reduces toxicity of reirradiation in recurrent high-grade glioma. Radiother. Oncol. 138, 99–105 (2019).
Furuse, M. et al. Boron neutron capture therapy and add-on bevacizumab in patients with recurrent malignant glioma. Jpn. J. Clin. Oncol. 52, 433–440 (2022).
Carrieri, F. A., Smack, C., Siddiqui, I., Kleinberg, L. R. & Tran, P. T. Tumor treating fields: At the crossroads between physics and biology for cancer treatment. Front. Oncol. 10, 575992 (2020).
Lombardi, G. et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 20, 110–119 (2019).
Sahebjam, S. et al. Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade gliomas: Results from a phase I study. Neuro. Oncol. 23, 677–686 (2021).
Brandes, A. A. et al. A randomized phase II trial (TAMIGA) evaluating the efficacy and safety of continuous bevacizumab through multiple lines of treatment for recurrent glioblastoma. Oncologist 24, 521–528 (2019).
Hovey, E. J. et al. Continuing or ceasing bevacizumab beyond progression in recurrent glioblastoma: An exploratory randomized phase II trial. Neurooncol. Pract. 4, 171–181 (2017).
Birzu, C. et al. Leptomeningeal spread in glioblastoma: Diagnostic and therapeutic challenges. Oncologist 25, e1763–e1776 (2020).
Le Rhun, E. et al. Thrombocytopenia limits the feasibility of salvage lomustine chemotherapy in recurrent glioblastoma: A secondary analysis of EORTC 26101. Eur. J. Cancer 178, 13–22 (2023).
McBain, C., Lawrie, T. A. & Rogozińska, E. Treatment options for progression or recurrence of glioblastoma: A network meta‐analysis. Cochrane Database Syst. Rev. (2021)
Taal, W. et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 15, 943–953 (2014).
Holt, D. E. et al. Tumor bed radiosurgery following resection and prior stereotactic radiosurgery for locally persistent brain metastasis. Front. Oncol. 5, 84 (2015).
Minniti, G. et al. Repeated stereotactic radiosurgery for patients with progressive brain metastases. J. Neurooncol. 126, 91–97 (2016).
Kohutek, Z. A. et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J. Neurooncol. 125, 149–156 (2015).
Minniti, G. et al. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 6, 48 (2011).
Moreau, J., Khalil, T., Dupic, G., Chautard, E. & Lemaire, J. J. Second course of stereotactic radiosurgery for locally recurrent brain metastases: Safety and efficacy. PLoS ONE 13, e0195608 (2018).
Lawrence, Y. R. et al. Radiation dose-volume effects in the brain. Int. J. Radiat. Oncol. Biol. Phys. 76, S20–S27. https://doi.org/10.1016/j.ijrobp.2009.02.091 (2010).
McKay, W. H. et al. Repeat stereotactic radiosurgery as salvage therapy for locally recurrent brain metastases previously treated with radiosurgery. J. Neurosurg. 127, 148–156 (2017).
Niyazi, M. et al. Independent validation of a new reirradiation risk score (RRRS) for glioma patients predicting post-recurrence survival: A multicenter DKTK/ROG analysis. Radiother. Oncol. 127, 121–127 (2018).
Clarke, J. et al. Multicenter, phase 1, dose escalation study of hypofractionated stereotactic radiation therapy with bevacizumab for recurrent glioblastoma and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 99, 797–804 (2017).
Stupp, R. et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 318, 2306–2316 (2017).
Brown, T. J. et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2, 1460–1469 (2016).
Brandes, A. A. et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: A phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemother. Pharmacol. 64, 769–775 (2009).
Brandes, A. A. et al. AVAREG: A phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro. Oncol. 18, 1304–1312 (2016).
Lombardi, G. et al. Regorafenib in recurrent glioblastoma patients: A large and monocentric real-life study. Cancers 13, 4731 (2021).
Reardon, D. A. et al. Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br. J. Cancer 107, 1481–1487 (2012).
Mandel, J. J. et al. Impact of IDH1 mutation status on outcome in clinical trials for recurrent glioblastoma. J. Neurooncol. 129, 147–154 (2016).
Qu, S., Liu, S., Qiu, W., Liu, J. & Wang, H. Screening of autophagy genes as prognostic indicators for glioma patients. Am. J. Transl. Res. 12, 5320–5331 (2020).
Mao, C., Huang, C., Hu, Z. & Qu, S. Transcription factor CASZ1 increases an oncogenic transcriptional process in tumorigenesis and progression of glioma cells. MedComm 3, e182 (2022).
Qu, S., Qiu, O., Huang, J., Liu, J. & Wang, H. Upregulation of hsa-miR-196a-5p is associated with MIR196A2 methylation and affects the malignant biological behaviors of glioma. Genomics 113, 1001–1010 (2021).
Funding
The study was supported in part by grants from the study program 105DHA0500184 of Taichung Veterans General Hospital.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: W.-C.Y. and H.-C.P. Data collection: H.-D.L. Data analysis and interpretation: H.-C.C. and W.-C.Y. Drafting the article: W.-C.Y. Critical revision of the article: W.-C.Y. and H.-C.P. Final approval of the version to be published: W.-C.Y. W.-C.Y. and H.-C.P. contributed equally to this work as the corresponding author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
You, WC., Lee, HD., Pan, HC. et al. Re-irradiation combined with bevacizumab for recurrent glioblastoma beyond bevacizumab failure: survival outcomes and prognostic factors. Sci Rep 13, 9442 (2023). https://doi.org/10.1038/s41598-023-36290-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36290-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.