Abstract
We investigated the prognostic impact of central blood pressure (BP) on outcomes in patients with embolic stroke of undetermined source (ESUS). The prognostic value of central BP according to ESUS subtype was also evaluated. We recruited patients with ESUS and data on their central BP parameters (central systolic BP [SBP], central diastolic BP [DBP], central pulse pressure [PP], augmentation pressure [AP], and augmentation index [AIx]) during admission. ESUS subtype classification was arteriogenic embolism, minor cardioembolism, two or more causes, and no cause. Major adverse cardiovascular event (MACE) was defined as recurrent stroke, acute coronary syndrome, hospitalization for heart failure, or death. Over a median of 45.8 months, 746 patients with ESUS were enrolled and followed up. Patients had a mean age of 62.8 years, and 62.2% were male. Multivariable Cox regression analysis showed that central SBP and PP were associated with MACE. All-cause mortality was independently associated with AIx. In patients with no cause ESUS, central SBP and PP, AP, and AIx were independently associated with MACE. AP and AIx were independently associated with all-cause mortality (all p < 0.05). We demonstrated that central BP can predict poor long-term prognosis in patients with ESUS, especially those with the no cause ESUS subtype.
Similar content being viewed by others
Introduction
Embolic stroke of undetermined source (ESUS) is a non-lacunar ischemic stroke without apparent arterial stenosis or major cardioembolic sources1. Of all patients with ischemic stroke, 9–25% are classified as ESUS2. Recurrent stroke and mortality rates of patients with ESUS are high at 3.9% and 4.5% per year, respectively2,3. Previous large randomized clinical trials found that treatment with direct oral anticoagulants of patients with ESUS was ineffective4,5. One reason might be the heterogeneity of ESUS6. Thus, a method of determining the prognosis according to ESUS subtype is needed. In addition, novel predictive markers for ESUS prognosis are warranted.
Prognosis of ESUS may be related to vascular risk factors such as blood pressure (BP). Central BP is the pressure in the aorta or the carotid artery. Central BP is more accurate and useful than peripheral BP because central BP may better represent the imposed load on the cerebral and coronary arteries. Furthermore, it shows a stronger relationship with vascular damage and the occurrence of cardiovascular diseases and mortality7,8. The Strong Heart Study supports this premise by demonstrating that central BP is a better predictor of future cardiovascular events than brachial pressure9. Central BP is also considered a clinical marker of silent cerebrovascular disease in an apparent general population10,11. Further studies have also shown that central BP is linked to poor short-term outcomes in patients with ischemic stroke12,13. However, the prognostic value of central BP for long-term outcomes in stroke, especially in patients with ESUS, has not been established.
In this regard, we hypothesized that hemodynamic parameters of central BP may predict poor long-term prognosis in patients with ESUS. We also investigated whether the association between central BP and long-term prognosis differed across ESUS subtypes.
Methods
Study population
From January 2012 to December 2018, patients with ESUS who were consecutively enrolled in the prospective registry were included. The definition of ESUS was based on the criteria proposed by the Cryptogenic Stroke/ESUS International Working Group1. Namely, patients with ESUS were defined as those who had non-lacunar infarctions, no cerebral artery stenosis, no major-risk cardioembolic sources (mechanical cardiac valve, mitral stenosis with atrial fibrillation, atrial fibrillation, left artrial/artrial appendage thrombus, sick sinus syndrome, recent myocardial infarction [< 4 weeks], left ventricular segment, atrial myxoma, infective endocarditis, atrial flutter, lone atrial fibrillation, bioprosthetic cardiac valve, mitral stenosis without atrial fibrillation, and nonbacterial thrombotic endocarditis), and no other uncommon causes (reversible cerebral artery vasoconstriction syndrome, vasculopathy, and cancer). During hospitalization, the patients underwent computed tomography, brain magnetic resonance imaging, and/or cerebral angiography. For standard evaluation, chest radiography, 12-lead electrocardiography, and blood tests, including lipid profiling, were performed. Central BP measurement and transesophageal echocardiography (TEE) were also performed as standard evaluations, except as otherwise restricted by applicable exclusion criteria14.
We classified patients with ESUS into (1) arteriogenic embolism, (2) minor cardioembolism, (3) two or more causes (arteriogenic embolism + minor cardioembolism), or (4) no cause. Arteriogenic embolism included complex aortic plaque (≥ 4 mm) and non-stenotic (< 50%) relevant artery plaque. Minor cardioembolism included mitral valve prolapse, mitral annulus calcification, left atrial turbulence (smoke), atrial septal aneurysm, patent foramen ovale, congestive heart failure, hypokinetic left ventricular segment, and myocardial infarction (> 4 weeks but < 6 months)15.
We collected information on baseline characteristics (age, sex, and National Institutes of Health Stroke Scale [NIHSS] score at admission), infarct location, risk factors, lipid profile, and secondary prevention. The presence of patent foramen ovale may affect stroke prognosis16 and was therefore included in the demographic characteristics. To obtain parameters for central BP, we performed pulse wave analysis of the radial artery using a commercially available applanation tonometer (SphygmoCor, Pulse Wave Analysis System, AtCor Medical, Sydney, Australia)17. Central BP parameters included central systolic BP (SBP), central diastolic BP (DBP), central pulse pressure (PP), augmentation pressure (AP), and augmentation index (AIx). AIx normalized to a heart rate of 75 beats per minute was used for analysis.
Follow-up and outcomes
Patient follow-up was conducted at 3 months after discharge and annually via an outpatient clinic or structured telephone interview. The occurrence of a major adverse cardiovascular event (MACE) was the primary outcome and was defined as any occurrence of recurrent stroke, acute coronary syndrome, hospitalization for heart failure, or death. The secondary outcomes were recurrent stroke and all-cause mortality. Recurrent stroke was determined as a new neurologic deficit with relevant lesions on brain imaging 7 days after an index stroke or discharge. In addition, the date and cause of death were collected from the Korean National Statistical Office, which were determined based on the identification numbers of the death certificates. The censoring date was December 31, 2019. If the last visit occurred before that date, the last visit date was replaced with the censoring date14.
Statistical analysis
Statistical analysis was performed using SPSS (version 26, IBM, Chicago, IL, USA) and R version 4.3.0 (http://www.r-project.org). Significant intergroup differences were analyzed by one-way ANOVA or the Kruskal–Wallis test for continuous variables and chi-square test or Fisher’s exact test for categorical variables. Data were presented as mean ± standard deviation or median (interquartile range) for continuous variables and number (%) for categorical variables. The cutoff values for central SBP, DBP, and PP were set at ≥ 130 mmHg, ≥ 90 mmHg, and > 50 mmHg, respectively, based on previous studies18,19. Because no cutoff values for AP and AIx have been previously reported, the cutoff values for AP and AIx were set at > 13 mmHg and > 25%, respectively, based on the median value. Survival curves were plotted using the Kaplan–Meier method and compared using the log-rank test. Multivariable Cox proportional hazard regression analysis was performed after adjusting for baseline characteristics (age, sex, and NIHSS score), ESUS subtype, and variables with p < 0.05 in univariable analysis. Each multivariable Cox regression analysis was also conducted according to the ESUS subtype. Restricted cubic spline curves were plotted to visualize the impact of central BP on prognosis. Two-tailed p values < 0.05 were considered statistically significant.
Ethical approval
The Institutional Review Board of Severance Hospital of the Yonsei University Health System approved this study and waived the need for informed consent because of the retrospective design and observational nature of the study (Approval Number: 4-2021-1724). The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Results
Demographic characteristics
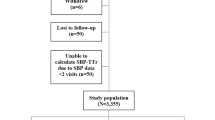
A total of 4,298 patients were registered during study period, we excluded those with stroke subtypes other than ESUS, including large artery atherosclerosis (n = 666), cardioembolism (n = 981), small vessel occlusion (n = 366), stroke of other etiology (n = 156), stroke with two or more causes (n = 579), and incomplete stroke evaluation (n = 61). Additionally, patients without TEE (n = 504) and central BP (n = 239) data were excluded. After exclusion, a final total of 746 patients with ESUS were included in this study, of which 155 (20.8%) had arteriogenic embolism, 200 (26.8%) had minor cardioembolism, 180 (24.1%) had two or more causes, and 211 (28.3%) had no cause (Fig. 1).
The patients had a mean age of 62.8 ± 13.1 years and a median NIHSS score of 2.0 (interquartile range, 1.0–4.0), and 464 (62.2%) were male. In terms of ESUS subtype, patients with arteriogenic embolism were the oldest, had the highest hypertension and diabetes rates, and had the highest central PP. Patients with two or more causes had the highest coronary artery disease rates. Conversely, patients with minor cardioembolism were the youngest and had the lowest central PP and AP. Patients with no cause had the lowest rates of hypertension, diabetes, and coronary artery disease but had the highest AP (all p values < 0.05) (Table 1).
Relationship between central BP and outcome
The patients were followed up for a median of 43.4 months (interquartile range, 27.0–64.5 months). A total of 100 patients suffered MACE (13.4%), including 60 (8.0%) recurrent strokes, 10 (1.3%) acute coronary syndrome cases, 1 (0.1%) hospitalization for heart failure, and 29 (3.9%) deaths during the study period (Table 1). The Kaplan–Meier survival curves showed that central SBP ≥ 130 mmHg and PP > 50 mmHg were significantly associated with an increased risk of MACE and recurrent stroke (log-rank test; all p values < 0.05). All-cause mortality was significantly associated with central PP > 50 mmHg and AIx > 25% (log-rank test; all p values < 0.05) (Fig. 2).
Kaplan–Meier survival plots for embolic stroke of undetermined source according to central blood pressure parameters. MACE (a) and stroke recurrence (b) according to central SBP. MACE (c), stroke recurrence (d), and all-cause mortality (e) according to central PP. All-cause mortality (f) according to AIx. AIx augmentation index, MACE major adverse cardiovascular event, PP pulse pressure, SBP systolic blood pressure.
Univariable Cox proportional hazards regression analysis showed that MACE was associated with old age, diabetes, no patent foramen ovale, central SBP, central SBP ≥ 130 mmHg, central PP, central PP > 50 mmHg, and AP. Recurrent stroke was significantly associated with old age, diabetes, no patent foramen ovale, central SBP, central SBP ≥ 130 mmHg, central PP, and central PP > 50 mmHg. All-cause mortality was significantly associated with old age, NIHSS score, diabetes, no patent foramen ovale, central PP, central PP > 50 mmHg, AP, AIx, and AIx > 25% (all p values < 0.05) (Supplementary Table 1).
Multivariable Cox proportional hazards regression analysis was performed to adjust for covariates (age, sex, NIHSS score, patent foramen ovale, hypertension, diabetes, coronary artery disease, anticoagulant, and ESUS subtype). Central SBP, PP, and PP > 50 mmHg were independently associated with an increased risk of MACE (central SBP: hazard ratio [HR] 1.014, 95% confidence interval [CI] 1.004‒1.024; central PP: HR 1.024, 95% CI 1.008‒1.040; central PP > 50 mmHg: HR 1.606, 95% CI 1.034‒2.496). In addition, central SBP, SBP ≥ 130 mmHg, DBP, and PP were independently associated with an increased risk of recurrent stroke (central SBP: HR 1.021, 95% CI 1.008‒1.034; central SBP ≥ 130 mmHg: HR 1.846, 95% CI 1.069‒3.188; central DBP: HR 1.022, 95% CI 1.002‒1.043; central PP: HR 1.029, 95% CI 1.009‒1.050). Meanwhile, all-cause mortality was associated with continuous and binary variables of AIx (AIx: HR 1.080, 95% CI 1.028‒1.134; AIx > 25%: HR 3.494, 95% CI 1.568‒7.782) (Table 2).
The impact of central BP parameters on the risk of the primary outcome is visualized in Fig. 3. Restricted cubic spline analysis for the HR of central BP showed that the adjusted risk for MACE increased with higher central BP parameters above the cutoff value.
Restricted cubic spline curves of the risk for the primary outcome. HRs (line) and 95% CIs (shading) of MACE associated with central SBP (a), central DBP (b), central PP (c), AP (d), and AIx (e) were calculated after adjusting for age, sex, NIHSS score at admission, patent foramen ovale, hypertension, diabetes mellitus, coronary artery disease, anticoagulant, and ESUS subtype. AIx augmentation index, AP augmentation pressure, CI confidence interval, DBP diastolic blood pressure, ESUS embolic stroke of undetermined source, HR hazard ratio, MACE major adverse cardiovascular event, NIHSS National Institutes of Health Stroke Scale, PP pulse pressure, SBP systolic blood pressure.
Prognostic impact of central BP by ESUS subtype
We further conducted multivariable Cox regression analysis based on the subtype of ESUS. Among the subtypes, no cause ESUS showed a strong association between central BP parameters and long-term outcomes. The cutoff values for central SBP, central PP, AP, and AIx showed an independent association with MACE (central SBP ≥ 130 mmHg: HR 2.690, 95% CI 1.100‒6.578; central PP > 50 mmHg: HR 6.943, 95% CI 2.560‒18.834; AP > 13 mmHg: HR 4.929, 95% CI 1.869‒13.001; AIx > 25%: HR 3.952, 95% CI 1.638‒9.534). The cutoff values for central SBP, central PP, and AP showed an independent association with recurrent stroke (central SBP ≥ 130 mmHg: HR 5.126, 95% CI 1.309‒20.077; central PP > 50 mmHg: HR 9.669, 95% CI 2.262‒41.319; AP > 13 mmHg: HR 7.797, 95% CI 1.826‒33.296). Furthermore, the cutoff values of AP and AIx showed an independent association with all-cause mortality (AP > 13 mmHg: HR 6.539, 95% CI 1.151‒37.144; AIx > 25%: HR 9.744, 95% CI 1.561‒60.813) (Table 3).
Discussion
We demonstrated that central BP parameters independently predicted poor long-term outcomes in patients with ESUS. Central SBP and PP were particularly associated with MACE and recurrent stroke, whereas AIx was linked to all-cause mortality. Interestingly, among the ESUS subtypes, patients with no cause ESUS showed a strong association between central BP and outcomes. Therefore, our study suggests that central BP is a useful prognostic marker for patients with ESUS, especially those with the no cause subtype.
Central BP is easily and noninvasively derived from radial artery waveform. Applanation tonometry is commonly used to detect radial artery waveform because of its simplicity and good tolerability. This tonometry utilizes a transfer function to calculate the aortic pressure waveform from the radial artery. The aortic pressure waveform can yield central BP parameters that reflect both arterial stiffness and wave reflection20.
Blood vessels lose flexibility and become stiffer with age, which impedes heart function. This is known as arterial stiffness. Arterial stiffness results from aging and arteriosclerosis. Inflammation also plays a major role in arteriosclerosis development and is consequently a major contributor to large artery stiffening21. Arterial stiffness is a well-established intermediate endpoint for cardiovascular outcome because it increases the risk of cardiovascular events, such as myocardial infarction, hypertension, heart failure, and stroke22. Our previous study also showed that increased stiffness predicted poor long-term prognosis in patients with cryptogenic stroke23. In stiff arteries, the reflected wave arrives back at the central arteries earlier and amplifies the forward wave, thereby augmenting the systolic pressure. A premature return of reflected waves in late systole increases the left ventricular load and myocardial oxygen demand, resulting in left ventricular hypertrophy24. Thus, both wave reflection and aortic stiffness are closely related to unfavorable outcomes25,26,27.
Previous studies have proven that central SBP and PP are significantly associated with an increased risk of subclinical cerebrovascular disease and future stroke10,28. An increased AIx was also associated with in-hospital outcomes, such as longer length of stay and lower Barthel index score in patients with ischemic stroke29. Similarly, prospective studies revealed that an increased AIx was an independent predictor of poor functional outcome after acute ischemic stroke12,13. In line with these reports, we found that central BP parameters were independently associated with poor long-term outcomes in patients with ESUS.
Poor long-term outcome may result from worsened aortic stiffness and wave reflection, which can be measured by central BP. We hypothesize possible mechanisms like below. First, increased central BP influences arterial remodeling in both the extracranial and intracranial arteries, which may result in increased carotid wall thickness30, the development of stenosis and plaques31, and the likelihood of plaque rupture32. Second, blood flow impedance in the cerebral artery is lower than that in other systemic arteries. Aortic stiffness can facilitate the transmission of pulsatile pressure into the cerebral microcirculation33. Furthermore, torrential flow and high-pressure fluctuations in the carotid and vertebral arteries can induce cerebrovascular damage11. Third, aortic stiffness and wave reflection are associated with poor left ventricular conditions, which may increase the risk of poor outcomes34. Fourth, aortic stiffness is linked to atherosclerosis because both share several risk factors, including hypertension35. The current study showed that hypertension was common in 72.5% of patients. Thus, atherosclerosis may contribute to a poor prognosis in ESUS in patients with high central BP.
A previous meta-analysis showed that central SBP and PP and AIx were significantly predictive of MACE, whereas all-cause mortality was associated only with AIx36. Similarly, we found that central SBP and PP were associated with MACE and stroke recurrence, whereas AIx was associated with all-cause mortality. The underlying cause for this is unclear, but it may arise from the fact that AIx values were negative in 18 (2.4%) patients in our cohort. Wave intensity analysis indicates that negative AIx is principally due to a forward travelling (re-reflected) decompression wave in mid-systole and should not be used as an estimate of wave reflection magnitude37. This loophole may attenuate the prognostic effect of AIx for MACE, including stroke recurrence. Additionally, ESUS patients with high central SBP or PP had higher levels of vascular risk factors including total cholesterol, triglyceride, and body mass index than those with high AIx (Supplementary Table 2). These factors may cause differences in the prognostic impact among central BP parameters. However, given the conflicting and limited data regarding the association between central BP and outcomes in ESUS patients, more studies are needed to define the predictive value of central BP in the population with ESUS.
Central BP parameters showed strong prognostic values for poor outcomes in no cause ESUS. This is an unexpected finding because no cause ESUS usually involves a hidden embolic source, such as atrial fibrillation. Conversely, central BP is a parameter of arterial stiffness and wave reflection. Our results suggest several potential reasons for this. First, patients with no cause ESUS had the highest AP among the subtypes. Previous studies have reported that increased arterial stiffness and wave reflection are related to newly developed atrial fibrillation38. Given the high AP, hidden atrial fibrillation may be involved in poor prognosis in no cause ESUS. In addition, AP more sensitively reflects ventricular ejection functions than other central BP parameters39, and thus high AP may precipitate the risk of recurrent stroke due to left ventricular dysfunction40. Second, patients with other ESUS subtypes were older and had higher rates of hypertension, diabetes, and coronary artery disease than those with no cause ESUS. These risk factors are well-known prognostic markers after stroke. Although we adjusted for these factors in the multivariable analysis, risk factors may increase the likelihood of poor outcomes in other ESUS subtypes than no cause ESUS and mitigate the effect size of central BP parameters on clinical outcomes41.
Our study has noteworthy strengths. We enrolled patients with comprehensive work-ups, including TEE and prolonged electrocardiography monitoring. All patients underwent TEE and transthoracic echocardiography was performed in 93.2% of patients. Prolonged heart rhythm evaluation was also performed in 93.6% of patients (Supplementary Table 3). ESUS working group investigators recommended a comprehensive stroke work-up, but they did not consider TEE and long-term electrocardiography monitoring as mandatory investigations42. TEE examination can uncover abnormal findings in more than half of cases, and such abnormalities significantly impact on the prognosis of ESUS patients43. Another strength is that we measured central BP using a device readily available in clinical practice. Measuring central BP is simple, non-invasive, and chief. Based on our finding on the prognostic value of central BP in ESUS, the central BP measurement may be recommended for identifying high-risk patients without an obvious etiology.
This study has several limitations. First, although we included consecutive patients with ESUS, the mandatory inclusion criteria may have excluded some patients with ESUS who did not undergo central BP measurement and TEE. Second, central BP may be affected by the type and dosage of antihypertensive medication26. We did not analyze these factors because too many types of antihypertensive drugs were used. Third, we only assessed patients of a single ethnicity and from a single center. Further prospective validation studies involving other ethnicities and larger cohorts are required. Despite these limitations, our study firstly reported the association between central BP and long-term prognosis of ESUS patients.
Conclusions
We found that central BP parameters in patients with ESUS were independently associated with unfavorable outcomes, including MACE, stroke recurrence, and mortality. The prognostic impact of these parameters was most evident in patients with no cause ESUS. Therefore, we propose that central BP measurement may be used as an important diagnostic and prognostic factor for patients with ESUS, especially those with no apparent embolic cause.
Data availability
The study data are available from the corresponding author upon reasonable request and with the permission of all contributing authors.
Abbreviations
- AIx:
-
Augmentation index
- AP:
-
Augmentation pressure
- DBP:
-
Diastolic blood pressure
- ESUS:
-
Embolic stroke of undetermined source
- PP:
-
Pulse pressure
- SBP:
-
Systolic blood pressure
- TEE:
-
Transesophageal echocardiography
References
Hart, R. G. et al. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 13, 429–438. https://doi.org/10.1016/S1474-4422(13)70310-7 (2014).
Hart, R. G., Catanese, L., Perera, K. S., Ntaios, G. & Connolly, S. J. Embolic stroke of undetermined source: A systematic review and clinical update. Stroke 48, 867–872. https://doi.org/10.1161/STROKEAHA.116.016414 (2017).
Ntaios, G. et al. Risk stratification for recurrence and mortality in embolic stroke of undetermined source. Stroke 47, 2278–2285. https://doi.org/10.1161/STROKEAHA.116.013713 (2016).
Diener, H. C. et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N. Engl. J. Med. 380, 1906–1917. https://doi.org/10.1056/NEJMoa1813959 (2019).
Hart, R. G. et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N. Engl. J. Med. 378, 2191–2201. https://doi.org/10.1056/NEJMoa1802686 (2018).
Kamel, H., Merkler, A. E., Iadecola, C., Gupta, A. & Navi, B. B. Tailoring the approach to embolic stroke of undetermined source: A review. JAMA Neuro. 76, 855–861. https://doi.org/10.1001/jamaneurol.2019.0591 (2019).
Pini, R. et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: The Icare Dicomano study. J. Am. Coll. Cardiol. 51, 2432–2439. https://doi.org/10.1016/j.jacc.2008.03.031 (2008).
Wang, K. L. et al. Central or peripheral systolic or pulse pressure: Which best relates to target organs and future mortality?. J. Hypertens. 27, 461–467. https://doi.org/10.1097/hjh.0b013e3283220ea4 (2009).
Roman, M. J. et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The strong heart study. Hypertension 50, 197–203. https://doi.org/10.1161/HYPERTENSIONAHA.107.089078 (2007).
Matsumoto, K. et al. Association between central blood pressure and subclinical cerebrovascular disease in older adults. Hypertension 75, 580–587. https://doi.org/10.1161/HYPERTENSIONAHA.119.13478 (2020).
Ochi, N. et al. Association of central systolic blood pressure with intracerebral small vessel disease in Japanese. Am. J. Hypertens. 23, 889–894. https://doi.org/10.1038/ajh.2010.60 (2010).
Gasecki, D. et al. Aortic stiffness predicts functional outcome in patients after ischemic stroke. Stroke 43, 543–544. https://doi.org/10.1161/STROKEAHA.111.633487 (2012).
Kowalczyk, K. et al. Changes of augmentation index early after ischaemic stroke predict functional outcome. Blood Press. 29, 327–335. https://doi.org/10.1080/08037051.2020.1769468 (2020).
Nam, H. S. et al. Long-term mortality in patients with stroke of undetermined etiology. Stroke 43, 2948–2956. https://doi.org/10.1161/STROKEAHA.112.661074 (2012).
Adams, H. P. et al. Classification of subtype of acute ischemic stroke: Definitions for use in a multicenter clinical trial TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24, 35–41. https://doi.org/10.1161/01.str.24.1.35 (1993).
Baik, M. et al. Patent foramen ovale and risk of recurrence in stroke of determined etiology. Ann. Neurol. 92, 596–606. https://doi.org/10.1002/ana.26449 (2022).
Russo, C. et al. Arterial stiffness and wave reflection: Sex differences and relationship with left ventricular diastolic function. Hypertension 60, 362–368. https://doi.org/10.1161/HYPERTENSIONAHA.112.191148 (2012).
Cheng, H. M. et al. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks. J. Am. Coll. Cardiol. 62, 1780–1787. https://doi.org/10.1016/j.jacc.2013.06.029 (2013).
Chung, J. W. et al. Reference values for the augmentation index and pulse pressure in apparently healthy Korean subjects. Korean Circ. J. 40, 165–171. https://doi.org/10.4070/kcj.2010.40.4.165 (2010).
Chen, C. H. et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 95, 1827–1836. https://doi.org/10.1161/01.cir.95.7.1827 (1997).
Mozos, I. et al. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front. Immunol. 8, 1058. https://doi.org/10.3389/fimmu.2017.01058 (2017).
Mitchell, G. F. et al. Arterial stiffness and cardiovascular events: The Framingham heart study. Circulation 121, 505–511. https://doi.org/10.1161/CIRCULATIONAHA.109.886655 (2010).
Han, M. et al. Brachial-ankle pulse wave velocity for predicting functional outcomes in patients with cryptogenic stroke. J. Clin. Neurosci. 69, 214–219. https://doi.org/10.1016/j.jocn.2019.07.050 (2019).
Laurent, S. et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 27, 2588–2605. https://doi.org/10.1093/eurheartj/ehl254 (2006).
Safar, M. E. et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension 39, 735–738. https://doi.org/10.1161/hy0202.098325 (2002).
Williams, B. et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the conduit artery function evaluation (CAFE) study. Circulation 113, 1213–1225. https://doi.org/10.1161/CIRCULATIONAHA.105.595496 (2006).
Weber, T. et al. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur. Heart J. 26, 2657–2663. https://doi.org/10.1093/eurheartj/ehi504 (2005).
Chuang, S. Y. et al. Blood pressure, carotid flow pulsatility, and the risk of stroke: A community-based study. Stroke 47, 2262–2268. https://doi.org/10.1161/STROKEAHA.116.013207 (2016).
Soiza, R. L., Davie, M. M. & Williams, D. J. Use of the augmentation index to predict short-term outcome after acute ischemic stroke. Am. J. Hypertens. 23, 737–742. https://doi.org/10.1038/ajh.2010.66 (2010).
Salonen, R. & Salonen, J. T. Determinants of carotid intima-media thickness: A population-based ultrasonography study in eastern Finnish men. J. Intern. Med. 229, 225–231. https://doi.org/10.1111/j.1365-2796.1991.tb00336.x (1991).
Zureik, M. et al. Common carotid intima-media thickness predicts occurrence of carotid atherosclerotic plaques: Longitudinal results from the aging vascular study (EVA) study. Arterioscler. Thromb. Vasc. Biol. 20, 1622–1629. https://doi.org/10.1161/01.atv.20.6.1622 (2000).
Cheng, G. C., Loree, H. M., Kamm, R. D., Fishbein, M. C. & Lee, R. T. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions: A structural analysis with histopathological correlation. Circulation 87, 1179–1187. https://doi.org/10.1161/01.cir.87.4.1179 (1993).
O’Rourke, M. F. & Safar, M. E. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 46, 200–204. https://doi.org/10.1161/01.HYP.0000168052.00426.65 (2005).
London, G. M. et al. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation 90, 2786–2796. https://doi.org/10.1161/01.cir.90.6.2786 (1994).
Acampa, M. et al. Non-stenosing carotid atherosclerosis and arterial stiffness in embolic stroke of undetermined source. Front. Neurol. 11, 725. https://doi.org/10.3389/fneur.2020.00725 (2020).
Vlachopoulos, C. et al. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur. Heart J. 31, 1865–1871. https://doi.org/10.1093/eurheartj/ehq024 (2010).
Hughes, A. D. et al. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS ONE 8(3), e59371. https://doi.org/10.1371/journal.pone.0059371 (2013).
Tsang, T. S. et al. Abstract 3163: Central pulse pressure as a robust predictor of first atrial fibrillation: Study of atrial fibrillation in high risk elderly (SAFFIHRE). Circulation 118, 1106 (2008).
Fok, H. et al. Augmentation pressure is influenced by ventricular contractility/relaxation dynamics: Novel mechanism of reduction of pulse pressure by nitrates. Hypertension 63(5), 1050–1055. https://doi.org/10.1161/HYPERTENSIONAHA.113.02955 (2014).
Park, H. K. et al. Left ventricular diastolic dysfunction in ischemic stroke: Functional and vascular outcomes. J. Stroke 18(2), 195–202. https://doi.org/10.5853/jos.2015.01697 (2016).
Li, H. Q. et al. Causal relations between exposome and stroke: A Mendelian randomization study. J. Stroke 24, 236–244. https://doi.org/10.5853/jos.2021.01340 (2022).
Tsivgoulis, G. et al. Embolic strokes of undetermined source: theoretical construct or useful clinical tool?. Ther. Adv. Cardiovasc. Dis. 12, 1756286419851381. https://doi.org/10.1177/1756286419851381 (2019).
Cho, H. J. et al. Transoesophageal echocardiography in patients with acute stroke with sinus rhythm and no cardiac disease history. J. Neurol. Neurosurg. Psychiatry 81, 412–415. https://doi.org/10.1136/jnnp.2009.190322 (2010).
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1A2C1007948).
Author information
Authors and Affiliations
Contributions
Research conception and design, M.H., H.S.N.; Data acquisition, M.H., J.H., I.H.L., J.H.K., H.L., J.W.J., I.H.L., S.H.H., Y.D.K., H.S.N.; Data analysis and interpretation, M.H., H.S.N.; Statistical analysis, M.H.; Drafting the manuscript, M.H., H.S.N.; Critical revision of the manuscript, M.H., H.S.N.; All authors approval the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, M., Heo, J., Lee, I.H. et al. Prognostic value of central blood pressure on the outcomes of embolic stroke of undetermined source. Sci Rep 13, 9550 (2023). https://doi.org/10.1038/s41598-023-36151-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36151-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.