Abstract
Gulf War illness (GWI) is an important exemplar of environmentally-triggered chronic multisymptom illness, and a potential model for accelerated aging. Inflammation is the main hypothesized mechanism for GWI, with mitochondrial impairment also proposed. No study has directly assessed mitochondrial respiratory chain function (MRCF) on muscle biopsy in veterans with GWI (VGWI). We recruited 42 participants, half VGWI, with biopsy material successfully secured in 36. Impaired MRCF indexed by complex I and II oxidative phosphorylation with glucose as a fuel source (CI&CIIOXPHOS) related significantly or borderline significantly in the predicted direction to 17 of 20 symptoms in the combined sample. Lower CI&CIIOXPHOS significantly predicted GWI severity in the combined sample and in VGWI separately, with or without adjustment for hsCRP. Higher-hsCRP (peripheral inflammation) related strongly to lower-MRCF (particularly fatty acid oxidation (FAO) indices) in VGWI, but not in controls. Despite this, whereas greater MRCF-impairment predicted greater GWI symptoms and severity, greater inflammation did not. Surprisingly, adjusted for MRCF, higher hsCRP significantly predicted lesser symptom severity in VGWI selectively. Findings comport with a hypothesis in which the increased inflammation observed in GWI is driven by FAO-defect-induced mitochondrial apoptosis. In conclusion, impaired mitochondrial function—but not peripheral inflammation—predicts greater GWI symptoms and severity.
Introduction
Environmentally-triggered chronic multisymptom illnesses (CMI) impose a mounting burden on society, and share many clinical features and biomarkers—with new environmental and pharmacological triggers continuing to be identified1,2,3. Gulf War illness (GWI) is of interest both in itself, and as an exemplar of broader CMI. It may also, perhaps, serve as a model of accelerated aging: Numerous exposures such as would normally be distributed over decades (if experienced at all) were compressed into a short time span4,5,6 with marked increase in many aging-related symptoms7,8,9,10 and health conditions (like metabolic syndrome features6,8,11,12,13,14, cardiovascular disease13,15,16, and heightened infection risk15).
Nearly 700,000 U.S. troops were deployed to the 1990–1991 Persian Gulf theater of operations, of whom approximately a third developed chronic illness attributable to deployment—i.e. GWI—with symptoms encompassing fatigue, sleep problems, muscle pain and weakness, cognitive compromise, mood disturbance, diarrhea and other gastrointestinal complaints, respiratory symptoms, and dermatological disturbance7, among others. Evidence implicates drug, chemical, and environmental exposures which were of unprecedented variety in that conflict.
Unique exposures included pyridostigmine bromide, a nerve agent pretreatment adjunct given as a pill to ~ 250,000 of those deployed20; nerve gas, to which an estimated ~ 100,000 were exposed to low levels, following the Khamisiyah munitions depot demolition17 and possibly following other episodes18; botulinum toxoid vaccine6; a high number of multiple vaccines5; and oil fire smoke, among others.
New exposures included depleted uranium, with potential heavy metal and low-level radioactivity toxicity, used in that conflict for the first time to gird tanks and missiles, leading to aerosolized and sometimes shrapnel exposure following friendly fire episodes; anthrax vaccine19, with limited production oversight at the time; and permethrin-impregnated uniforms, among others.
Excessive exposures encompassed pesticides including acetylcholinesterase inhibiting carbamate and organophosphate pesticides20,21,22—with pesticide overexposure acknowledged to have occurred in tens of thousands23; nuclear biological chemical agent protection suits (NBC suits), which were used and reused after chemical alarm episodes; heat; fine sand (the finest in the world, with particles less than 0.5 microns, small enough to penetrate into the alveoli and carry toxins and microorganisms); and burn pits, among others.
Although stress had been emphasized early on as a hypothesized cause of GWI24,25, the ground war lasted only 4 days26 and many of those deployed never saw combat. Combat stress is not an independent predictor in regressions that adjust for other exposures26. In contrast, chemical exposures such as acetylcholinesterase inhibitor exposures have been documented to play a role20. Evidence for a causal role includes dose–response information20, as well as gene-environment interaction support27.
While routine laboratory tests are typically normal in veterans with GWI (VGWI), a number of objective markers have now been shown to be abnormal in GWI. Two leading hypotheses for major mechanisms underlying GWI have emerged: inflammation and mitochondrial impairment (not necessarily mutually exclusive). Inflammation has had more adherents28,29,30,31,32 perhaps in part because of greater awareness of inflammation as a contributor to health problems. CRP, a marker of peripheral inflammation, is reported to be elevated in VGWI relative to healthy controls, a replicated finding28,29. (There is now a human study also supporting increased neuroinflammation32.) However, values are typically within normal limits. Moreover, anti-inflammatory drugs like NSAIDs (non-steroidal anti-inflammatories) have not been generally reported to relieve symptoms associated with GWI. Mitochondrial impairment, long proposed33, has more recently had the benefit of supporting data9,34,35,36,37,38,39, including objective evidence of impaired bioenergetic function34,35 and favorable response to treatment with coenzyme Q10 (a mitochondrial coenzyme) relative to placebo. Coenzyme Q10 significantly alleviated GWI symptoms and improved objectively measured physical function, in a double-blind randomized controlled trial9. Among possibilities, (1) mitochondrial impairment and inflammation could concurrently and independently relate to GWI; (2) alternatively, mitochondrial impairment could relate only through the induction of inflammation e.g., via triggering of oxidative stress-induced intrinsic pathway (aka “mitochondrial”) apoptosis40 with resulting inflammation41; or (3) mitochondrial impairment could be the principal factor driving symptoms, with inflammation (e.g., arising from mitochondrial apoptosis) as a side product with limited independent contribution.
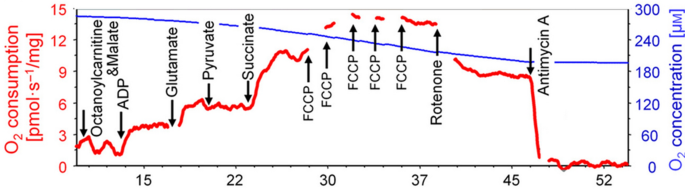
The present study sought to assess muscle mitochondrial indices from freshly procured muscle biopsy tissue in VGWI and healthy controls, and to assess how implicated mitochondrial indices compare to hsCRP in predicting GWI symptoms and summed symptom severity. Mitochondria are complex organelles that not only regulate cellular signaling but utilize a variety of fuel sources with coupled respiration to ATP production. Assessing coupled respiration with multiple fuel sources provides optimal assessments of mitochondrial resilience in tissues. We focused on various mitochondrial respiratory indices with electron transport complex I and II substrates with glucose and fatty acids as the fuel sources. Additional inhibitors and uncouplers were also utilized to assess residual mitochondrial respiration and maximal uncoupled respiration. The directed protocols for mitochondrial assessments systematically determine the efficiency of electron transport in muscle tissue to see how healthy and resilient the tissue is. Here we will focus more particularly on four mitochondrial indices: CI&CII&FAOCI/CIIOXPHOS refers to assessment of that aspect of mitochondrial function determined by using fatty acids as the fuel source with complex I and II substrates malate, glutamate, pyruvate, succinate and adenosine diphosphate (ADP) to drive respiration. CII&FAOETS refers to assessment of that aspect of mitochondrial function determined by using fatty acids as the fuel source with rotenone to inhibit complex I to look at complex II respiration. FAOMaxUC refers to assessment of that aspect of mitochondrial function determined by using fatty acids as the fuel source with FCCP as an uncoupler. CI&CIIOXPHOS refers to assessment of that aspect of mitochondrial function determined by using glucose as the fuel source with complex I and II substrates malate, glutamate, pyruvate, and succinate, with ADP to drive respiration. Particular attention attaches to CI&CIIOXPHOS. This study makes use of Oroboros, and to orient readers since Results are included before Methods, we provide a figure (Fig. 1), illustrating Oroboros methodology, from the Oroboros website.
Results
Forty-two participants (21 VGWI and 21 controls) were enrolled and showed for the biopsy visit. For 1 participant (control) who showed for the biopsy visit, no biopsy was performed because hygiene issues led to concern about potential infection risk with this procedure. Of the 41 participants in whom biopsies were performed, biopsy materials were too damaged and usable data could not be secured for 2 cases and 3 controls. This provided for 36 participants (19 cases and 17 controls) with an adequate muscle tissue sample, enabling assessment of mitochondrial respiratory chain indices. Of this “full sample” of 36 participants, 28 (14 per group) contributed to a matched-pair subset analysis. In addition to the 5 for whom the designated match had a degraded sample, for 3 participants (2 cases, 1 control), their prospective match had been recruited but not yet seen at the time of the Covid-19 associated research shutdown. Matched pair analyses are subsidiary, with the focus on cross-sectional assessments of relations of objective mitochondrial and inflammatory markers to summed symptom severity, based on the full group of biopsy participants.
Nonetheless, cases and controls in the retained full sample do not significantly differ on match characteristics (Table 1). Most participants, consistent with Gulf War deployment demographics11, were white and male. As seen in other studies42,43,44, cases were significantly more likely to be married. Perhaps because of their challenging condition, social support is needed to add research participation to an already complicated life for those with GWI. A nonsignificant trend toward higher BMI was observed in VGWI (consistent with past reports of increased weight gain in Gulf War veterans6).
Both hsCRP (a marker of peripheral inflammation) and highlighted mitochondrial indices showed trends toward disparities in VGWI relative to healthy controls, in the predicted direction—increased hsCRP and depressed mitochondrial function indices in VGWI (Table 2). Indices were selected as those with strong trends toward significance. All had p-values, averaging unpaired and paired analyses, of < 0.085 on one-sided testing, which is noted here to make more apparent the trends in the expected direction for these indices. (For case–control comparisons for all indices, see Supplement 1.) Continuous relations (of potential mechanistic variables to summed GWI symptom severity) are better powered than binarized comparisons and are the focus of this paper. The case–control comparisons are shown only to underscore that trends support prior reports of depressed bioenergetics and elevated inflammation in VGWI.
Relations of mitochondrial indices to one another and to hsCRP are shown in Table 3. Mitochondrial fatty acid oxidation impairment strongly correlated with hsCRP in VGWI, in a fashion not observed in controls. CI&CIIOXPHOS also strongly correlated to fatty acid oxidation indices in cases, with these relations absent or markedly blunted (and nonsignificant) in controls. The most exaggerated case–control difference is in the relation between CI&CIIOXPHOS and FAOMaxUC, showing a strong and highly significant correlation in cases and essentially no correlation in controls.
Of the candidate mitochondrial indices, CI&CIIOXPHOS was the most dissociated from hsCRP, so was selected for further comparison to hsCRP against symptoms. This dissociation aids in reducing the impact of collinearity in contrasting the mitochondrial and inflammatory indices. CI&CIIOXPHOS showed broad relationships to many symptoms and generally the strongest relation to cognitive (and certain other) symptoms. A couple of fatty acid oxidation indices related slightly more strongly to muscle indices, with CII&FAOETS the strongest: correlation coefficients (p-values) for low energy, muscle weakness and fatigue with exertion were r = − 0.41 (p = 0.029), r = − 0.43 (p = 0.021) and r = − 0.45 (p = 0.014) respectively. CIIMaxUC (aspect of mitochondrial function determined by using glucose as the fuel source with FCCP as an uncoupler) related to cold limbs, significantly in the total sample and with borderline significance in VGWI assessed separately: r = − 0.40 (p = 0.033) and r = − 0.47 (p = 0.065), respectively. In contrast, as Tables 4 and 5 show, cold limbs bore ~ no relation to CI&CIIOXPHOS.
Tables 4 and 5 show the correlation of CI&CIIOXPHOS, and of hsCRP, to each of the 20 symptoms of the UCSD GWI symptom survey—in the total sample and in VGWI separately. As this table makes evident, symptoms show a far stronger relationship to the mitochondrial measure than to inflammation. In the total sample, 20 symptoms out of 20 show the predicted direction of relation to CI&CIIOXPHOS (vs. 0 in the opposite direction): sign test two-sided p = 0.000002 (i.e. strongly different from expectation by chance). Similarly, hsCRP correlates with 19 of the 20 symptoms in the predicted direction, with only one in the opposite direction: sign test p = 0.00004. However, as we look in greater detail at the strength of the relationship to symptoms and also at the extent to which these findings are upheld in veterans with Gulf War illness, a very different picture emerges. For CI&CIIOXPHOS, of the 20 symptoms, 17 (predicted direction) versus 0 (opposite direction) showed at least a borderline significant relation to CI&CIIOXPHOS (sign test p < 0.0001); and 11 of these correlations showed frank significance (that is, symptoms showed two-sided p < 0.05) in the predicted direction versus 0 in the opposite direction: sign test p < 0.001. In contrast, for hsCRP, in the total sample, only one symptom was significant or borderline significant in the predicted direction (tiredness) versus 0 in the opposite direction: sign test nonsignificant (not meaningfully testable). This may relate to the fact that a major cause of tiredness is (often undiagnosed) sleep apnea, which in turn relates strongly—as both cause and consequence—to greater body mass index/fat, and adipose tissue is a major source of inflammatory mediators.
Considering VGWI cases separately, the sign of relationship of CI&CIIOXPHOS was in the predicted direction for 18 out of 20 symptoms versus two in the opposite direction, highly unlikely to be attributable to chance: sign test p < 0.001. The smaller sample size reduced power for individual symptoms to attain significance. Nonetheless, six were of at least borderline significance in the predicted direction vs. none in the opposite direction: sign test p = 0.031. In contrast, for hsCRP, more symptoms than not showed the “wrong” direction relationship within VGWI separately (seven predicted direction vs. 13 opposite direction, nonsignificant on sign test). Moreover, in affected Gulf War veterans assessed separately, only one symptom correlated to hsCRP with significance or borderline significance, and the relationship was in the direction opposite to prediction: Greater inflammation was tied to lesser problems with the (cognitive) symptom. Thus, evidence supports a far more robust relation of the mitochondrial measure to GWI-relevant symptoms, in the overall sample and in VGWI separately, than does inflammation—which appears entirely unrelated to GWI symptoms within VGWI themselves.
Tables 6 and 7 show the central analysis, evaluating the relationship of CI&CIIOXPHOS and of hsCRP to overall summed symptom severity (indexed by the summed 20-item symptom score) assessed via regression with robust standard errors, considering the mitochondrial and inflammatory variables as predictors in models separately and together, in the total sample and in VGWI (cases) separately. Lower mitochondrial CI&CIIOXPHOS significantly predicted greater summed GWI symptoms in all four assessments, in each case with significance or strong statistical significance. In contrast, hsCRP did not positively predict symptoms in any of the four analyses. It showed no apparent relationship to summed symptom severity in the total sample, with or without adjustment for the mitochondrial measure, or in VGWI separately in the analysis unadjusted for the mitochondrial measure. Unexpectedly, within VGWI, the independent relation of hsCRP (after adjusting for the mitochondrial measure) to summed (GWI) symptom severity was strongly significant, but in the opposite direction to that which has long been hypothesized. That is, greater inflammation after controlling for the mitochondrial measure was significantly tied to less GWI symptom severity. (Addition of BMI adjustment, see Table 7 legend, did not materially affect any of these findings.)
Supplement Figs. 1 and 2 show scatterplot values of GWI severity (summed symptom severity) vs CI&CIIOXPHOS in the full sample (Supplement Fig. 1) and in VGWI separately (Supplement Fig. 2).
Discussion
This is the first study to directly assess mitochondrial respiratory chain function on muscle biopsy in VGWI—and to assess it together with inflammation. Findings suggest a radical rethinking of the respective roles of mitochondrial impairment and inflammation in this condition. While both bioenergetic impairment and elevated (peripheral) inflammation discriminate VGWI in prior studies and also show apparent case–control differences in this study, it is seen here for the first time that the two correlate closely to one another in VGWI but not in controls—but also, for the first time, that despite this correlation, only mitochondrial impairment relates either to individual GWI symptoms or to overall GWI-relevant symptom severity. To restate, although elevated hsCRP correlates to mitochondrial impairment in VGWI, elevated hsCRP does not relate positively to GWI symptoms individually, or to GWI severity overall. When adjusted for the mitochondrial predictor, an independent association of hsCRP was unmasked (confined to VGWI)—but, unexpectedly, more elevated hsCRP was significantly tied to less GWI severity.
The mitochondrial finding has internal support, with a relation to symptom severity of high significance in the total sample and in VGWI separately; and both for univariable prediction and in regression adjusted for hsCRP. The hsCRP finding has high novelty, and the relation of mitochondrial indices to hsCRP selectively in VGWI, yet the opposite direction independent relation to symptoms, is intriguing and suggestive of mechanisms supported by other evidence.
The mitochondrial findings are not only internally supported but supported by external evidence. Data show that organophosphates depress signaling for fatty acid oxidation46, and depress enzymes critical for fatty acid oxidation47: Organophosphates are a key GWI-relevant exposure believed to be causal20,48 and used in many GWI animal models49,50,51,52,53,54,55,56. In fact, consistent with evidence suggesting that pesticides beyond organophosphates (including pyrethroids, organochlorines) may be implicated in GWI48, multiple pesticide classes share metabolomic signatures consistent with fatty acid oxidation involvement57. Gulf War illness animal model muscle biopsy data show a reduction in succinate dehydrogenase activity (used as an index of complex II activity)36.
Another finding is that the relation of select other mitochondrial indices to fatty acid oxidation indices is markedly strengthened in VGWI, consistent with yoked depressions in these indices in GWI. It is fatty acid oxidation indices selectively that relate potently to elevated hsCRP, consistent with prior evidence that depressed fatty acid oxidation promotes apoptosis58,59,60,61,62,63,64,65; and apoptosis promotes inflammation41. We hypothesize, therefore, that the increased inflammation observed in VGWI is a consequence of apoptosis (promoted by reduced fatty acid oxidation), which may serve an adaptive function, clearing impaired cells that are a drain on energy while not contributing favorably to function. This would account both for the specific correlation of inflammation to fatty acid oxidation indices in VGWI, and for the favorable independent association of inflammation (after accounting for mitochondrial impairment) to GWI severity.
Also relevant to the yoked depression in mitochondrial indices is the observation that depression in different mitochondrial indices relate differentially to GWI symptoms: A mitochondrial measure reliant on sugar bears the strongest relation to cognitive indices (consistent with the knowledge that the brain, at ~ 2% of the body weight, uses ~ 50% of the total body glucose66); while a fatty acid oxidation measure related most strongly to muscle-energetics relevant symptoms (low energy, muscle weakness, fatigue with exertion), consistent with the important role of fatty acid oxidation in muscle energetics and indeed the primacy of its role for resting muscle67,68. Yet a different mitochondrial measure related most strongly to cold limbs (CIIMaxUC). This measure relates to uncoupled energy production (i.e. in the presence of an uncoupler), the foundation for thermogenesis69. The fit to expectation of these relationships adds further triangulating support for study findings.
Findings need not preclude a causal role for inflammation in conditions known to be elevated in GWI (like cardiovascular disease): However, mitochondrial impairment could also explain these relations given that myocardial infarction, congestive heart failure, and stroke each arise from inadequate cell energy; and mitochondrial supportive supplements (which have also benefited GWI9, as below) have shown significant protection for each of these conditions in randomized placebo-controlled trials70,71,72,73,74.
A role for neuroinflammation separately was not assessed—so is neither supported nor precluded by these findings. However, findings comport powerfully with a primary role for mitochondrial impairment as an underlying basis for GWI. Moreover, the observation of elevated (peripheral) CRP helped drive the inflammatory hypothesis of GWI28,29,75,76. (Of note, apoptosis triggers both inflammation and also coagulation activation41; both inflammation and coagulation activation are reportedly elevated in VGWI29,75).
Our findings are consistent with others’ findings documenting increased inflammation in VGWI relative to controls, although the implications proposed are different. We do not see a relationship between inflammation and symptom severity in VGWI. We have identified only one study that reported a relationship between inflammation and symptom severity within veterans with "Gulf War illness"76, but this can be readily understood based on case-selection practices in that study relative to this one. We restricted to veterans that met both CDC and Kansas criteria for VGWI, to enhance specificity of the sample. (Kansas criteria are far more specific4). Furthermore, we excluded late-onset GWI, because there are no data on whether late-onset development of symptoms occurs more often in Gulf-deployed or whether it relates to GWI mechanisms. In contrast, the James et al. study did not exclude late-onset GWI, and accepted in their “GWI” category individuals who met either the non-specific CDC criteria or the more specific Kansas criteria.
Already within a decade after the Gulf conflict, when Era and deployed veterans were still relatively young, studies reported that 33% and 36% of Era non-Gulf deployed personnel met CDC symptom criteria12,77, and with 20 further years of aging, it may be expected that a materially higher fraction would now do so. (CDC criteria merely require that any symptom, however mild, be present for the last six months, in two of the three categories of fatigue/sleep, mood/cognitive and musculoskeletal.) Thus, early data already found that more than half of Gulf War veterans meeting these (CDC) criteria would be expected to do so irrespective of whether they had been deployed to the Gulf (with corresponding symptoms thus not attributable to Gulf exposures and associated mechanisms), and the fraction may be expected to be higher now, when veterans are older. Thus, Gulf-independent conditions and mechanisms, including inflammatory conditions leading to symptoms, would be expected to be present in more than half the veterans selected for that study’s sample. This could readily produce the appearance of a connection between inflammation and symptoms, arising from the potentially-majority fraction of participants whose symptoms were not attributable to their Gulf-deployment. (Future studies could examine the relation of inflammation and of mitochondrial impairment to symptoms in those with early onset symptoms who fully meet Kansas (and CDC) Gulf War illness criteria, compared to those who meet CDC but not Kansas criteria; and in those with late-onset GWI. Whether late-onset symptom accrual in deployed personnel differs from that in non-deployed and legitimately relates to GWI mechanisms is unknown.)
Mitochondrial impairment is a fit for all of the distinctive features of GWI, and indeed it is this that led us to first examine a role for mitochondrial impairment in this condition. Gulf War illness is characterized by high symptom multiplicity within individuals7,78 and high symptom variability across individuals7,78, consistent with mitochondrial impairment79,80,81. While symptoms are protean and traverse many domains, there is a focus on fatigue as well as brain and muscle symptoms8—i.e. a preferential effect on post-mitotic organs with high energy demand, as expected in mitochondrial impairment82,83,84,85,86,87. Variable latency to symptom onset (for veterans, following Gulf theater exposures88) is a well-characterized feature in mitochondrial illness, even extending to heritable mitochondrial conditions in a single kindred89. This variable latency is recognized to arise from the concurrent mitochondrial constructs of heteroplasmy (variability in mitochondrial compromise within and across tissues within an individual, differing across individuals) and clinical threshold effects90,91,92 (in which a certain degree of mitochondrial impairment and/or resulting cell loss is required for energetic or cellular reserves to be overcome and symptoms to emerge). Magnification of symptoms and defects following exercise in VGWI is also a fit93—as energetic demand would more decisively surpass supply in that setting, and as needed energy substrates are diverted from other tissues to strive to meet heightened energy needs of exercised muscle. Also consistent with mitochondrial impairment is the worsening (often reported by VGWI) of symptoms and impairments following exposure to any of a wide array of drugs and chemicals that increase oxidative stress or mitochondrial impairment. (Many drugs and chemicals, irrespective of their nominal specific mechanism of action, confer much or most of their toxicity through mitochondrial and oxidative stress mechanisms2,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112.) Mitochondrial impairment is a leading hypothesis for aging83,91,113,114,115,116,117,118, and the resemblance of GWI to accelerated aging has been noted, by us as well as by others16—including increases in aging-related conditions like cardiovascular disease (tied to impaired energy and alleviated by mitochondrial supports70,71,72,119); and metabolic syndrome factors like hypertension (tied to impaired energy120 and alleviated by mitochondrial supports121).
Some inflammatory mediators have been reported to increase fatty acid oxidation59, which could conceivably represent a further mechanism by which increased inflammation may be tied to lesser GWI severity, after mitochondrial impairment is considered. This could conceivably further contribute to the observed independent association of inflammation to lesser GWI severity, after accounting for mitochondrial impairment (indexed by CI&CIIOXPHOS). Findings may or may not relate to reports of symptom improvement with infection/inflammatory state in a subset of patients with mitochondrial-related disorders such as autism spectrum disorder122,123 (in which a subset show fatty acid oxidation defects124, and where fever has been tied to improved symptoms in a subset125,126).
We have previously suggested apoptosis as a route to inflammation in GWI9,127,128. Data here, showing a strong correlation between fatty acid oxidation defects and increased inflammation specific to VGWI, comport with this hypothesis: as above, fatty acid oxidation has been reported to protect against apoptosis in cell types where this has been examined58,59,60,61,62,63,64,65. Low fatty acid oxidation in VGWI leads to loss of apoptosis protection by this mechanism, resulting in increased cell death and consequent inflammation. Further consistent with this hypothesis, reduced fatty acid oxidation has been tied to increased ceramide synthesis58: increased ceramides were the most striking metabolomic alteration that separated VGWI from controls in our prior metabolomic study128. This is relevant because ceramides are pivotal drivers of mitochondrial apoptosis129.
Additional evidence favoring apoptosis as a feature in GWI comes from prostaglandin findings. Prostaglandin F2α, an eicosanoid known to protect against myocyte apoptosis130, was found to be strongly and significantly depressed in VGWI127. The degree of depression related strongly and significantly to the degree of self-rated muscle weakness127. (Self-rated muscle weakness has been validated against actual physical function9, and as in the methods, has also been shown to relate significantly to mitochondrial/bioenergetic impairment in VGWI assessed by post-exercise phosphocreatine recovery time constant determined on 31Phosphorus Magnetic Resonance Spectroscopy. Phosphocreatine is a backup muscle energy source that is depressed with exercise and the rate of recovery is known to relate to the rate of ATP production131). Though significantly depressed in VGWI, prostaglandin F2α did not relate to overall GWI symptom severity, just as we find here to be the case for inflammation. As above, the apparent protective association of hsCRP to GWI severity in VGWI selectively (after accounting for mitochondrial CI&CIIOXPHOS) could be compatible with a protective role of apoptosis against some symptoms. Of course, ongoing apoptosis in tissues with poor regenerative potential (like brain and to a lesser extent muscle) can, over time, cause problems—and brain atrophy is observed in VGWI93,132. If the mechanisms proposed here are operative, interventions should be prioritized for testing that support mitochondrial function/integrity and thereby limit “need” (and drive) for such apoptosis. Examples include coenzyme Q10 (ubiquinone) which in fact was reported to benefit symptoms and function in VGWI, in a double-blind RCT9. Other agents with reported tentative benefit (small placebo-controlled crossover study) such as curcumin and resveratrol133,134, though reportedly chosen for their anti-inflammatory properties, may alternatively have conferred relief in GWI through their documented benefits to mitochondrial status including fatty acid oxidation124,135,136,137,138,139,140,141,142,143,144. (Where alleviation of inflammation is produced through benefit to impaired mitochondrial function, clinical benefit would be expected under the mitochondrial-impairment hypothesis for GWI. Indeed, treatments that benefit impaired mitochondrial status may be expected to improve clinical outcomes, whether or not inflammation is reduced through a distinct pathway145).
As a secondary comment meriting follow-up, we note that sleep was the one symptom that did not, in the prior coenzyme Q10 study, show the favorable direction association with coenzyme Q10 versus placebo (the relation was almost exactly neutral)—and that here it was one of two symptoms not showing the predicted direction association to CI&CIIOXPHOS in VGWI. In the total sample, moreover, “tiredness” (which is commonly related to sleep problems) related significantly to inflammation—although no relation was observed in VGWI separately.
Given apparent trends toward lesser mitochondrial function, possibly spanning mitochondrial measures, reduced mitochondrial content (or mitochondrial DNA) could be one contributor. (However, while correlations among some mitochondrial indices appear more positive in VGWI than in controls, consistent with coordinated shifts, this is not a consistent finding across all indices). Future studies can assess this and other potential foundations for the observed findings.
The study is of modest size, but it is desirable to keep sample size to a minimum for a study employing an invasive procedure. Despite the modest size sample, a strongly significant relation between depressed mitochondrial function and increased GWI severity was observed, underscoring the large effect size. This study did not assess markers of inflammation besides hsCRP, which could bear different relations to GWI. However, the previously reported relation of increased CRP to GWI contributed to the dominance of the inflammatory hypothesis of GWI28,29,75,76, a hypothesis that the present findings appear to controvert. Regarding non-CRP inflammatory markers, studies have shown inconsistent findings: For instance, one study reported increased IL-4 and IL-13146 and another study reported decreased IL-4 and IL-13 in GWI147. Findings do not necessarily preclude a role for inflammation in some conditions noted to be elevated in GWI. Moreover, this study does not assess neuroinflammation, or its relation to mitochondrial impairment or to symptoms.
GWI is an exemplar of a growing cadre of environmentally-triggered chronic multisymptom illnesses, most if not all of which share at their core exposure to mitochondrially toxic agents, rendering findings in Gulf War veterans of broader relevance. Illustrative of the broad scope of mitochondrially toxic factors that have been tied to chronic multisymptom illness are organophosphate pesticides3,148,149,150, fluroquinolones2,151, carbon monoxide152,153,154, (long) Covid155,156,157 and non-ionizing radiation toxicity including so-called “Havana Syndrome”1,158. Both organophosphates and fluroquinolones are among implicated agents in GWI, that bear relevance to civilian and other military settings. Thus, our findings in GWI have potential relevance to chronic multisymptom fatiguing illness outside of GWI, both due to agents implicated in GWI, and other agents bearing shared mechanisms of toxicity. Additionally, these findings may have implications for aging—and may prove relevant to interpreting (or reinterpreting) the basis of the relation of inflammation to other health conditions. (For instance, inflammation is tied to cardiovascular disease—but anti-inflammatory agents promote rather than prevent cardiovascular events.)
To summarize, for the first time, direct muscle biopsy data affirm the presence of mitochondrial impairment in VGWI, specify the character of that compromise, and document a powerful relation of mitochondrial compromise to GWI severity. For the first time, data document a strong relation of mitochondrial impairment to inflammation in VGWI selectively, and clarify that mitochondrial impairment but not inflammation positively predicts GWI symptoms in VGWI. Indeed, we document an apparent protective association of inflammation to GWI severity in VGWI, once mitochondrial impairment is considered: This highly novel finding clearly requires replication. We speculate that the inflammation may arise from and serve as a proxy for an association to mitochondrial apoptosis, perhaps adaptively triggered—which may help to clear impaired cells. Further research is needed to affirm or refute this hypothesis.
Materials and methods
Ethics statement
The study protocol was approved by the University of California, San Diego, Human Research Protections Program (Project #151863) and Department of Defense Human Research Protections Office (Project #A-18779). All subjects gave written informed consent to participate. All methods were performed in accordance with relevant guidelines and regulations.
Forty-two participants included 21 veterans meeting both CDC and Kansas criteria for GWI and 21 healthy controls.
Cases: To qualify as a GWI case, screened prospective participants were required to have been deployed to the Persian Gulf theater of operations at any time from August 1, 1990 through July 31, 1991, inclusive. They were required to meet both CDC8 and Kansas7 symptom inclusion criteria for GWI. Both criteria require chronic symptoms of at least six months duration, arising during or after Gulf-theater deployment. CDC criteria require that these symptoms are present in at least two of the three domains of fatigue, mood/cognitive, and musculoskeletal. Kansas criteria are more discriminating and require symptoms in at least three of the six categories of fatigue/sleep, pain, neurological, respiratory, gastrointestinal and dermatologic. In addition, for a domain to qualify toward Kansas GWI criteria, symptoms within the domain must be at least moderate in severity (not mild) and/or multiple symptoms must be present in the category.
Exclusions encompass: (1) concurrent conditions (like lupus or multiple sclerosis) that can cause symptoms that might be confused with GWI; (2) general self-rated health prior to the Gulf War (cases) or in 1990 (controls) retrospectively rated as less than “very good” or “excellent” (in order to exclude those that might have had other processes affecting their health already present at that time); (3) late onset symptoms, on grounds that GWI criteria were only shown to be discriminating in the early time period after the Gulf War (tapping ~ early onset symptoms in primarily young veterans) and accrual of symptoms with extended time and aging might spuriously cause some individuals to meet GWI criteria in whom GWI processes are not involved. It is unknown whether gradual accrual of symptoms encompassing several domains in the many years following the Gulf War as participants age need necessarily relate to GWI, to Gulf War deployment, or to GWI processes. That is, GWI case criteria have not been validated for those with late-onset GWI. At about one fourth through study enrollment, a requirement was added that cases must have at least moderate muscle weakness, to address mitochondrial “heteroplasmy” (in which there can be variable involvement of mitochondrial impairment across organs, necessitating matching of the organ assessed to an organ with clinical compromise159). It is understood to be preferable to sample an affected tissue if assessing for mitochondrial impairment90,91,92. Conversely, if sampling a specific tissue, participants symptomatic in that tissue should be the focus.
Controls: To qualify as a control, participants were required to be non-veterans (to exclude those with a forme fruste of GWI, since evidence suggests that some veterans continue to newly meet criteria for GWI years later, and it is unknown whether these too in fact have GWI). Controls were required to meet neither CDC nor Kansas symptom criteria. To be eligible as a control, participants must have had a maximum of one symptom of at least moderate severity or two “mild” symptoms. Exceptions were considered in selected circumstances. For instance, if an individual had significant joint pain related to a discrete injury, that was clearly not related to systemic processes, this need not be exclusionary. Controls were also required not to have any Kansas exclusionary condition (like lupus or multiple sclerosis). Controls were selected to match 1:1 to a qualified case on age (within 5 years), sex, and race/ethnicity. (Half matches on ethnicity were accepted in recognition of the reality of mixed ethnicities.)
Because headache is not uncommonly tied to mitochondrial/bioenergetic impairment or energy supply/demand mismatch160,161,162,163,164, prospective controls who reported headache were excluded.
Assessments: Each participant underwent phlebotomy on the same day as the muscle biopsy.
HsCRP, a measure of peripheral inflammation, was assessed at the UCSD clinical laboratory using the latex-enhanced immunoturbidimetric method165. HsCRP is preferred over CRP for the range 0.5–10 mg/L (with CRP preferred for the range 10–1000 mg/L). Other studies have reported increased CRP in VGWI relative to selected healthy controls, but have noted that most values are in the normal range, usually defined as < 3 mg/L. Therefore, hsCRP was chosen as the preferred metric for inflammation with superior validity and sensitivity within the expected range.
Questionnaires: Participants completed Qualtrics surveys providing information on demographics, military history, exposure history, and symptoms/GWI severity.
Summed symptom severity: The UCSD GWI Symptom Survey was administered, assessing 20 symptoms each found to be present in ≥ 50% of VGWI in a prior study9. Severity of each symptom over the prior two weeks was rated 0–10.
This symptom survey has undergone validation as have a number of the individual symptom queries within the survey. The summed symptom score shows convergent validity, strongly correlating with the summed score from Kansas GWI criteria in a mixed sample of VGWI and controls (r = 0.91, p < 0.0001) and general self-rated health by visual analog scale (r = − 0.75, p < 0.0001).
Muscle weakness assessment: A muscle weakness self-rating of > 5 (out of 10) defined muscle weakness that was of at least moderate severity. The single item self-rating of muscle weakness (as above a qualifying criterion for GWI cases) was previously validated against objectively measured physical function assessed by the lower extremity Summary Performance Score166 (r = − 0.47, p = 0.001) procured in Gulf War veterans in a previous study based on averages of duplicated measurements of each (assessed ~ 1 week apart)9; and found to relate to bioenergetics assessed by post-exercise phosphocreatine recovery time constant (PCr-R) on 31Phosphorus Magnetic Resonance Spectroscopy: muscle weakness rating 0–5, PCr-R mean (SD) = 31.1 (6.95); muscle weakness rating 6–10, PCr-R mean (SD) = 45.4 (13.0), difference p = 0.01. It has also been shown to be sensitive to change with (double-blinded) treatment with an agent with potential to adversely affect muscle167.
Percutaneous skeletal muscle biopsy was performed (by co-I Taub) using the modified Bergström technique168 at the UCSD Altman Clinical and Translational Research Institute with a nurse experienced in assisting in the biopsy procedure. A single muscle biopsy was performed for each participant, and all mitochondrial assessments were conducted on the resulting sample. No more than one biopsy was performed on a given day. Participants were instructed not to take blood thinning agents (such as aspirin, nonsteroidal anti-inflammatory agents, clopidogrel) for 7 days prior to the procedure. Participants were prepped for the procedure using the Universal Protocol Checklist. The procedure field was sterilized with chlorhexidine and the operator employed hand hygiene, a protective gown, and sterile gloves. A partial body drape was used to keep the biopsy field sterile and a ~ 20 cm diameter area proximal to the knee joint was cleaned three times with chlorhexidine and dried. A 1 cm area in the center of the sterilized field was anesthetized twice with ~ 10 cc of 1% lidocaine, first subcutaneously, then intramuscularly into the vastus lateralis muscle. After waiting ~ 5 min for the anesthetic to take effect, an incision was made to the depth of a #11 scalpel blade. Using a Bergström needle, one rapid pass was made while suction was being provided to the inner trochar into the vastus lateralis muscle, retrieving approximately 150–220 mg of muscle.
Mitochondrial assessment was conducted under the supervision of co-I Patel, using three published protocols169. The first was fatty acids with sequential substrates, the second was MiR05 (mitochondrial respiration medium) with sequential substrates, and the final was MiR05 with rotenone and complex II substrates only. Fresh muscle biopsies from subjects were placed directly in ice-cold BIOPS solution. (The muscle biopsy was dissected, and 9 pieces of muscle fiber were put into 9 separate ports. Groups of 3 ports then underwent coupled (CI through CII or CII directly with CI inhibition) assays with fatty acids or sugar as substrates.) BIOPS, a medium designed to relax and preserve muscle biopsy samples, comprised 10 mM Ca-EGTA buffer, 0.1 µM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM K-MES (2-(N-morpholino)ethanesulfonic acid, a buffering agent), 0.5 mM dithiothreitol, 6.56 mM MgCl2, 5.77 mM ATP, 15 mM phosphocreatine, pH 7.1. After transporting the samples to the laboratory, skeletal muscle fibers were manually dissected under a microscope in BIOPS and then incubated with saponin (50 mg/mL) in BIOPS for 20 min. Then the sample was equilibrated in MiR05 respiratory buffer (110 mM D-sucrose, 60 mM lactobobionic acid, 20 mM taurine, 10 mM KH2PO4, 3 mM MgCl2, 20 mM HEPES, 0.5 mM EGTA, and 0.1 mg/mL bovine serum albumin at pH 7.1) at 37 °C for 5 min. Tissue weight was obtained after lightly patting with weighing paper, and 0.6–1.2 mg of muscle fiber was added to each chamber of an Oroboros O2k high-resolution respirometry system containing MiR05 respiratory buffer. Responses and changes in oxygen flux were normalized to mass of tissue in the chamber. Before addition of tissue, O2 was injected in each Oxygraph-2k chamber and equilibration of the gas phase with MiR05 was set to 400–450 µM before addition of muscle fibers. O2 flux measurements were typically made with 200–400 µM O2. The chamber was allowed to equilibrate for 10 min after addition of muscle fibers. After equilibration, coupled pathway assessments were made with and without fatty acids (octanoylcarnitine, [0.8 mM]) through mitochondrial complexes (“C”) by assessing leak (CI substrates glutamate [10 mM]/malate [2 mM], “L”), oxidative phosphorylation (ADP [1.25 mM] added, “Ox”), followed by addition of CI substrate pyruvate [5 mM] (“CI Ox”), then CII substrate succinate [10 mM] (CI&CII Ox), then carbonilcyanide p-trifluoromethoxyphenylhydrazone (FCCP, [0.5 µM] steps until max uncoupling) to uncouple mitochondria (mUC), then addition of rotenone [0.5 µM] to inhibit CI to assess CII uncoupled (“CII ETS”), and antimycin A [2.5 µM] to inhibit CII and CIII activity to assess residual oxygen consumption (“ROX”). CII coupled respiration was assessed without other substrates. Oxygen flux trace and rate determinations were obtained using DatLab 7 software. Cytochrome c [10 µM] was added after ADP to assess mitochondrial quality. The preparation was not utilized if respiration increased greater than 10% after addition of cytochrome c. All assessments were performed in technical triplicate.
As per the Introduction, based on preliminary analyses, four indices of mitochondrial function are highlighted in this study. (To ease understanding of the abbreviations based on component, CI and CII refer to mitochondrial complexes I and II, respectively; FAO refers to fatty acid oxidation; ETS refers to respiratory capacity of the electron transport system: MaxUC refers to the uncoupled state.) CI&CII&FAOCI/CIIOXPHOS refers to assessment of that aspect of mitochondrial function determined by using fatty acids as the fuel source with complex I and II substrates malate, glutamate, pyruvate, succinate and adenosine diphosphate ADP to drive respiration. CII&FAOETS refers to assessment of that aspect of mitochondrial function determined by using fatty acids as the fuel source with rotenone to inhibit complex I to look at complex II respiration. FAOMaxUC refers to assessment of that aspect of mitochondrial function determined by using fatty acids as the fuel source with FCCP as an uncoupler. CI&CIIOXPHOS refers to assessment of that aspect of mitochondrial function determined by using glucose as the fuel source with complex I and II substrates malate, glutamate, pyruvate, succinate with ADP to drive respiration.
Blinding of mitochondrial assessments: All mitochondrial assessments were conducted blinded to case–control status.
Analysis:
-
I.
Descriptive statistics were assessed for participant characteristics, for all participants and for VGWI and controls separately. These included means (standard deviations) for continuous variables and proportions for categorical variables. Separate analyses were performed for matched case–control pairs, and for the expanded sample who contributed to the primary cross-sectional analyses of relations of mitochondrial and inflammatory measures to outcomes. T-tests of difference in means (continuous variables) and chi-square tests (categorical variables) assessed case–control differences; however, case–control comparisons are not the focus of the present analyses, which are directed to prediction of summed symptom severity.
-
II.
Case–control differences in important candidate mitochondrial markers and in hsCRP were assessed in matched pairs (paired t-tests) and in the full sample (unpaired t-tests). Because of prior evidence of impaired mitochondrial function and increased hsCRP in VGWI, for this peripheral assessment, one-sided p-values may be justified, and are mentioned (legend only) to assist in determining whether the present study is concordant with prior findings showing elevated inflammation and mitochondrial impairment in GWI; and in helping to characterize the nature of mitochondrial respiratory chain impairment.
-
III.
The interrelation of mitochondrial markers to one another and to inflammation was provisionally examined via correlation of candidate mitochondrial markers and hsCRP to one another, using Pearson’s correlation coefficients.
-
IV.
Correlations of candidate mitochondrial markers and of hsCRP to each of the twenty symptoms of the UCSD GWI symptom survey were also assessed in all participants, and separately in cases and controls. This focused on the mitochondrial marker of interest that showed the most dissociation from hsCRP, to provide better discrimination (to reduce collinearity).
-
V.
Sign tests were employed to assess whether the sign of the correlation was more often in the predicted than the converse direction (more mitochondrial impairment or inflammation tied to greater symptoms is predicted for symptoms overall), as well as considering those symptoms with at least borderline significant correlations (two-sided p < 0.1), and those with a frankly significant correlation (two-sided p < 0.05) considering mitochondrial indices of relevance and hsCRP. Although the sign test is simple, it has critical advantages: It makes no distributional assumptions and carries high authority. Indeed, statistics experts state that “if the Sign-test indicates a significant difference, and another test does not, you should seriously rethink whether the other test is valid”170.
-
VI.
Summed symptom severity in the total sample and within VGWI (cases) was assessed via regression analysis with robust (heteroskedasticity-independent) standard errors171, examining univariable prediction by the chosen mitochondrial marker and hsCRP, then in joint analysis.
-
VII.
Study power: The originally proposed sample size of 54 provided 80% power with two-sided alpha of 0.05 to detect a difference between cases and controls of 0.4 standard deviations. Shutdown of the study due to Covid-19, and enforced extended delays in study resumption due to the requirement for sustained close proximity of multiple parties for the biopsy procedure, led the study to be suspended prior to initial recruitment targets being achieved. Findings were deemed sufficiently striking that the determination was made not to subject additional individuals to the invasive muscle biopsy procedure. For the paired sample, with the achieved sample size, there was 80% power with two-sided alpha of 0.05 to detect an effect size of 0.56 standard deviations. However, a focus on the relation of mitochondrial indices to summed symptom severity rather than dichotomous outcomes more strongly supports study power by capitalizing on the continuous nature of both the mechanistic (mitochondrial and inflammatory) and summed symptom severity variables.
-
VIII.
All statistical analyses employed Stata® (versions 8.0 and 13.0, College Station, Texas). P-values are two-sided except where otherwise stated. P-values < 0.05 designated statistical significance. Adjustment for multiple comparisons rests on the presumption that chance is the first order explanation for significance of findings, if observed172. This presumption is obviated given past evidence of bioenergetic/mitochondrial impairment in VGWI, as well as in animal models of GWI, and multiple other features of GWI that comport with known features of mitochondrial illness (see Discussion). Thus, no adjustment for multiple comparisons was undertaken.
Data availability
Data will be made available on request. Participants were advised that their data would be kept confidential, with only group-level data published. Because specific demographic and symptom information could potentially identify individual veterans, we prefer to share the data on an as-requested basis, with assurances of confidentiality protections, rather than in a public-use database. Requests for data should be made to Dr. Golomb (bgolomb@ucsd.edu).
References
Golomb, B. A. Diplomats’ mystery illness and pulsed radiofrequency/microwave radiation. Neural Comput. 30, 1–104. https://doi.org/10.1162/neco_a_01133 (2018).
Golomb, B. A., Koslik, H. J. & Redd, A. J. Fluoroquinolone-induced serious, persistent, multisymptom adverse effects. BMJ Case Rep. https://doi.org/10.1136/bcr-2015-209821 (2015).
Davies, D. R., Ahmed, G. M. & Freer, T. Chronic organophosphate induced neuropsychiatric disorder (COPIND): Results of two postal questionnaire surveys. J Nutr. Environ. Med. 9, 123–134 (1999).
Binns, J. H. et al. Research Advisory Committee on Gulf War Veterans' Illnesses: Scientific Progress in Understanding Gulf War Veterans’ Illnesses: Report and Recommendations 1–141 (2004).
Cherry, N. et al. Health and exposures of United Kingdom Gulf war veterans. Part II: The relation of health to exposure. Occup. Environ. Med. 58, 299–306 (2001).
Gray, G. C., Reed, R. J., Kaiser, K. S., Smith, T. C. & Gastanaga, V. M. Self-reported symptoms and medical conditions among 11,868 Gulf War-era veterans: the Seabee Health Study. Am. J. Epidemiol. 155, 1033–1044 (2002).
Steele, L. Prevalence and patterns of Gulf War illness in Kansas veterans: Association of symptoms with characteristics of person, place, and time of military service. Am. J. Epidemiol. 152, 992–1002 (2000).
Fukuda, K. et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. J. Am. Med. Assoc. 280, 981–988 (1998).
Golomb, B. A. et al. Coenzyme Q10 benefits symptoms in Gulf War veterans: Results of a randomized double-blind study. Neural Comput. 26, 2594–2651. https://doi.org/10.1162/NECO_a_00659 (2014).
Cherry, N. et al. Health and exposures of United Kingdom Gulf war veterans. Part I: The pattern and extent of ill health. Occup. Environ. Med. 58, 291–298 (2001).
Kang, H. K., Mahan, C. M., Lee, K. Y., Magee, C. A. & Murphy, F. M. Illnesses among United States veterans of the Gulf War: A population-based survey of 30,000 veterans. J. Occup. Environ. Med. 42, 491–501 (2000).
Unwin, C. et al. Health of UK servicemen who served in Persian Gulf War. Lancet 353, 169–178 (1999).
McCauley, L. A., Lasarev, M., Sticker, D., Rischitelli, D. G. & Spencer, P. S. Illness experience of Gulf War veterans possibly exposed to chemical warfare agents. Am. J. Prev. Med. 23, 200–206 (2002).
Steele, L. et al. Brain-immune interactions as the basis of Gulf War illness: Clinical assessment and deployment profile of 1990–1991 Gulf War Veterans in the Gulf War Illness Consortium (GWIC) multisite case-control study. Brain Sci. 11, 1132. https://doi.org/10.3390/brainsci11091132 (2021).
Simmons, R., Maconochie, N. & Doyle, P. Self-reported ill health in male UK Gulf War veterans: A retrospective cohort study. BMC Public Health 4, 27 (2004).
Zundel, C. G. et al. Rates of chronic medical conditions in 1991 Gulf War veterans compared to the general population. Int. J. Environ. Res. Public Health 16, 949. https://doi.org/10.3390/ijerph16060949 (2019).
Berardocco, D. DoD, CIA release Khamisiyah modeling data. GulfNEWS 1, 3 (1997).
General Accounting Office. Gulf War illnesses: DOD's conclusions about U.S. Troops' exposure cannot be adequately supported. In: GAO report number GAO-04-159. http://www.gao.gov/htext/d04159.html (2004).
Schumm, W. R. et al. Self-reported changes in subjective health and anthrax vaccination as reported by over 900 Persian Gulf War era veterans. Psychol. Rep. 90, 639–653 (2002).
Golomb, B. A. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc. Natl. Acad. Sci. USA 105, 4295–4300. https://doi.org/10.1073/pnas.0711986105 (2008).
Cecchine, G., Golomb, B. A., Hilborne, L. H., Spektor, D. M. & Anthony, R. A. A Review of the Scientific Literature as it Pertains to Gulf War Illnesses: Pesticides Vol. 8 (RAND, Santa Monica, 2000).
Fricker, R. D. et al. Pesticide Use During the Gulf War: A Survey of Gulf War Veterans (RAND, Santa Monica, 2000).
Department of Defense. Environmental exposure report. Pesticides. In: Final Report. http://www.gulflink.osd.mil/pest_final (2003).
Presidential Advisory Committee on Gulf War Veterans' Illnesses. Presidential Advisory Committee on Gulf War Veterans' Illnesses: Final Report. (U.S. Government Printing Office, 1996).
Committee on the Evaluation of the Department of Defense Clinical Evaluation Program Division of Health Promotion and Disease Prevention Institute of Medicine. Adequacy of the Comprehensive Clinical Evaluation Program: A Focused Assessment (National Academy Press, 1997).
Binns, J. H. et al. Gulf War illness and the health of Gulf War veterans. In: Scientific Findings and Recommendations 1–454 (U.S. Government Printing Office, 2008).
Steele, L., Lockridge, O., Gerkovich, M. M., Cook, M. R. & Sastre, A. Butyrylcholinesterase genotype and enzyme activity in relation to Gulf War illness: preliminary evidence of gene-exposure interaction from a case-control study of 1991 Gulf War veterans. Environ. Health 14, 4. https://doi.org/10.1186/1476-069X-14-4 (2015).
Butterick, T. A. et al. Gulf War illness-associated increases in blood levels of interleukin 6 and C-reactive protein: Biomarker evidence of inflammation. BMC Res. Notes 12, 816. https://doi.org/10.1186/s13104-019-4855-2 (2019).
Johnson, G. J., Slater, B. C., Leis, L. A., Rector, T. S. & Bach, R. R. Blood biomarkers of chronic inflammation in Gulf War illness. PLOS ONE 11, e0157855. https://doi.org/10.1371/journal.pone.0157855 (2016).
Locker, A. R. et al. Corticosterone primes the neuroinflammatory response to Gulf War illness-relevant organophosphates independently of acetylcholinesterase inhibition. J. Neurochem. 142, 444–455. https://doi.org/10.1111/jnc.14071 (2017).
James, L. M. et al. Human Leukocyte Antigen (HLA) and Gulf War Illness (GWI): HLA-DRB1*13:02 spares subcortical atrophy in Gulf War veterans. EBioMedicine 26, 126–131. https://doi.org/10.1016/j.ebiom.2017.11.005 (2017).
Alshelh, Z. et al. In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain Behav. Immun. 87, 498–507. https://doi.org/10.1016/j.bbi.2020.01.020 (2020).
Golomb, B. A. Oxidative stress and mitochondrial injury in chronic multisymptom conditions: From Gulf War illness to autism spectrum disorder. Nat. Proc. https://doi.org/10.1038/npre.2012.6847.1 (2012).
Koslik, H. J., Hamilton, G. & Golomb, B. A. Mitochondrial dysfunction in Gulf War illness revealed by 31 phosphorus magnetic resonance spectroscopy: A case-control study. PLOS ONE 9, e92887 (2014).
Chen, Y. et al. Role of mitochondrial DNA damage and dysfunction in veterans with Gulf War Illness. PLOS ONE 12, e0184832. https://doi.org/10.1371/journal.pone.0184832 (2017).
Nguyen, H. et al. Exposure to Gulf War illness-related agents leads to the development of chronic pain and fatigue. Life Sci. 283, 119867. https://doi.org/10.1016/j.lfs.2021.119867 (2021).
Abdullah, L. et al. Translational potential of long-term decreases in mitochondrial lipids in a mouse model of Gulf War illness. Toxicology 372, 22–33. https://doi.org/10.1016/j.tox.2016.10.012 (2016).
Shetty, G. A. et al. Chronic oxidative stress, mitochondrial dysfunction, Nrf2 activation and inflammation in the hippocampus accompany heightened systemic inflammation and oxidative stress in an animal model of Gulf War illness. Front. Mol. Neurosci. 10, 182. https://doi.org/10.3389/fnmol.2017.00182 (2017).
Zakirova, Z. et al. Complementary proteomic approaches reveal mitochondrial dysfunction, immune and inflammatory dysregulation in a mouse model of Gulf War Illness. Prot. Clin. Appl. 11, 1600190. https://doi.org/10.1002/prca.201600190 (2017).
Braga, M. et al. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis 13, 822–832. https://doi.org/10.1007/s10495-008-0216-7 (2008).
Reutelingsperger, C. P. & van Heerde, W. L. Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell. Mol. Life Sci. 53, 527–532 (1997).
Golomb, B. A. et al. Lower blood malondialdehyde is associated with past pesticide exposure: Findings in Gulf War illness and healthy controls. Military Med Res (2021).
Golomb, B. A. et al. A pilot study of bioenergetic marker relationships in Gulf War illness: Phosphocreatine recovery vs. Citric acid cycle intermediates. Int. J. Environ. Res. Public Health 18, 1635. https://doi.org/10.3390/ijerph18041635 (2021).
Golomb, B. A., Nguyen, E. & Dinkeloo, E. Radiation exposure predicts reported vaccine adverse effects in veterans with Gulf War illness. Int. J. Environ. Res. Public Health 17, 7136. https://doi.org/10.3390/ijerph17197136 (2020).
Ismail, K. et al. Chronic fatigue syndrome and related disorders in UK veterans of the Gulf War 1990–1991: Results from a two-phase cohort study. Psychol. Med. 38, 953–961 (2007).
Yan, J. et al. Induction of lipid metabolism dysfunction, oxidative stress and inflammation response by tris(1-chloro-2-propyl)phosphate in larval/adult zebrafish. Environ Int 160, 107081. https://doi.org/10.1016/j.envint.2022.107081 (2022).
Xiang, D. & Wang, Q. PXR-mediated organophorous flame retardant tricresyl phosphate effects on lipid homeostasis. Chemosphere 284, 131250. https://doi.org/10.1016/j.chemosphere.2021.131250 (2021).
Sullivan, K. et al. Neuropsychological functioning in military pesticide applicators from the Gulf War: Effects on information processing speed, attention and visual memory. Neurotoxicol. Teratol. 65, 1–13. https://doi.org/10.1016/j.ntt.2017.11.002 (2018).
Ojo, J. O. et al. Exposure to an organophosphate pesticide, individually or in combination with other Gulf War agents, impairs synaptic integrity and neuronal differentiation, and is accompanied by subtle microvascular injury in a mouse model of Gulf War agent exposure. Neuropathology 34, 109–127. https://doi.org/10.1111/neup.12061 (2014).
Phillips, K. F. & Deshpande, L. S. Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness. Neurotoxicology 52, 127–133. https://doi.org/10.1016/j.neuro.2015.11.014 (2016).
Phillips, K. F. & Deshpande, L. S. Chronic neurological morbidities and elevated hippocampal calcium levels in a DFP-based rat model of Gulf War illness. Mil. Med. 183, 552–555. https://doi.org/10.1093/milmed/usx148 (2018).
Scremin, O. U. et al. Low-dose cholinesterase inhibitors do not induce delayed effects on cerebral blood flow and metabolism. Pharmacol. Biochem. Behav. 80, 529–540. https://doi.org/10.1016/j.pbb.2004.12.013 (2005).
Nutter, T. J., Johnson, R. D. & Cooper, B. Y. A delayed chronic pain like condition with decreased Kv channel activity in a rat model of Gulf War Illness pain syndrome. Neurotoxicology 51, 67–79. https://doi.org/10.1016/j.neuro.2015.09.010 (2015).
Repine, J. E. et al. Inhalation of two putative Gulf War toxins by mice. J. Environ. Sci. Health B 51, 366–373. https://doi.org/10.1080/03601234.2016.1142318 (2016).
Husain, K. & Somani, S. M. Persistent/delayed toxic effects of low-dose sarin and pyridostigmine under physical stress (exercise) in mice. Indian J. Physiol. Pharmacol. 48, 150–164 (2004).
O’Callaghan, J. P., Kelly, K. A., Locker, A. R., Miller, D. B. & Lasley, S. M. Corticosterone primes the neuroinflammatory response to DFP in mice: Potential animal model of Gulf War illness. J. Neurochem. 133, 708–721. https://doi.org/10.1111/jnc.13088 (2015).
Yan, Q. et al. High-resolution metabolomic assessment of pesticide exposure in central valley, California. Chem. Res. Toxicol. 34, 1337–1347. https://doi.org/10.1021/acs.chemrestox.0c00523 (2021).
Yao, H. et al. Fatty acid oxidation protects against hyperoxia-induced endothelial cell apoptosis and lung injury in neonatal mice. Am. J. Respir. Cell Mol. Biol. 60, 667–677. https://doi.org/10.1165/rcmb.2018-0335OC (2019).
Guo, X. et al. IL-13 alleviates cardiomyocyte apoptosis by improving fatty acid oxidation in mitochondria. Front. Cell. Dev. Biol. 9, 736603. https://doi.org/10.3389/fcell.2021.736603 (2021).
Sun, R. et al. l-Carnitine protects against 1,4-benzoquinone-induced apoptosis and DNA damage by suppressing oxidative stress and promoting fatty acid oxidation in K562 cells. Environ. Toxicol. 35, 1033–1042. https://doi.org/10.1002/tox.22939 (2020).
Sun, J. et al. Berberine protects against palmitate-induced apoptosis in tubular epithelial cells by promoting fatty acid oxidation. Med. Sci. Monit. 24, 1484–1492. https://doi.org/10.12659/msm.908927 (2018).
Yang, W. M. & Lee, W. CTRP5 ameliorates palmitate-induced apoptosis and insulin resistance through activation of AMPK and fatty acid oxidation. Biochem. Biophys. Res. Commun. 452, 715–721. https://doi.org/10.1016/j.bbrc.2014.08.145 (2014).
Henique, C. et al. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J. Biol. Chem. 285, 36818–36827. https://doi.org/10.1074/jbc.M110.170431 (2010).
Wan, J. et al. Activation of PPARdelta up-regulates fatty acid oxidation and energy uncoupling genes of mitochondria and reduces palmitate-induced apoptosis in pancreatic beta-cells. Biochem. Biophys. Res. Commun. 391, 1567–1572. https://doi.org/10.1016/j.bbrc.2009.12.127 (2010).
Samudio, I. et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Investig. 120, 142–156. https://doi.org/10.1172/jci38942 (2010).
Fehm, H. L., Kern, W. & Peters, A. The selfish brain: Competition for energy resources. Prog. Brain Res. 153, 129–140 (2006).
Tein, I. Lipid Storage Myopathies Due to Fatty Acid Oxidation Defects in Neuromuscular Disorders of Infancy, Childhood, and Adolescence 761–795 (Elsevier, 2015).
Tein, I. Metabolic myopathies. Semin. Pediatr. Neurol. 3, 59–98. https://doi.org/10.1016/s1071-9091(96)80038-6 (1996).
Wong, W. A signal to warm up to. Sci Signal. 9, ec190–ec190 (2016).
Mortensen, S. A. et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail 2, 641–649. https://doi.org/10.1016/j.jchf.2014.06.008 (2014).
Mortensen, A. L., Rosenfeldt, F. & Filipiak, K. J. Effect of coenzyme Q10 in Europeans with chronic heart failure: A sub-group analysis of the Q-SYMBIO randomized double-blind trial. Clin. Cardiol. 26, 147–156 (2019).
Singh, R. B. et al. Randomized, double-blind placebo-controlled trial of coenzyme Q10 in patients with acute myocardial infarction. Cardiovasc. Drugs Ther. 12, 347–353 (1998).
Alehagen, U., Johansson, P., Bjornstedt, M., Rosen, A. & Dahlstrom, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 167, 1860–1866. https://doi.org/10.1016/j.ijcard.2012.04.156 (2013).
Alehagen, U., Aaseth, J., Alexander, J. & Johansson, P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLOS ONE 13, e0193120. https://doi.org/10.1371/journal.pone.0193120 (2018).
Johnson, G. J., Leis, L. A., Slater, B. C. & Bach, R. R. Elevated platelet count, C-reactive protein and thromboxane analog-induced platelet aggregation in patients with Gulf War veterans’ illnesses: Evidence of a chronic inflammatory state?. Blood Coagul. Fibrinolysis 7, 736–741. https://doi.org/10.1097/MBC.0b013e328362627f (2013).
James, L. M., Engdahl, B. E., Johnson, R. A. & Georgopoulos, A. P. Gulf War illness and inflammation: Association of symptom severity with C-reactive protein. J. Neurol. Neuromed. 4, 15–19 (2019).
Proctor, S. P., Harley, R., Wolfe, J., Heeren, T. & White, R. F. Health-related quality of life in Persian Gulf War veterans. Mil Med 166, 510–519 (2001).
Doebbeling, B. N. et al. Is there a Persian Gulf War syndrome? Evidence from a large population-based survey of veterans and nondeployed controls. Am. J. Med. 108, 695–704 (2000).
Siciliano, G. et al. Functional diagnostics in mitochondrial diseases. Biosci. Rep. 27, 53–67. https://doi.org/10.1007/s10540-007-9037-0 (2007).
Tarnopolsky, M. A. & Raha, S. Mitochondrial myopathies: diagnosis, exercise intolerance, and treatment options. Med. Sci. Sports Exerc. 37, 2086–2093 (2005).
Tsujita, Y. et al. A surviving case of mitochondrial cardiomyopathy diagnosed from the symptoms of multiple organ dysfunction syndrome. Int. J. Cardiol. 128, e43-45. https://doi.org/10.1016/j.ijcard.2007.05.072 (2008).
De Flora, S. et al. DNA adducts and chronic degenerative disease. Pathogenetic relevance and implications in preventive medicine. Mutat. Res. 366, 197–238 (1996).
Sastre, J., Pallardo, F. V. & Vina, J. The role of mitochondrial oxidative stress in aging. Free Radic. Biol. Med. 35, 1–8 (2003).
De Vivo, D. C. & DiMauro, S. Mitochondrial defects of brain and muscle. Biol. Neonate 58(Suppl 1), 54–69 (1990).
Pinto, M. & Moraes, C. T. Mitochondrial genome changes and neurodegenerative diseases. Biochem. Biophys. Acta 1842, 1198–1207. https://doi.org/10.1016/j.bbadis.2013.11.012 (2014).
Gardner, A. & Boles, R. G. Symptoms of somatization as a rapid screening tool for mitochondrial dysfunction in depression. Biopsychosoc. Med. 2, 7 (2008).
Myhill, S., Booth, N. E. & McLaren-Howard, J. Chronic fatigue syndrome and mitochondrial dysfunction. Int. J. Clin. Exp. Med. 2, 1–16 (2009).
Kroenke, K., Koslowe, P. & Roy, M. Symptoms in 18,495 Persian Gulf War veterans. Latency of onset and lack of association with self-reported exposures. J. Occup. Environ. Med. 40, 520–528 (1998).
Crimmins, D. et al. Mitochondrial encephalomyopathy: Variable clinical expression within a single kindred. J. Neurol. Neurosurg. Psychiatry 56, 900–905 (1993).
Dimauro, S. & Davidzon, G. Mitochondrial DNA and disease. Ann. Med. 37, 222–232 (2005).
Wei, Y. H. Mitochondrial DNA mutations and oxidative damage in aging and diseases: an emerging paradigm of gerontology and medicine. Proc. Natl. Sci. Counc. Repub. China B 22, 55–67 (1998).
Vattemi, G. et al. Overexpression of TNF-alpha in mitochondrial diseases caused by mutations in mtDNA: Evidence for signaling through its receptors on mitochondria. Free Radic. Biol. Med. 63, 108–114. https://doi.org/10.1016/j.freeradbiomed.2013.04.025 (2013).
Rayhan, R. U. et al. Exercise challenge in Gulf War illness reveals two subgroups with altered brain structure and function. PLOS ONE 8, e63903 (2013).
Golomb, B. A. & Evans, M. A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 8, 373–418 (2008).
Amin, A. & Hamza, A. A. Oxidative stress mediates drug-induced hepatotoxicity in rats: A possible role of DNA fragmentation. Toxicology 208, 367–375. https://doi.org/10.1016/j.tox.2004.11.039 (2005).
Das, G. C., Bacsi, A., Shrivastav, M., Hazra, T. K. & Boldogh, I. Enhanced gamma-glutamylcysteine synthetase activity decreases drug-induced oxidative stress levels and cytotoxicity. Mol Carcinog 45, 635–647. https://doi.org/10.1002/mc.20184 (2006).
Denicola, A. & Radi, R. Peroxynitrite and drug-dependent toxicity. Toxicology 208, 273–288 (2005).
Fosslien, E. Adverse effects of nonsteroidal anti-inflammatory drugs on the gastrointestinal system. Ann. Clin. Lab. Sci. 28, 67–81 (1998).
McMillian, M. et al. Drug-induced oxidative stress in rat liver from a toxicogenomics perspective. Toxicol. Appl. Pharmacol. 207, 171–178. https://doi.org/10.1016/j.taap.2005.02.031 (2005).
Shuhendler, A. J., Pu, K., Cui, L., Uetrecht, J. P. & Rao, J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat. Biotechnol. 32, 373–380. https://doi.org/10.1038/nbt.2838 (2014).
Tafazoli, S., Spehar, D. D. & O’Brien, P. J. Oxidative stress mediated idiosyncratic drug toxicity. Drug Metab. Rev. 37, 311–325 (2005).
Verma, P., Bhattacharya, S. N., Banerjee, B. D. & Khanna, N. Oxidative stress and leukocyte migration inhibition response in cutaneous adverse drug reactions. Indian J. Dermatol. Venereol. Leprol. 78, 664. https://doi.org/10.4103/0378-6323.100519 (2012).
Joshi, G. et al. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: Insight into chemobrain. Free Radic. Res. 39, 1147–1154 (2005).
Kovacic, P. & Cooksy, A. L. Unifying mechanism for toxicity and addiction by abused drugs: electron transfer and reactive oxygen species. Med. Hypotheses 64, 357–366. https://doi.org/10.1016/j.mehy.2004.07.021 (2005).
Varga, Z. V., Ferdinandy, P., Liaudet, L. & Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 309, H1453-1467. https://doi.org/10.1152/ajpheart.00554.2015 (2015).
Bastianon, C., Zanoni, R., Miolo, G., Caffieri, S. & Reddi, E. Mitochondria and plasma membrane as targets of UVA-induced toxicity of neuroleptic drugs fluphenazine, perphenazine and thioridazine. Int. J. Biochem. Cell Biol. 37, 901–908 (2005).
Boelsterli, U. A. & Lim, P. L. Mitochondrial abnormalities: A link to idiosyncratic drug hepatotoxicity?. Toxicol. Appl. Pharmacol. 220, 92–107 (2007).
de Mendoza, C., Sanchez-Conde, M., Ribera, E., Domingo, P. & Soriano, V. Could mitochondrial DNA quantitation be a surrogate marker for drug mitochondrial toxicity?. AIDS Rev. 6, 169–180 (2004).
Finsterer, J. & Zarrouk-Mahjoub, S. Mitochondrial toxicity of cardiac drugs and its relevance to mitochondrial disorders. Expert Opin. Drug Metab. Toxicol. 11, 15–24. https://doi.org/10.1517/17425255.2015.973401 (2015).
Foli, A. et al. Direct analysis of mitochondrial toxicity of antiretroviral drugs. AIDS 15, 1687–1694 (2001).
Swartz, M. N. Mitochondrial toxicity–new adverse drug effects. N. Engl. J. Med. 333, 1146–1148 (1995).
Wallace, K. B. & Starkov, A. A. Mitochondrial targets of drug toxicity. Annu. Rev. Pharmacol. Toxicol. 40, 353–388 (2000).
Wallace, D. C., Brown, M. D., Melov, S., Graham, B. & Lott, M. Mitochondrial biology, degenerative diseases and aging. BioFactors 7, 187–190 (1998).
Wallace, D. C. Mitochondrial DNA in aging and disease. Sci. Am. 277, 40–47 (1997).
Wallace, D. C. Mitochondrial genetics: A paradigm for aging and degenerative diseases?. Science 256, 628–632 (1992).
Wallace, D. C. et al. Mitochondrial DNA mutations in human degenerative diseases and aging. Biochem. Biophys. Acta 1271, 141–151 (1995).
Melov, S., Schneider, J. A., Coskun, P. E., Bennett, D. A. & Wallace, D. C. Mitochondrial DNA rearrangements in aging human brain and in situ PCR of mtDNA. Neurobiol. Aging 20, 565–571 (1999).
Wallace, D. C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407 (2005).
Ramezani, M., Sahraei, Z., Simani, L., Heydari, K. & Shahidi, F. Coenzyme Q10 supplementation in acute ischemic stroke: Is it beneficial in short-term administration?. Nutr. Neurosci. 23, 640–645. https://doi.org/10.1080/1028415x.2018.1541269 (2020).
Sun, F., Cui, J., Gavras, H. & Schwartz, F. A novel class of tests for the detection of mitochondrial DNA-mutation involvement in diseases. Am. J. Hum. Genet. 72, 1515–1526 (2003).
Rosenfeldt, F. L. et al. Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. J. Hum. Hypertens. 21, 297–306 (2007).
Finsterer, J. Autism spectrum disorder: A mitochondrial disorder. Iran. J. Child Neurol. 15, 115–117. https://doi.org/10.22037/ijcn.v16i2.33066 (2021).
Wen, Y. & Yao, Y. The Mitochondria Connection in Autism Spectrum Disorders (ed A. M. Grabrucker) (Exon Publications Copyright: The Authors, 2021).
Barone, R. et al. Mitochondrial fatty acid β-oxidation and resveratrol effect in fibroblasts from patients with autism spectrum disorder. J. Pers. Med. 11, 510. https://doi.org/10.3390/jpm11060510 (2021).
Curran, L. K. et al. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 120, e1386-1392. https://doi.org/10.1542/peds.2007-0360 (2007).
Grzadzinski, R., Lord, C., Sanders, S. J., Werling, D. & Bal, V. H. Children with autism spectrum disorder who improve with fever: Insights from the Simons Simplex Collection. Autism Res. 11, 175–184. https://doi.org/10.1002/aur.1856 (2018).
Golomb, B. A. et al. Depressed prostaglandins and leukotrienes in veterans with Gulf War illness. J. Environ. Sci. Health B 54(8), 623–639. https://doi.org/10.1080/03601234.2019.1596001 (2019).
Naviaux, R. K. et al. Metabolic features of Gulf War illness. PLOS ONE 14, e0219531. https://doi.org/10.1371/journal.pone.0219531 (2019).
Tirodkar, T. S. & Voelkel-Johnson, C. Sphingolipids in apoptosis. Exp. Oncol. 34, 231–242 (2012).
Jansen, K. M. & Pavlath, G. K. Prostaglandin F2alpha promotes muscle cell survival and growth through upregulation of the inhibitor of apoptosis protein BRUCE. Cell Death Differ. 15, 1619–1628. https://doi.org/10.1038/cdd.2008.90 (2008).
Thompson, C. H., Kemp, G. J., Sanderson, A. L. & Radda, G. K. Skeletal muscle mitochondrial function studied by kinetic analysis of postexercise phosphocreatine resynthesis. J. Appl. Physiol. 78, 2131–2139 (1995).
Heaton, K. J. et al. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology 28, 761–769 (2007).
Hodgin, K. S. et al. A placebo-controlled, pseudo-randomized, crossover trial of botanical agents for Gulf War illness: Resveratrol (Polygonum cuspidatum), Luteolin, and Fisetin (Rhus succedanea). Int. J. Environ. Res. Public Health 18, 2483. https://doi.org/10.3390/ijerph18052483 (2021).
Donovan, E. K. et al. A placebo-controlled, pseudo-randomized, crossover trial of botanical agents for Gulf War illness: Curcumin (Curcuma longa), Boswellia (Boswellia serrata), and French maritime pine bark (Pinus pinaster). Int. J. Environ. Res. Public Health 18, 2468. https://doi.org/10.3390/ijerph18052468 (2021).
Zhao, D. et al. Curcumin improves adipocytes browning and mitochondrial function in 3T3-L1 cells and obese rodent model. R Soc Open Sci 8, 200974. https://doi.org/10.1098/rsos.200974 (2021).
Zhang, J. et al. Curcumin attenuates D-galactosamine/lipopolysaccharide-induced liver injury and mitochondrial dysfunction in mice. J. Nutr. 144, 1211–1218. https://doi.org/10.3945/jn.114.193573 (2014).
Lan, J. et al. Curcumin alleviates arsenic-induced injury in duck skeletal muscle via regulating the PINK1/Parkin pathway and protecting mitochondrial function. Toxicol Appl Pharmacol 434, 115820. https://doi.org/10.1016/j.taap.2021.115820 (2022).
Li, X. et al. Resveratrol protects renal damages induced by periodontitis via preventing mitochondrial dysfunction in rats. Oral Dis. https://doi.org/10.1111/odi.14148 (2022).
Hyatt, J. K., de Cabo, R. & Mattison, J. A. Resveratrol blunts mitochondrial loss in slow and mixed skeletal muscle phenotypes of non-human primates following a long-term high fat/sugar diet. J. Diet Suppl. https://doi.org/10.1080/19390211.2022.2039340 (2022).
Chen, L. L. et al. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial β-oxidation. Metabolism 60, 1598–1609. https://doi.org/10.1016/j.metabol.2011.04.002 (2011).
Niu, W., Wang, H., Wang, B., Mao, X. & Du, M. Resveratrol improves muscle regeneration in obese mice through enhancing mitochondrial biogenesis. J Nutr Biochem 98, 108804. https://doi.org/10.1016/j.jnutbio.2021.108804 (2021).
Cheng, K. et al. Effects of early resveratrol intervention on skeletal muscle mitochondrial function and redox status in neonatal piglets with or without intrauterine growth retardation. Oxid. Med. Cell Longev. 2020, 4858975. https://doi.org/10.1155/2020/4858975 (2020).
Wang, D. et al. Resveratrol improves muscle atrophy by modulating mitochondrial quality control in STZ-induced diabetic mice. Mol Nutr Food Res 62, e1700941. https://doi.org/10.1002/mnfr.201700941 (2018).
Zheng, J. et al. Resveratrol improves insulin resistance of catch-up growth by increasing mitochondrial complexes and antioxidant function in skeletal muscle. Metabolism 61, 954–965. https://doi.org/10.1016/j.metabol.2011.11.005 (2012).
Attaluri, S. et al. Oral nano-curcumin in a model of chronic Gulf War illness alleviates brain dysfunction with modulation of oxidative stress, mitochondrial function, neuroinflammation, neurogenesis, and gene expression. Aging Dis. 13, 583–613. https://doi.org/10.14336/ad.2021.0829 (2022).
Haines, D. D., Ottenweller, J. E., Dickens, B. F., Mahmoud, F. F. & Levine, P. H. Activity of paraoxonase/arylesterase and butyrylcholinesterase in peripheral blood of Gulf War era veterans with neurologic symptom complexes or post-traumatic stress disorder. J. Occup. Environ. Med. 59, 1000–1006. https://doi.org/10.1097/JOM.0000000000001129 (2017).
Khaiboullina, S. F. et al. Cytokine expression provides clues to the pathophysiology of Gulf War illness and myalgic encephalomyelitis. Cytokine 72, 1–8. https://doi.org/10.1016/j.cyto.2014.11.019 (2015).
Le, Y., Shen, H., Yang, Z., Lu, D. & Wang, C. Comprehensive analysis of organophosphorus flame retardant-induced mitochondrial abnormalities: Potential role in lipid accumulation. Environ Pollut 274, 116541. https://doi.org/10.1016/j.envpol.2021.116541 (2021).
Farkhondeh, T., Mehrpour, O., Forouzanfar, F., Roshanravan, B. & Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. Int. 27, 24799–24814. https://doi.org/10.1007/s11356-020-09045-z (2020).
Leung, M. C. K. & Meyer, J. N. Mitochondria as a target of organophosphate and carbamate pesticides: Revisiting common mechanisms of action with new approach methodologies. Reprod. Toxicol. 89, 83–92. https://doi.org/10.1016/j.reprotox.2019.07.007 (2019).
U.S. Food and Drug Administration. FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. In: FDA Drug Safety Communications July 26 (2016).
Bleecker, M. L. Carbon monoxide intoxication. Handb. Clin. Neurol. 131, 191–203. https://doi.org/10.1016/b978-0-444-62627-1.00024-x (2015).
Knobeloch, L. & Jackson, R. Recognition of chronic carbon monoxide poisoning. WMJ 98, 26–29 (1999).
Wright, J. Chronic and occult carbon monoxide poisoning: We don’t know what we’re missing. Emerg. Med. J. 19, 386–390. https://doi.org/10.1136/emj.19.5.386 (2002).
Díaz-Resendiz, K. J. G. et al. Loss of mitochondrial membrane potential (ΔΨ(m) ) in leucocytes as post-COVID-19 sequelae. J. Leukoc. Biol. https://doi.org/10.1002/jlb.3ma0322-279rrr (2022).
Stefano, G. B. et al. Editorial: The pathogenesis of long-term neuropsychiatric COVID-19 and the role of microglia, mitochondria, and persistent neuroinflammation: A hypothesis. Med. Sci. Monit. 27, e933015. https://doi.org/10.12659/msm.933015 (2021).
Pozzi, A. COVID-19 and mitochondrial non-coding RNAs: New insights from published data. Front. Physiol. 12, 805005. https://doi.org/10.3389/fphys.2021.805005 (2021).
National Academies of Sciences Engineering and Medicine. An Assessment of Illness in U.S. Government Employees and Their Families at Overseas Embassies (National Academies Press (US) Copyright 2020 by the National Academy of Sciences. All Rights Reserved, 2020).
Stewart, J. B. & Chinnery, P. F. The dynamics of mitochondrial DNA heteroplasmy: Implications for human health and disease. Nat. Rev. Genet. 16, 530–542. https://doi.org/10.1038/nrg3966 (2015).
Zaki, E. A. et al. Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia 29, 719–728. https://doi.org/10.1111/j.1468-2982.2008.01793.x (2009).
Koillinen, H., Jaaskelainen, S. & Koski, K. Mitochondrial disorder underlying headache symptoms. Duodecim 125, 297–300 (2009).
Rosen, N. Headache and mitochondrial disorders. Headache 48, 733–734. https://doi.org/10.1111/j.1526-4610.2008.01118.x (2008).
Koga, Y. & Nataliya, P. Migraine headache and mitochondrial DNA abnormality. Nihon. Rinsho. 63, 1720–1726 (2005).
Wang, Q. et al. Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. Am. J. Med. Genet. A 131, 50–58. https://doi.org/10.1002/ajmg.a.30323 (2004).
Keevil, B. G., Nicholls, S. P. & Kilpatrick, E. S. Evaluation of a latex-enhanced immunoturbidimetric assay for measuring low concentrations of C-reactive protein. Ann. Clin. Biochem. 35(Pt 5), 671–673. https://doi.org/10.1177/000456329803500512 (1998).
Guralnik, J. M. et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 49, M85–M94 (1994).
Golomb, B. A. & Koperski, S. Who becomes weak on statins? Effect modification exposed in a RCT by risk factor compounding. Circulation 127, AP072 (2013).
Shanely, R. A. et al. Human skeletal muscle biopsy procedures using the modified Bergström technique. J. Vis. Exp. https://doi.org/10.3791/51812 (2014).
Schöpf, B. et al. Oxidative phosphorylation and mitochondrial function differ between human prostate tissue and cultured cells. FEBS J. 283, 2181–2196. https://doi.org/10.1111/febs.13733 (2016).
University of Virginia. Sign Test. http://www.fon.hum.uva.nl/Service/CGI-Inline/HTML/Statistics/Sign_Test.html. Access Date 8–28–2019 (2019).
White, H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 48, 817–838 (1980).
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46 (1990).
Acknowledgements
This study was funded by the Department of Defense Congressionally Directed Medical Research Programs (award #GW140045). Dr. Patel was also funded by a Research Career Scientist award from the Veterans Administration (award #BX005229). The study sponsors had no involvement in study design; collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit for publication. We thank the nurses at the Altman Clinical Translational Research Institute where biopsies were conducted. We thank the following individuals for their assistance with aspects of this study: Janis Ritchie BSN (IRB submissions, staff oversight, administrative and logistical support); Alexis Messner (now RN, study set-up); Alexander Fung (now DDS, conducted initial study visits); Arthur Pavlovsky BS (assistance with study visits); Eero Dinkeloo MPH (some early statistical analyses); Jun Hee Han BS (data validation and editorial assistance); and Franklin Tran BS (validation of table entries against statistical output). We gratefully thank our Gulf War veteran participants and the healthy controls for their kind participation in this study.
Author information
Authors and Affiliations
Contributions
B.A.G. designed research, performed research, analyzed data and wrote the paper. R.S. performed research (screened prospective participants, scheduled and ran study visits). J.M.S., M.D. and M.J.F. performed research (conducted mitochondrial assessments under the supervision of co-I Patel). B.K.B. analyzed data, and modified the manuscript to address journal requirements. B.J.M. analyzed data, managed the database and oversaw duplicate data entry. P.R.T. performed research (conducted muscle biopsies). H.P. performed research (designed and oversaw all aspects of mitochondrial assessments). All authors reviewed the paper for critical intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Golomb, B.A., Sanchez Baez, R., Schilling, J.M. et al. Mitochondrial impairment but not peripheral inflammation predicts greater Gulf War illness severity. Sci Rep 13, 10739 (2023). https://doi.org/10.1038/s41598-023-35896-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35896-w
This article is cited by
-
Bioenergetic impairment in Gulf War illness assessed via 31P-MRS
Scientific Reports (2024)
-
Susceptibility to radiation adverse effects in veterans with Gulf War illness and healthy civilians
Scientific Reports (2024)
-
The effect of disease misclassification on the ability to detect a gene-environment interaction: implications of the specificity of case definitions for research on Gulf War illness
BMC Medical Research Methodology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.