Abstract
Immune reconstitution after hematopoietic stem cell transplantation (HSCT) is a complex and extremely variable process. The Ikaros transcription factor plays an important role in hematopoiesis in several cell lines, especially in the lymphoid lineage. We hypothesized that Ikaros might influence immune reconstitution, and consequently, the risk of opportunistic infections, relapse, and graft versus host disease (GVHD). Samples were collected from the graft and from the peripheral blood (PB) of the recipients 3 weeks after neutrophil recovery. Real-time polymerase chain reaction (RT-PCR) was performed to analyze the absolute and relative Ikaros expression. Patients were divided into two groups, according to Ikaros expression in the graft and in the recipients’ PB based on the ROC curves for moderate/severe cGVHD. A cutoff of 1.48 was used for Ikaros expression in the graft, and a cutoff of 0.79 was used for Ikaros expression in the recipients’ PB. Sixty-six patients were included in this study. Median age of patients was 52 years (range 16–80 years), 55% of them were male, and 58% of them had acute leukemia. Median follow-up period was 18 months (range 10–43 months). There was no association between Ikaros expression and the risk of acute GVHD, relapse, or mortality. However, a significant association was observed with the risk of chronic GVHD. Higher Ikaros expression in the graft was associated with a significantly higher cumulative incidence (CI) of moderate/severe chronic GVHD according to the National Institute of Health (NIH) classification at two years (54% vs. 15% for patients with lower expression, P = 0.03). A higher Ikaros expression in the recipients’ PB 3 weeks after engraftment was also associated with a significantly higher risk of moderate/severe chronic GVHD (65% vs. 11%, respectively, P = 0.005). In conclusion, Ikaros expression in the graft and in the recipients’ PB after transplantation was associated with a higher risk of moderate/severe chronic GVHD. Ikaros expression should be evaluated in larger prospective trials as a potential biomarker for chronic GVHD.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative therapy approach for several malignant and non-malignant diseases and, in some cases, the only with curative intent1. Unfortunately, non-relapse mortality is still very high, mainly due to infections and acute and chronic graft-versus-host disease (aGVHD and cGVHD, respectively)2,3. cGVHD can affect up to 50% of patients and is also responsible for significant comorbidities and low quality of life after HSCT4. The diagnosis of chronic GVHD is based on specific clinical features, although not all patients exhibit these signs and symptoms, and other nonspecific features may be the main manifestation. In doubtful cases, there are only a few laboratory tests that may be useful for diagnosis5. Several biomarkers have been studied to help establish and predict diagnosis and prognosis of cGVHD; however, to date, no biomarkers have been validated for clinical practice6,7.
Ikaros transcription factor could be a good candidate as a prognostic biomarker for the risk of cGVHD. Ikaros is a member of a family of zinc finger transcription factors encoded by IKZF1 gene. It is an essential regulator of hematopoiesis8, with an important role in T and B cell differentiation and their mature cell function9,10,11, as well as in cells of the myeloid lineage, in erythroid and neutrophil differentiation12,13.
IKZF1 haploinsufficiency due to germline mutations can be responsible for common variable immunodeficiency with a decrease in B cell lymphocytes, but can also lead to a more pronounced immunodeficiency with low eosinophils, neutrophils, and myeloid dendritic cells, and a dysfunction in T cells and monocytes14,15. As an essential hematopoietic transcription factor implicated in lymphocyte and myeloid differentiation, IKZF1 activity may be a critical component in immune reconstitution and in acute and cGVHD pathophysiology. To our knowledge, IKZF1 expression has not yet been studied in the context of HSCT. In the present study, we explored whether IKZF1 expression in mononuclear cells in the graft and in the recipients’ peripheral blood (PB) after engraftment could be associated with the risk of aGVHD or cGVHD.
Patients and methods
Study population
This was a non-interventional prospective study that included patients older than 16 years who underwent allogeneic HSCT between January 2017 and January 2020 in two transplant centers, Hospital Sirio-Libanes and Hospital Sao Paulo, both in Sao Paulo, Brazil. Conditioning regimen, graft source, GVHD prophylaxis, time to transplantation, and all other clinical decisions were made according to each center’s guidelines. Prophylaxis, diagnosis and treatment of GVHD are based on established consensus and do not differ between the two centers.
All subjects provided written informed consent prior to enrollment. The study was conducted in accordance with the Declaration of Helsinki and was approved by the research ethics committees of each center (CEP-UNIFESP of Hospital Sao Paulo and CEPesq/HSL of Hospital Sirio-Libanes).
Sample preparation and analysis
All blood samples were prospectively collected. Samples were taken from the graft immediately before infusion on the day of the transplant (graft) and from the recipients’ PB 3 weeks after engraftment (engraftment + 21). Initially, our goal was to identify a readily detectable and cost-effective biomarker that could have practical applications in our country of origin where cost is a common practical limitation. In prior studies conducted by our team, we found that a three-week post-transplantation period was adequate to identify immune reconstitution, which strongly correlated with transplant outcomes16,17. We observed stronger correlations between the analyzed biomarkers and the outcomes from samples obtained 3 weeks after transplant compared to samples obtained at other post-transplant time-points.
Mononuclear cells were separated and stored according to institutional guidelines. Briefly, the collected material was immediately sent for freezing at the laboratory of the Institute of Education and Research at Hospital Sírio-Libanês. All collected material was individually processed to extract peripheral blood mononuclear cells (PBMCs) from each sample. Using Ficoll-Paque (SigmaAldrich, Darmstadt, Germany) as the separation gradient, the samples were centrifuged to form a buffy coat layer above the gradient. The cells in the buffy coat were separated at room temperature using a sterile pipette and subjected to three cycles of washing and resuspension in sterile phosphate buffered saline (PBS), with centrifugation between washes. The material from each patient was then preserved in a fetal bovine serum solution with 10% dimethyl sulfoxide (DMSO), stored in vials, and gradually frozen in a glycerol box at − 20 °C and subsequently at − 80 °C. Finally, they were stored in a liquid nitrogen tank until used in all subsequent tests. Ikaros expression was measured using real-time PCR. Total RNA was extracted from mononuclear cells using PureLink™ Micro (Thermo Fisher Scientific, Waltham, MA) or llustra RNAspin Mini (GE Healthcare Life Sciences, Chicago, IL) reagent and cDNA transcripts were quantified using the Superscript III Cells Direct cDNA (Life Technologies, Carlsbad, CA) kit. Reactions were amplified using a 7500 Fast Real-Time PCR System (Life Technologies, Carlsbad, CA) using TaqMan probes (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer’s instructions. The value of 2−ΔΔCt was used to calculate the fold change in gene expression, according to the studies of Schmittgen et al. and Vandesompele et al.18,19.
Endpoint definitions and statistical analysis
The primary endpoint was the correlation between Ikaros expression and the incidence and severity of cGVHD. Secondary endpoints include the correlation between Ikaros expression and the incidence and severity of aGVHD, as well as overall survival (OS), progression-free survival (PFS), relapse incidence (RI), and non-relapse mortality (NRM). The severity of aGVHD was graded based on the Mount Sinai Acute GVHD International Consortium20, while cGVHD was scored as mild, moderate, or severe according to NIH standards5. Patients’ comorbidities and disease risk were classified as previously published21,22.
The median Ikaros relative expression and receiver operating characteristic (ROC) curves were used to divide patients into two groups based on high or low Ikaros expression levels in both the graft and the PB after engraftment, and to correlate these results with the presence of aGVHD and cGVHD.
PFS and OS probabilities were calculated using the Kaplan–Meier method and compared using the log-rank test. Cumulative incidence (CI) rates were calculated for aGVHD, cGVHD, NRM, and relapse/progression, with death considered a competing event. Ninety-five percent confidence intervals (95% CIs) were estimated using the Greenwood formula. Adjusted probabilities for outcomes after transplantation were estimated using the Cox proportional hazards method (PFS and OS) and the Fine-Gray risk regression model (aGVHD, cGVHD, NRM, and relapse/progression). The statistical analyses were performed using SPSS version 20 (SPSS Inc., Chicago, IL), R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria, 2023; https://www.R-project.org/), and RStudio version 2023.03.0 + 386 'Cherry Blossom' (RStudio, PBC, Boston, MA; http://www.rstudio.com/).
Results
Patients and graft demographics
Between January 2017 and January 2020, 95 patients underwent allogeneic HSCT at the two transplant centers. Chimerism data were evaluated during first 3 months after HSCT. Full donor chimerism was defined as the presence of more than 95% of cells of donor origin. Patients who did not achieve neutrophil recovery or had neutrophil recovery but not full donor chimerism (n = 17), those who died 1 week after engraftment or earlier (n = 10), or those who were lost to follow-up (n = 2) were excluded from the analyses. There were no significant differences between the included and excluded patient groups regarding any of the clinical features (data not shown).
A total of 66 patients were included in final analysis, 43 from Hospital Sirio-Libanes and 23 from Hospital Sao Paulo. The main patient characteristics are presented in Table 1. Among patients included, 55% were male, and the median age at the time of transplant was 52 years (range 16–80 years). The HCT comorbidity index was 0 or 1 in all but four patients. The diagnosis was acute myelogenous leukemia (AML) in 41%, ALL in 17%, MDS/MPN in 20%, lymphoma in 12%, and aplastic anemia in 10% of patients. The disease risk index was low/intermediate in 83% of the patients. Most of the patients (77%) received grafts from mobilized PB stem cells and underwent reduced-intensity conditioning regimens (82%). Donors were haploidentical in 48% of cases, matched related in 29%, and matched unrelated in 23%. GVHD prophylaxis consisted of a regimen containing cyclosporine, mycophenolate mofetil, and post-transplant cyclophosphamide in 58% of cases, and anti-thymocyte globulin was used in 26% of cases. The median follow-up period was 18 months (range 10–43 months).
Median Ikaros relative expression in mononuclear cells in the graft sample was 0.298 (range 0.002–25.683), while median Ikaros relative expression in mononuclear cells from the recipients’ engraftment + 21 samples was 0.073 (range 0.002–1.870).
There was no significant difference in the median of Ikaros relative expression between grafts obtained from bone marrow (0.536) or mobilized PB stem cells (0.298, P = 0.66).
The relative expression of Ikaros in the engraftment + 21 samples was not significantly different between patients who received post-transplant cyclophosphamide (median, 0.081) and those who did not (median: 0.072; p = 0.61). There was also no significant difference in the relative expression of Ikaros in the engraftment + 21 samples of patients who received antithymocyte globulin compared to those who did not (median, 0.085 vs. 0.021, respectively, P = 0.18).
Patients were then divided into two groups, according to Ikaros expression in the graft and in the recipients’ PB based on the ROC curves for moderate/severe cGVHD. A cutoff of 1.48 was used for Ikaros expression in the graft, and a cutoff of 0.79 was used for Ikaros expression in the recipients’ PB.
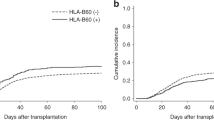
There was no difference in aGVHD and overall cGVHD between groups. However, the CI of moderate/severe cGVHD was significantly higher in patients with higher Ikaros expression in the graft (71%) than in patients with lower expression in the graft (38%, P = 0.04, Fig. 1). In addition, the CI of moderate/severe cGVHD was also significantly higher in patients with higher Ikaros expression in the recipients’ engraftment + 21 samples (68%) than in patients with lower Ikaros expression (24%, P = 0.006, Fig. 2).
Cumulative incidence of moderate/severe cGVHD in patients with higher (dotted line) or lower (solid line) Ikaros expression in the graft. In the multivariate analysis, after correction for source of hematopoietic progenitor cells, use of thymoglobulin, and post-transplant cyclophosphamide, the expression of Ikaros did not remain an independent risk factor.
The median Ikaros relative expression in the engraftment + 21 samples was significantly higher in patients with moderate/severe cGVHD (0.982; range 0.054–1.870) compared to patients without this complication (0.610; range 0.002–1.236, P = 0.004, Fig. 3).
In multivariate analysis, higher Ikaros expression in the graft did not remain statistically associated with an increased risk of moderate/severe cGVHD after adjusting for stem cell source (PB vs. bone marrow), use of ATG or post-transplant cyclophosphamide (hazard ratio = 1.82; 95% confidence interval 0.79–4.19; P = 0.16).
However, higher Ikaros expression in the engraftment + 21 sample remained an independent risk factor for moderate/severe cGVHD (hazard ratio = 2.84; 95% confidence interval 1.10–7.34; P = 0.03), after adjusting for the same covariates.
There was also no significant association between Ikaros expression in the graft or in the recipients’ engraftment + 21 samples for the other analyzed outcomes (OS, PFS, relapse/progression, and NRM).
Discussion
In the present study, we identified that a higher Ikaros expression in mononuclear cells in the recipients’ PB after engraftment is correlated with a higher risk of moderate/severe cGVHD.
Despite all advances in HSCT in recent years, including significant improvements in survival, cGVHD remains the most important cause of long-term morbidity and mortality23. The management of post-transplant immunosuppression is extremely difficult due to the delicate balance between the risks of opportunistic infections and relapse, and the risk of GVHD24. Although several studies have investigated possible biomarkers for cGVHD, none are yet available for daily clinical practice25. Moderate/severe cGVHD, which was associated with a higher Ikaros expression in our study, is generally treated through systemic immunosuppression and carries a high risk of morbidity and mortality. Predicting the risk of moderate/severe cGVHD is of particular interest as a possible biomarker because the possibility of early intervention might be effective in reducing associated long-term morbidity and complications. On the other hand, biomarkers are probably less important for mild cGVHD, since they are not associated with an increased risk of serious complications and are generally associated with the beneficial graft-versus-tumor effect26.
In our study, we did not observe any association between Ikaros expression and the risk of aGVHD, whose pathophysiology differs significantly from that of cGVHD27,28. In aGVHD, there is an initial phase with tissue damage due to the conditioning regimen used and/or the presence of infectious complications, which causes the activation and proliferation of donor T lymphocytes stimulated by antigen-presenting cells, followed by an effector phase dependent on cellular and soluble inflammatory mediators such as TNF-α, IFN-γ, and IL-1 which causes tissue damage and activation of downstream pro-inflammatory pathways27,28. In contrast, cGVHD pathophysiology is characterized by a deficiency in the immune tolerance system, such as T and B cells, involved in chronic inflammatory activity, with subsequent development of fibrosis and, therefore, a wide variety of organs and tissues may be affected24. Some risk factors for cGVHD, such as the source of hematopoietic stem cells29 and the use of anti-thymocyte globulin, are less important for aGVHD30,31. Retrospective studies have shown that haploidentical stem cell transplantation with high-dose cyclophosphamide post-transplant also had lower rates of cGVHD, with no difference in the risk of aGVHD when compared to related and unrelated donors32,33. These data support the idea that graft characteristics and events in the early phase after graft infusion might play an important role in cGVHD, and Ikaros expression might be a significant contributing factor to this pathophysiology.
To the best of our knowledge, this is the first study that has explored the role of Ikaros in immune reconstitution after HSCT. Ikaros is one of the most important transcription factors involved in hematopoiesis regulation8 and it influences differentiation of several cell lines, including lymphoid and myeloid cell lines11,13,14,15,34, with particular importance in B cell lymphoid development8,9.
B cell lymphocytes and the presence of alloreactive antibodies can be an essential part of cGVHD pathophysiology35,36. Female donor-to-male receptor is a well-established risk factor for cGVHD, in part due to antibodies directed against epitopes encoded on the Y chromosome37. In addition, B cell activation factor (BAFF) is generally elevated in cGVHD, resulting in the rescue of autoreactive B lymphocytes, providing them with greater activity38,39,40. Ikaros can contribute to this increase in BAFF-induced B-cell activation. Patients with systemic lupus erythematosus also have elevated levels of BAFF, which leads to greater activation and proliferation of B lymphocytes. An in vitro study showed that the reduction of Aiolos and Ikaros reduced this effect of BAFF41.
In our study, we could not identify the specific cell type responsible for this increased Ikaros expression. Future studies focusing on specific analyses of B cells, CD4 and CD8 T cell lymphocytes, monocytes, dendritic cells, or other cells, could address this topic. On the other hand, the simpler collection and analysis procedures presented in this study could be more easily replicated and even used in clinical practice.
Multivariate analysis confirmed the statistical significance of the engraftment + 21 but not the graft sample. With a small number of patients, it could be hypothesized that the sample size was not sufficient to identify the statistical significance of the graft. It can also be assumed that the graft, which has not yet been exposed to host antigens, may not have sufficient stimulus for the activation of Ikaros. Further studies should be conducted to confirm the relevance of Ikaros expression in the graft.
Among the previously described biomarkers for cGVHD, only a few were collected from the graft and in the early phase after transplantation, as in our study. This characteristic is particularly favorable for decision-making during the management of immunosuppression after HSCT. One of the main risk factors for moderate/severe cGVHD is progression after aGVHD42. In patients with elevated Ikaros expression, aGVHD treatment could be intensified, given that they already have a higher risk of cGVHD. Another possible intervention could be the intensification of prophylactic immunosuppression, such as the addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based prophylaxis, which has been shown to result in lower cGVHD rates without higher relapse rates43. Possibly, with a better stratification of patients’ GVHD risk, we can move from the actual “one size fits all” kind of GVHD prophylaxis to a more personalized strategy. Donor selection may also be improved if the patient has more than one donor available, assuming that the donor's baseline Ikaros expression also influences cGVHD, a strategy that could be investigated in a prospective trial in the future.
Our study has several limitations. Initially, the small number of cases reduces the power of subgroup analysis, as discussed above. Another concern was pre-analytical laboratory errors, as samples were frozen for later analysis. To minimize the possible risk of interfering with Ikaros expression, samples were frozen for the shortest possible time after collection. In addition, the same technician processed the samples both during freezing and in the final analysis. The higher Ikaros expression in the graft than in the PB after engraftment provides evidence that the laboratory analysis is representative of its activity in vivo. The graft is an environment with greater cell proliferation and differentiation and it is expected to have significantly higher Ikaros expression than in the PB after engraftment. Additionally,, while our study cohort is very heterogeneous, it is quite comparable to other case series in the literature, including those analyzing data on cGVHD44. We had a slightly higher number of haploidentical transplants than most of the previous studies, but this seems to be a trend worldwide44. The limited number of each type of transplant including related, unrelated, and haploidentical, prevented a comprehensive subgroup analysis, which should be addressed in future studies. There is also an excessive number of reduced-intensity conditioning regimens, largely explained by an institutional protocol that prospectively analyzed the results of exclusively reduced-intensity conditioning regimens in one of the transplant centers. Additionally, all patients diagnosed with lymphoproliferative diseases, SMD/MPN, or aplastic anemia only received RIC regimens at their physician’s discretion. As we had a low number of patients who received myeloablative conditioning regimens, the possible effects of Ikaros expression in this scenario remain unclear.
In conclusion, a higher Ikaros expression in mononuclear cells in the PB after engraftment was significantly correlated with a higher risk of moderate/severe cGVHD, supporting its use as a prognostic biomarker. Further studies should be conducted to confirm these findings and to identify how to incorporate this marker in clinical practice.
Data availability
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
References
Gratwohl, A. et al. Hematopoietic stem cell transplantation: A global perspective. JAMA 303(16), 1617–1624 (2010).
Phelan, R., Arora, M., & Chen, M. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides (2020).
Arai, S. et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: A report from the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transp. 21(2), 266–274 (2015).
Wolff, D. et al. Post-transplant multimorbidity index and quality of life in patients with chronic graft-versus-host disease-results from a joint evaluation of a prospective German multicenter validation trial and a cohort from the National Institutes of Health. Bone Marrow Transp. 56(1), 243–256 (2021).
Jagasia M.H., et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transp. 21(3), 389–401.e1 (2015).
Paczesny, S., et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. The 2014 biomarker working group report. Biol. Blood Marrow Transp. 21(5), 780–792 (2015).
Wolff, D. et al. Biomarkers in chronic graft-versus-host disease: quo vadis?. Bone Marrow Transplant. 53(7), 832–837 (2018).
Heizmann, B., Kastner, P. & Chan, S. The Ikaros family in lymphocyte development. Curr. Opin. Immunol. 51, 14–23 (2018).
Georgopoulos, K. et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell 79(1), 143–156 (1994).
Molnár, A. et al. The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse. J. Immunol. 156(2), 585–592 (1996).
Kirstetter, P. et al. Ikaros is critical for B cell differentiation and function. Eur. J. Immunol. 32(3), 720–730 (2002).
Nichogiannopoulou, A., Trevisan, M., Friedrich, C. & Georgopoulos, K. Ikaros in hemopoietic lineage determination and homeostasis. Semin. Immunol. 10(2), 119–125 (1998).
Dumortier, A., Kirstetter, P., Kastner, P. & Chan, S. Ikaros regulates neutrophil differentiation. Blood 101(6), 2219–2226 (2003).
Kuehn, H. S. et al. Loss of B cells in patients with heterozygous mutations in IKAROS. N. Engl. J. Med. 374(11), 1032–1043 (2016).
Boutboul, D. et al. Dominant-negative IKZF1 mutations cause a T, B, and myeloid cell combined immunodeficiency. J. Clin. Invest. 128(7), 3071–3087 (2018).
Gonçalves, M. V. et al. Low counts of plasmacytoid dendritic cells after engraftment are associated with high early mortality after allogeneic stem cell transplantation. Biol. Blood Marrow Transp. 21(7), 1223–1229 (2015).
Carneiro T.X., et al. Circulating microparticles as predictive biomarkers of acute graft versus host disease. In: American Society of Hematology Annual Meeting, 2016, San Diego. 58th ASH Annual Meeting Abstracts, v. 128. p. 5778 (2016).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3(6), 1101–1108 (2008).
Vandesompele J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3(7), RESEARCH0034 (2002).
Sorror, M. L. et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 106(8), 2912–2919 (2005).
Armand, P. et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 123(23), 3664–3671 (2014).
Schoemans H.M. et al. EBMT (European Society for Blood and Marrow Transplantation) Transplant Complications Working Party and the “EBMT−NIH (National Institutes of Health)−CIBMTR (Center for International Blood and Marrow Transplant Research) GvHD Task Force”. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transp. 53(11), 1401–1415 (2018).
Jamil, M. O. & Mineishi, S. State-of-the-art acute and chronic GVHD treatment. Int. J. Hematol. 101(5), 452–466 (2015).
Cooke, K. R. et al. The biology of chronic graft-versus-host disease: A task force report from the national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol. Blood Marrow Transp. 23(2), 211–234 (2017).
Ren, H. G., Adom, D. & Paczesny, S. The search for drug-targetable diagnostic, prognostic and predictive biomarkers in chronic graft-versus-host disease. Expert. Rev. Clin. Immunol. 14(5), 389–404 (2018).
Grube, M. et al. Risk factors and outcome of chronic graft-versus-host disease after allogeneic stem cell transplantation-results from a single-center observational study. Biol. Blood Marrow Transp. 22(10), 1781–1791 (2016).
Teshima, T., Reddy, P. & Zeiser, R. Acute graft-versus-host disease: novel biological insights. Biol. Blood Marrow Transp. 22(1), 11–16 (2016).
Zeiser, R. Advances in understanding the pathogenesis of graft-versus-host disease. Br. J. Haematol. 187(5), 563–572 (2019).
Anasetti, C. et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N. Engl. J. Med. 367(16), 1487–1496 (2012).
Finke, J. et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 10(9), 855–864 (2009).
Rubio, M. T. et al. Impact of in vivo T cell depletion in HLA-identical allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission conditioned with a fludarabine Iv-busulfan myeloablative regimen: A report from the EBMT acute leukemia working party. J. Hematol. Oncol. 10(1), 31 (2017).
Kanate, A. S. et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood 127(7), 938–947 (2016).
Ahmed, S. et al. Lower graft-versus-host disease and relapse risk in post-transplant cyclophosphamide-based haploidentical versus matched sibling donor reduced-intensity conditioning transplant for Hodgkin lymphoma. Biol. Blood Marrow Transp. 25(9), 1859–1868 (2019).
Wong, L. Y., Hatfield, J. K. & Brown, M. A. Ikaros sets the potential for Th17 lineage gene expression through effects on chromatin state in early T cell development. J. Biol. Chem. 288(49), 35170–35179 (2013).
Schultz, K. R., Paquet, J., Bader, S. & HayGlass, K. T. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transp. 16(2), 289–295 (1995).
Patriarca, F. et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp. Hematol. 34(3), 389–396 (2006).
Nakasone, H. et al. Allogeneic HY antibodies detected 3 months after female-to-male HCT predict chronic GVHD and non relapse mortality in humans. Blood 125(20), 3193–3201 (2015).
Allen, J. L. et al. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood 120(12), 2529–2536 (2012).
Allen, J. L. et al. Increased BCR responsiveness in B cells from patients with chronic GVHD. Blood 123(13), 2108–2115 (2014).
Sarantopoulos, S. et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin. Cancer Res. 13(20), 6107–6114 (2007).
Nakayama, Y. et al. Aiolos overexpression in systemic lupus erythematosus B cell subtypes and BAFF-induced memory B cell differentiation are reduced by CC-220 modulation of Cereblon activity. J. Immunol. 199(7), 2388–2407 (2017).
Kuzmina, Z. et al. Significantly worse survival of patients with NIH-defined chronic graft-versus-host disease and thrombocytopenia or progressive onset type: Results of a prospective study. Leukemia 26(4), 746–756 (2012).
Sandmaier, B. M. et al. Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: A multicentre, randomised, phase 3 trial. Lancet Haematol. 6(8), e409–e418 (2019).
D’Souza, A., Lee, S., Zhu, X. & Pasquini, M. Current use and trends in hematopoietic cell transplantation in the United States. Biol. Blood Marrow Transp. 23(9), 1417–1421 (2017).
Funding
Funding was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES).
Author information
Authors and Affiliations
Contributions
A.D.P., V.C.M. and C.A.A.R. wrote the main manuscript text. A.R.B.M.F., L.T., Y.N. and C.A.A.R. were the physicians that treated all patients included and helped in the data collection. M.S.P., A.F.P. did all sample analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira, A.D., de Molla, V.C., Fonseca, A.R.B.M. et al. Ikaros expression is associated with an increased risk of chronic graft-versus-host disease. Sci Rep 13, 8458 (2023). https://doi.org/10.1038/s41598-023-35609-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35609-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.