Abstract
In patients with type 2 diabetes mellitus (T2DM) arterial stiffness is associated with increased cardiovascular and total mortality. Little is known about determinants of arterial stiffness in clinical routine. Identification of potential determinants of arterial stiffness will help to address treatment targets for patients in the early state of T2DM. This is a cross-sectional analysis of arterial stiffness in 266 patients in the early stage of T2DM who did not have cardiovascular or renal complications. Parameters of arterial stiffness such as central systolic blood pressure (cSBP), central pulse pressure (cPP) and pulse wave velocity (PWV) were measured with the SphygmoCor System (AtCor Medical). We investigated the influence of parameters of glucose metabolism, lipid status, body constitution, blood pressure (BP) and inflammation on the stiffness parameters using multivariate regression analysis. The study cohort consisted of male and female patients aged 61 ± 8 years with mean diabetes duration of 6.4 ± 5.1 years, mean HbA1c 7.1 ± 0.9%, mean cSBP 121 ± 12 mmHg, mean cPP 44 ± 10 mmHg and mean PWV 8.9 ± 1.8 m/s. Multiple regression analysis identified waist circumference (WC) (beta = 0.411, p = 0.026), LDL-cholesterol (beta = 0.106, p = 0.006), systolic office BP (beta = 0.936, p < 0.001) and diabetes duration (beta = 0.233, p = 0.043) as potential determinants of cSBP. cPP was determined by sex (beta = 0.330, p = 0.008), age (beta = 0.383, p < 0.001), systolic office BP (beta = 0.370, p < 0.001) and diabetes duration (beta = 0.231, p = 0.028) whereas for PWV the following determinants could be identified: age (beta = 0.405, p < 0.001), systolic office BP (beta = 0.421, p < 0.001) and diabetes duration (beta = 0.073, p = 0.038). In addition to the known parameters age, sex and systolic office BP serum LDL-cholesterol, WC and diabetes duration have been identified as determinants of arterial stiffness in patients with T2DM. Treatment of patients in the early stage of T2DM should focus on these clinical parameters to prevent progression of arterial stiffness and as a consequence reduce cardiovascular mortality.
Trial registration: The patients included in the analysis participated in one of the following clinical trials NCT02752113 (registered 26.4.2016), NCT02383238 (09.03.2015), NCT02471963 (15.06.2015), NCT01319357 (21.03.2011) (http://www.clinicaltrials.gov).
Similar content being viewed by others
Introduction
Vascular changes represent a key prognostic factor in patients with type 2 diabetes mellitus (T2DM). The idea of palpating the wrist, a time honored tradition in medicine, goes back to antiquity and was used for centuries to estimate the health of the circulation1. In the past decades several devices have been developed and are clinically used to estimate arterial stiffness in humans1. It has been recognized in the 2018 ESC/ESH guidelines on the management of arterial hypertension, that arterial stiffness, measured using pulse wave velocity (PWV) and pulse wave analysis (PWA) is an established intermediate surrogate endpoint of vascular dysfunction2. The effect of diabetes on arterial stiffness has been shown to equal in its magnitude 6–15 years of chronological aging on vessels3,4. In patients with T2DM arterial stiffness has been shown to predict cardiovascular events independent of traditional risk factors such as glycemic control and 24 h ambulatory blood pressure (BP)5. Moreover, arterial stiffness has been repeatedly shown to predict cardiovascular and all-cause mortality in patients with high cardiovascular risk profile6 and especially in patients with T2DM7,8,9. The REBOUND study, a large multicenter prospective observational cohort study with a median follow-up of 8.6 years, demonstrated that increased arterial stiffness measured by PWV predicts the risk of cardiovascular and all-cause mortality in patients with T2DM9.
However, the precise pathological mechanism how T2DM increases vascular stiffness in still unknown. Previous data suggest that increased arterial stiffness provides a potential mechanistic link between vascular changes and the development of diastolic dysfunction, a typical target organ damage in T2DM1. Arterial stiffness is known to cause premature wave reflection leading to increased mid-to-late systolic load and thus left ventricular diastolic dysfunction10. The following parameters determining arterial stiffness have been previously investigated: (1) Age: Several animal and human studies demonstrated that large elastic arteries, such as the aorta, show increases in arterial stiffness with aging, which correlates with histological and biochemical changes within the arterial wall4. (2) High blood pressure (BP): High BP is known to cause vascular damage and elastin fragmentation, which leads to increased arterial stiffness4. (3) Sex: Interestingly, vascular stiffening is sexually dimorphic. Whereas the age-related increases in vascular stiffness are less in women before menopause than those in age-matched men, after menopause woman show a greater increase in vascular stiffness compared to men11. Besides age, sex and BP other mechanisms have been proposed to mediate vascular stiffness especially in patients with T2DM: Non-enzymatic advanced glycation of proteins (AGEs), increased oxidative stress, increased incidence of atherosclerosis and displayed endothelial dysfunction. In summary, the current literature identified age, sex and systolic BP as determinants of arterial stiffness. However, there is a lack of analysis of further treatment target parameters related to vascular stiffness in patients with T2DM such as parameters of body shape [BMI, waist circumference (WC)] glucose control (HbA1c), blood lipids (serum LDL-cholesterol, serum triglycerides, serum Non-HDL-cholesterol), parameters of inflammation (hsCRP) and diabetes duration. Identification of determinants of arterial stiffness might help to address further treatment targets in patients in the early stage of T2DM. In addition, patients with yet unknown very high cardiovascular risk might be identified. Thus, treatment might be initiated earlier and the progression of arterial stiffness and as a consequence cardiovascular mortality reduced. Our cross-sectional analysis explores these potential determinants of arterial stiffness in a cohort of 266 patients in the early stage of T2DM.
Methods
Study design
This is a cross-sectional analysis of baseline parameters of patients in the early state of T2DM, who participated in one of the following randomized clinical trials (http://www.clinicaltrials.gov) during 2005–2021: “Effects of Empagliflozin + Linagliptin vs Metformin + Insulin Glargine on Renal and Vascular Changes in Type 2 Diabetes (ELMI)” (NCT02752113), “Effect of Dapagliflozin on Microvascular and Macrovascular Circulation and Total Body Sodium Content (Dapa)” (NCT02383238), “Effect of Empagliflozin on Macrovascular and Microvascular Circulation and on Endothelium Function (EMPA)” (NCT02471963), “Effects of Saxagliptin on Endothelial Function (ESENDI)” (NCT01319357). The clinical trials were conducted at the Clinical Research Centre of the Department of Nephrology and Hypertension, University Hospital Erlangen-Nuremberg, Germany (www.crc-erlangen.de). The patients were recruited from the University outpatient clinic, local newspaper advertisement and referring physicians. The study protocols have been approved by the local Ethics Committee of the University of Erlangen-Nuremberg. Before study inclusion written informed consent was obtained from each subject. The studies were conducted according to the principles of good clinical practice guidelines and tenets of the Declaration of Helsinki.
Study population
The study population consisted of patients aged 28–76 years with T2DM and without overt end-organ damage. All patients fulfilled the following criteria: No treatment with insulin, HbA1c < 11% or fasting plasma glucose < 240 mg/dl, eGFR > 50 ml/min/1.73 m2, no cardiovascular event within the last 3 months. 166 out of 266 patients had diagnosed hypertension and 112 out of 266 patients hypercholesterinemia.
Assessment of vascular stiffness
Blood pressure and heart rate were measured according to European Society of Hypertension/European Society of Cardiology guideline recommendations in standard fashion by validated devices in a seated position after 5 min of rest2.
The non‐invasive assessment of central aortic pulse wave and PWV was performed in a quiet temperature-controlled examination room with the patient being in supine position. The SphygmoCor XCEL System (AtCor Medical, Sydney, Australia) was used to assess parameters of vascular stiffness under resting conditions. Details about the system have been previously published12,13,14. In brief, the system is a validated highly reliable method with coefficient of variation below 10%, which allows the calculation of central BP based on brachial artery waveforms15,16. Peripheral brachial BP is measured with a conventional brachial oscillometric device. The device records the volumetric displacement related to the volume of the brachial artery within the cuff around the upper arm. Afterwards the system calculates the central aortic pressure wave from the peripheral signal by a validated transfer function17. This allows the measurement of the parameters cSBP and cDBP, cPP, augmentation pressure, augmentation index (normalized to a heart rate of 75 beats per minute), forward and reflected pressure pulse height.
Carotid-femoral pulse wave velocity (PWV) is the gold-standard to measure arterial stiffness non-invasively. The SpygmoCor XCEL system allows the validated determination of the aortic PWV. The system simultaneously uses the carotid pulse acquired by applanation tonometry and the femoral pulse acquired by a femoral cuff around the upper thigh. The software uses the foot‐to‐foot transit time between carotid and femoral pulse divided by the physical distance measured to calculate the PWV. Details about the method have been previously published18,19.
Vascular parameters under ambulatory conditions (24-h ABPM) such as 24-h cSBP, 24-h augmentation index and 24-h PWV were assessed using the Mobilograph™ (IEM, Stollberg, Germany)20.
Statistical analysis
Normal distribution of data was confirmed by histogram and Kolmogorov–Smirnov test prior to further analysis. Data were expressed as mean ± standard deviation (SD) in text and tables. A two-sided p-value < 0.05 was considered statistically significant.
Determinants of arterial stiffness have been evaluated in three steps. First, multivariate regression analysis was performed including the parameters sex, age, BMI, WC, systolic office BP, HbA1c, serum LDL-cholesterol, serum triglycerides, hsCRP and diabetes duration. Vascular stiffness parameters entered our model as an independent variable. cSBP (office and 24-h ABPM), cPP (office and 24-h ABPM), and PWV (office and 24-h ABPM). A separate multiple regression analysis was performed for each of the six independent variables mentioned. Potential collinearity between the dependent variables in our model has been excluded by calculating correlation coefficients between the dependent variables.
Secondly, the study population has been separated into different groups based on the median of the following parameters: age, BMI, WC, systolic office BP, HbA1c, serum LDL-cholesterol, serum triglycerides, hsCRP and diabetes duration. Then unpaired t-test has been performed for the parameters cSBP (office and 24-h ABPM), cPP (office and 24-h ABPM), and PWV (office and 24-h ABPM), comparing parameters ≥ median to < median of the mentioned parameters.
Thirdly, bivariate correlation analyses were performed using Pearson’s test and partial correlation was used to adjust for confounding parameter age and systolic office BP.
All analyses were performed using IBM SPSS Statistics 22 (SPSS Inc, Chicago, IL/USA).
Ethics approval and consent to participate
The study protocols have been approved by the local Ethics Committee of the University of Erlangen-Nuremberg. Before study inclusion written informed consent was obtained from each subject. The studies were conducted according to the principles of good clinical practice guidelines and tenets of the Declaration of Helsinki.
Results
General characteristics of the study population
The study cohort consisted of male and female patients with mean age of 61 ± 8 years, mean diabetes duration of 6.4 ± 5.1 years and mean HbA1c 7.1 ± 0.9%. 220 out of 266 patients (86.5%) took antidiabetic medication, such as metformin (220 patients, 84.6%), DPP4-I (14 patients, 5.3%) or sulfonylurea (4 patients, 1.5%). Further clinical baseline parameters of the study population are shown in Table 1.
Mean WC and body mass index (BMI) were elevated in the study population (Table 1). 166 out of 266 patients had previously diagnosed hypertension. Antihypertensive medication was frequent: 67 patients took one antihypertensive substance, 49 patients two substances, 36 patients three substances and 14 patients four or more antihypertensive substances. The following classes of antihypertensive substances were represented: ACE-I/ARB: 136 patients (51.1%), diuretics: 65 patients (24.4%), betablocker: 57 patients (21.4%), calcium antagonists: 65 patients (24.4%), aldosterone antagonists: 6 patients (2.3%). Mean BP under office conditions was 132 ± 13/77 ± 7.9 mmHg and under 24-h ambulatory conditions 130 ± 11/79 ± 7.7 mmHg. Parameters of central BP under office and 24-h conditions are shown in Table 1.
112 out of 266 patients had diagnosed hypercholesterinemia, whereas 79 out of the 112 patients with hypercholesterinemia took statins. Mean LDL-cholesterol and mean triglycerides of the study population indicate that dyslipidemia was still present in many patients (Table 1).
Multiple regression analysis
Multiple regression analysis was performed for independent parameters of pulse wave analysis [cSBP (Table 2) and cPP (Table 3)] and for PWV (Table 4) both under office and 24-h ambulatory conditions. The description focuses on modifiable risk factors such as factors determining body shape (BMI, waist circumference), diabetes control (HbA1c, diabetes duration), lipid metabolism (LDL-cholesterol, triglycerides), blood pressure (systolic OBP) and inflammation (hsCRP). Figure 1 shows an overview of the parameters integrated in the multiple regression analysis as dependent parameters. For the full panel of potential determinants calculated please see Tables 2, 3, 4, which also include age and sex as non-modifiable factors.
Beyond age and sex the following parameters emerged as determinants:
-
Pulse wave analysis parameters cSBP and cPP under 24-h ambulatory conditions: diabetes duration, systolic office BP, WC (only for cSBP), serum LDL-cholesterol (only for cSBP) (Tables 2, 3)
-
Pulse wave analysis parameters cSBP and cPP under office conditions: systolic office BP and LDL-cholesterol (only for cSBP) (Tables 2, 3).
-
Pulse wave velocity under 24 h ambulatory conditions: systolic office BP, diabetes duration (Table 4)
-
Pulse wave velocity under office conditions: systolic office BP (Table 4)
Stiffness parameters based on the median of potential determinants
In addition, the study population was divided according to the median of the potential determinants entered in the multiple regression analysis (Table 5). For pulse wave analysis under 24-h ambulatory conditions the following parameters beyond age revealed significant differences between the groups: serum triglycerides and systolic office BP (Table 5A,B). For pulse wave analysis under office conditions only systolic office BP emerged as significant determinant (Table 5A,B). The same was true for pulse wave velocity under 24 h ambulatory conditions as well as office conditions (Table 5C).
Correlations
Age
Patients’ age correlated with cSBP (r = 0.126, p = 0.041), cPP (r = 0.306, p < 0.001) and PWV (r = 0.395, p < 0.001).
BMI
There was a correlation between BMI and cPP (r = 0.127, p = 0.039), which persisted after adjustment for age (r = 0.185, p = 0.003) as well as after adjustment for age and systolic office BP (r = 0.133, p = 0.034) and cSBP (r = 0.078, p = 0.204, after adjustment for age: r = 0.161, p = 0.010), but not PWV (r = 0.027, p = 0.663).
WC
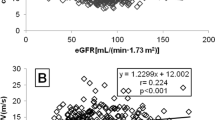
There was a correlation between WC and PWV (r = 0.110, p = 0.080, after adjustment for age: r = 0.145, p = 0.022), but not with cSBP (r = 0.045, p = 0.473) and cPP (r = − 0.028, p = 0.657).
HbA1c
There was no correlation between HbA1c and cSBP (r = 0.045, p = 0.464), cPP (r = 0.055, p = 0.371) and PWV (r = 0.037, p = 0.646).
Diabetes duration
There was a correlation between diabetes duration and PWV (r = 0.326, p < 0.001, which did not persist after adjustment for age (r = 0.085, p = 0.289). No correlation was present between diabetes duration and cSBP (r = 0.048, p = 0.434) as well as cPP (r = 0.101, p = 0.102).
Lipids
There was a correlation between serum LDL-cholesterol and cSBP (r = 0.125, p = 0.045), which persisted after adjustment for age (r = 0.136, p = 0.027) as well as PWV (r = 0.145, p = 0.020), which also persisted after adjustment for age (r = 0.190, p = 0.002) and age and systolic office BP (r = 0.142, p = 0.024)). There was no correlation between LDL-cholesterol and cPP (r = 0.089, p = 0.147). Serum triglyceride concentration correlated with PWV (r = 0.158, p = 0.011, age adjusted r = 0.210, p = 0.001, age and systolic office BP adjusted: r = 0.182, p = 0.004), but there was no correlation with cSBP and cPP. Serum non-HDL-cholesterol correlated with PWV (r = 0.137, p = 0.028, age adjusted r = 0.188, p = 0.003, age and systolic office BP adjusted 0.135, p = 0.032) and cSBP (r = 0.134, p = 0.030, age adjusted r = 0.188, p = 0.026). No correlation was present between non-HDL-cholesterol and cPP (r = 0.071, p = 0.251).
hsCRP
No correlation was present between hsCRP and cSBP (r = 0.157, p = 0.128) as well as cPP (r = 0.100, p = 0.337) and PWV (r = 0.112, p = 0.283).
Systolic office BP
There was a correlation between systolic office BP and cSBP (r = 0.684, p < 0.001, age adjusted: r = 0.698, p < 0.001) as well as cPP (r = 0.502, p < 0.001, age adjusted: r = 0.504, p < 0.001) and PWV (r = 0.404, p < 0.001, age adjusted: r = 0.382, p < 0.001).
Sub-group-analysis in patients with hypercholesterinemia
A sub-group-analysis was performed comparing parameters of arterial stiffness in patients with diagnosed hypercholesterinemia (n = 112) compared to patients without diagnosed hypercholesterinemia (n = 154). There was a significant difference in baseline parameters between both groups: age, LDL-cholesterol, hsCRP (Table 6). No difference was found for the baseline parameters of office BP, heart rate, diabetes duration and eGFR between the groups (Table 6). Patients with T2DM and hypercholesterinemia showed higher PWV (9.1 ± 1.1 m/s) compared to patients without hypercholesterinemia (8.6 ± 1.3 m/s, p = 0.007). However, when adjusting for the baseline parameters the difference in PWV did not remain significant (p = 0.644). There was no difference in cSBP (119 ± 9.7 vs. 120 ± 8.3 mmHg, p = 0.260, padj = 0.272) and cPP (50 ± 8.9 vs. 50 ± 8.6 mmHg, p = 0.759, padj = 0.183) between patients with and without hypercholesterinemia.
Discussion
In our cross-sectional analysis in patients in the early state of T2DM serum LDL-cholesterol, serum triglycerides, non-HDL-cholesterol, waist circumference and diabetes duration emerged as determinants of arterial stiffness besides the known factors age, sex and systolic office BP. Interestingly, the potpourri of determinants differed depending on the parameter indicating arterial stiffness.
Previous studies identified age and sex as important determinants of arterial stiffness4. It is well known that aortic stiffness and arterial pressure are strongly correlated in hypertension with vascular stiffness being both, a cause and a consequence of hypertension21. The discussion will therefore focus on the other identified modifiable determinants such as serum LDL-cholesterol, serum triglycerides, WC and diabetes duration.
Dyslipidemia
Dyslipidemia is a common phenomenon in patients with T2DM22. In patients with T2DM the risk of CV disease is greater at any given level of serum cholesterol and its association with hypertriglyceridemia is stronger than in the general population23. Dyslipidemia is known to impair endothelium-dependent dilation22. Endothelial dysfunction and a reduced contractile response to endothelin-1 trigger vasoconstriction have been previously proposed as important factors contributing to vascular stiffness24. Other studies indicate that formation of atherosclerotic plaques, oxidative stress, local and systemic inflammation and low nitric oxide bioavailability might relate PWV and dyslipidemia25,26.
In a small prospective cohort study in patients with T2DM increased PWV was associated with dyslipidemia27. This is in line with our results. We now also showed that serum LDL-cholesterol and triglyceride levels determine cSBP in patients with T2DM. Interestingly, the diagnosis of hypercholesterinemia did not determine arterial stiffness in our patient population; most likely because the patients with hypercholesterinemia were treated and therefore showed lower LDL-cholesterol compared to the patients without hypercholesterinemia. Patients with T2DM and hypercholesterinemia and/or hypertriglyceridemia even without end-organ damage are known to be at higher CV risk28. In patients with T2DM statin therapy has been shown to prevent atherosclerotic cardiovascular disease events and death from coronary heart disease28. In addition, statin induced improvement of arterial stiffness has been previously shown for PWV in a small cohort of male patients with T2DM29. According to the current guidelines our patient cohort with several risk factors but without overt end-organ damage would qualify for high-intensity statin therapy28. Even though our patients were “taking care of their diabetes” as they all participated in RCTs dealing with optimization of their T2DM treatment, only 79 out of the 112 patients (71%) with hypercholesterinemia took statins.
Waist circumference (WC)
Few data exist on the influence of WC or visceral adiposity on arterial stiffness in patients with T2DM. These data show a relation between WC and PWV in patients with T2DM, but do not investigate a relation between WC and either cSBP or cPP. For example, in a cross-sectional study in patients with T2DM “A Body Shape Index”, which is an indirect measure of visceral adiposity including WC in its calculation, has been shown to be associated with PWV in patients with T2DM30 Our results are in line with these findings. However, there are previous data in a large patient population indicating that the effect of increased BP on arterial stiffness is far greater that the influence of visceral adiposity31.
Diabetes duration
In our analysis antihyperglycemic control as judged with HbA1c did not emerge as determinant of arterial stiffness in patients with T2DM. This is in line with results from a large meta-analysis of 102 prospective studies. The meta-analysis showed that fasting blood glucose concentration did not significantly influence vascular disease prediction when added to information about conventional risk factors4. However, several preclinical and clinical studies propose the T2DM associated formation of advanced glycation end-products (AGEs) as important factor of vascular stiffening independent of age and other cardio-metabolic risk factors7. AGEs form in hyperglycemic environments and decrease arterial wall distensibility. They accumulate in the vessel wall and form cross-links with collagen and elastin fibers32. Formation of AGEs typically increases with diabetes duration4. To our knowledge this is the first time, diabetes duration has been identified as important determinant of arterial stiffness in our patient cohort.
Inflammation
Oxidative stress, is an important mechanism mediating increased vascular stiffness in T2DM and known to activate a number of pro-inflammatory pathways4. However, measurement of inflammatory markers is not part of the clinical routine work up in patients with T2DM. HsCRP can be considered a common parameter with is widely assessed in clinical routine. Thus, this parameter was chosen as surrogate marker for inflammation in our analysis. We did not see any association between parameters of arterial stiffness and hsCRP in our study cohort. This has to be interpreted with caution as hsCRP only reflects overall inflammation. There are other more specific inflammatory parameters such as IL6, which have not been measured in our analysis.
Limitations
Our results are based on a fairly small study cohort of patients in the early stage of T2DM. Further investigations in larger study populations are needed. A relevant proportion of patients in our study cohort were treated for the co-morbidities hypertension and hypercholesterinemia. Even though the medication has been kept stable for at least three months before study inclusion, overlapping effects might have influenced our analysis. Finally, the cross-sectional nature of our analysis does not allow to draw conclusions on causal relations.
Conclusion
In patients in the early state of T2DM arterial stiffness was determined by serum LDL-cholesterol, serum triglycerides, waist circumference and diabetes duration, besides the known factors age, sex and systolic office BP. Treatment of patients in the early stage of T2DM should focus also on these parameters to prevent progression of arterial stiffness and as a consequence cardiovascular mortality.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AGE:
-
Advanced glycation end product
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- cDBP:
-
Central diastolic blood pressure
- cPP:
-
Central pulse pressure
- cSBP:
-
Central systolic blood pressure
- CV:
-
Cardiovascular
- DD:
-
Diabetes duration
- HDL-cholesterol:
-
High density lipoprotein cholesterol
- hsCRP:
-
High sensitive c-reactive protein
- LDL-cholesterol:
-
Low density lipoprotein cholesterol
- PWA:
-
Pulse wave analysis
- PWV:
-
Pulse wave velocity
- TAG:
-
Triglycerids
- T2DM:
-
Type 2 diabetes mellitus
- WC:
-
Waist circumference
References
Budoff, M. J. et al. Clinical applications measuring arterial stiffness: An expert consensus for the application of cardio-ankle vascular index. Am. J. Hypertens. 35(5), 441–453 (2022).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 36(10), 1953–2041 (2018).
Loehr, L. R. et al. Prediabetes and diabetes are associated with arterial stiffness in older adults: The ARIC study. Am. J. Hypertens. 29(9), 1038–1045 (2016).
Vatner, S. F. et al. Vascular stiffness in aging and disease. Front. Physiol. 12, 762437 (2021).
Cardoso, C. R., Ferreira, M. T., Leite, N. C. & Salles, G. F. Prognostic impact of aortic stiffness in high-risk type 2 diabetic patients: The Rio de Janeiro Type 2 Diabetes Cohort Study. Diabetes Care 36(11), 3772–3778 (2013).
Vlachopoulos, C., Aznaouridis, K., Terentes-Printzios, D., Ioakeimidis, N. & Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension 60(2), 556–562 (2012).
Emerging Risk Factors, C., Sarwar, N., Gao, P., Seshasai, S. R., Gobin, R., Kaptoge, S., et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 375(9733), 2215–2222 (2010).
Gaede, P., Lund-Andersen, H., Parving, H. H. & Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 358(6), 580–591 (2008).
Kim, J. M. et al. Arterial stiffness is an independent predictor for risk of mortality in patients with type 2 diabetes mellitus: The REBOUND study. Cardiovasc. Diabetol. 19(1), 143 (2020).
Osawa, K. et al. Correlation of arterial stiffness with left atrial volume index and left ventricular mass index in young adults: Evaluation by coronary computed tomography angiography. Heart Lung Circ. 28(6), 932–938 (2019).
Ogola, B. O. et al. New insights into arterial stiffening: Does sex matter?. Am. J. Physiol. Heart Circ. Physiol. 315(5), H1073–H1087 (2018).
Karamanoglu, M., O’Rourke, M. F., Avolio, A. P. & Kelly, R. P. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur. Heart J. 14(2), 160–167 (1993).
Wilkinson, I. B. et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J. Hypertens. 16(12 Pt 2), 2079–2084 (1998).
Pauca, A. L., O’Rourke, M. F. & Kon, N. D. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 38(4), 932–937 (2001).
Palatini, P. et al. Arterial stiffness, central hemodynamics, and cardiovascular risk in hypertension. Vasc. Health Risk Manag. 7, 725–739 (2011).
Schultz, M. G. et al. Validation study to determine the accuracy of central blood pressure measurement using the Sphygmocor Xcel Cuff device. Hypertension 76(1), 244–250 (2020).
Chilton, R. et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes. Metab. 17(12), 1180–1193 (2015).
Kolwelter, J. et al. Effects of the sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function in patients with chronic heart failure. ESC Heart Fail. 8(6), 5327–5337 (2021).
Striepe, K. et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation 136(12), 1167–1169 (2017).
Weber, T. et al. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension 60(2), 534–541 (2012).
Humphrey, J. D., Harrison, D. G., Figueroa, C. A., Lacolley, P. & Laurent, S. Central artery stiffness in hypertension and aging: A problem with cause and consequence. Circ. Res. 118(3), 379–381 (2016).
Schofield, J. D., Liu, Y., Rao-Balakrishna, P., Malik, R. A. & Soran, H. Diabetes dyslipidemia. Diabetes Ther. 7(2), 203–219 (2016).
Stamler, J., Vaccaro, O., Neaton, J. D. & Wentworth, D. Diabetes, other risk factors, and 12-year cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16(2), 434–444 (1993).
Rizzoni, D. et al. Endothelial dysfunction in small resistance arteries of patients with non-insulin-dependent diabetes mellitus. J. Hypertens. 19(5), 913–919 (2001).
Zieman, S. J., Melenovsky, V. & Kass, D. A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 25(5), 932–943 (2005).
Tolezani, E. C. et al. Determinants of functional and structural properties of large arteries in healthy individuals. Arq. Bras. Cardiol. 103(5), 426–432 (2014).
Monteiro, C. I. et al. Arterial stiffness in type 2 diabetes: Determinants and indication of a discriminative value. Clinics (Sao Paulo). 76, e2172 (2021).
American Diabetes A. 10. Cardiovascular disease and risk management: Standards of medical care in diabetes-2021. Diabetes Care. 44(Suppl 1), S125–S50 (2021).
Davenport, C. et al. The effects of atorvastatin on arterial stiffness in male patients with type 2 diabetes. J. Diabetes Res. 2015, 846807 (2015).
Bouchi, R. et al. Indirect measure of visceral adiposity “A Body Shape Index” (ABSI) is associated with arterial stiffness in patients with type 2 diabetes. BMJ Open Diabetes Res. Care. 4(1), e000188 (2016).
Bouchi, R. et al. Is visceral adiposity a modifier for the impact of blood pressure on arterial stiffness and albuminuria in patients with type 2 diabetes?. Cardiovasc. Diabetol. 15, 10 (2016).
Goldin, A., Beckman, J. A., Schmidt, A. M. & Creager, M. A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 114(6), 597–605 (2006).
Acknowledgements
We gratefully acknowledge the expert technical assistance of Dorothea Bader-Schmieder, Ingrid Fleischmann, Kerstin Fröhlich-Endreß, Sabine Thümmler, Simone Pejkovic, Ulrike Heinritz and Wiebke Maurer.
Funding
We acknowledge financial support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding programme “Open Access Publication Funding”. Open Access funding enabled and organized by Projekt DEAL. None.
Author information
Authors and Affiliations
Contributions
M.St.—analyzed data, wrote the manuscript. C.O.—recruited patients, performed statistical review. D.K.—was responsible for funding, data analysis, statistical review. K.S.—recruited patients, organized funding. M.Sc.—performed internal review, was responsible for funding. R.S.—was responsible for statistical review, recruitment of patients, internal review. A.B.—performed statistical analysis, internal review, was responsible for patient recruitment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Staef, M., Ott, C., Kannenkeril, D. et al. Determinants of arterial stiffness in patients with type 2 diabetes mellitus: a cross sectional analysis. Sci Rep 13, 8944 (2023). https://doi.org/10.1038/s41598-023-35589-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35589-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.