Abstract
The clinical benefits of noninvasive ventilation (NIV) for patients with acute hypoxemic respiratory failure (AHRF) is still inconclusive. We aimed to evaluate the effect of NIV compared with conventional oxygen therapy (COT)/high-flow nasal cannula (HFNC) in this patient population. We searched for relevant studies from PubMed, Embase, Cochrane Library, ClinicalTrials.gov, CINHAL, Web of Science up to August 2019 for randomized controlled trials (RCTs) that compared NIV with COT/HFNC in AHRF. The primary outcome was the tracheal intubation rate. Secondary outcomes were intensive care unit (ICU) mortality, and hospital mortality. We applied the GRADE approach to grade the strength of the evidence. Seventeen RCTs that recruited 1738 patients were included in our meta-analysis. When comparing NIV versus COT/HFNC, the pooled risk ratio (RR) for the tracheal intubation rate was 0.68, 95% confidence interval (CI) 0.52–0.89, p = 0.005, I2 = 72.4%, low certainty of evidence. There were no significant differences in ICU mortality (pooled RR = 0.87, 95% CI 0.60–1.26), p = 0.45, I2 = 64.6%) and hospital mortality (pooled RR = 0.71, 95% CI 0.51–1.00, p = 0.05, I2 = 27.4%). Subgroup analysis revealed that NIV application with helmet was significantly associated with a lower intubation rate than NIV with face mask. NIV did not show a significant reduction in intubation rate compared to HFNC. In conclusion, NIV application in patients with medical illness and AHRF was associated with a lower risk of tracheal intubation compared to COT. NIV with helmet and HFNC are promising strategies to avoid tracheal intubation in this patient population and warrant further studies. NIV application had no effect on mortality.
The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42018087342).
Similar content being viewed by others
Introduction
Noninvasive ventilation (NIV) refers to the delivery of mechanical ventilation with techniques that do not require an invasive endotracheal airway1. NIV has been increasingly used worldwide. Currently, it is the first-line preferred therapy for acute on chronic ventilatory failure, mainly for exacerbations of chronic obstructive pulmonary disease (COPD). However, the use of NIV in the treatment of acute hypoxemic respiratory failure (AHRF) remains controversial. AHRF is a major problem in acute care settings in adult patients, often leading to endotracheal intubation and invasive mechanical ventilation. The hallmark of AHRF is severe hypoxemia (PaO2/FiO2 ≤ 300 mmHg) that requires high levels of oxygen and is accompanied by clinical signs of respiratory distress. The clinical evidence supporting the use of NIV in this condition have yielded conflicting results. NIV may improve oxygenation, facilitate ventilation, reduce work of breathing and dyspnea, avoid intubation, and reduce complications associated with invasive mechanical ventilation2. An important concern of using NIV for the indication of AHRF is the delay in endotracheal intubation that leads to higher mortality in patients with NIV failure3. Furthermore, concerns about the safety of NIV for patients with AHRF have recently been raised based on possible associations with higher tidal volumes leading to perpetuating lung injury in patients with high respiratory effort and increased mortality in some studies4,5,6. Recently, high-flow nasal cannula (HFNC) therapy has been shown to offer several advantages compared with NIV, including easier application and better patient tolerance7,8. Despite all these concerns, recent epidemiological data show that NIV is routinely used in patients with hypoxemic ARF and can be applied as first-line ventilatory support in 15–30% of them9,10,11.
Because of the controversy regarding the effectiveness of NIV in patients with AHRF, the safety concern of NIV, and the arrival of HFNC, we conducted a systematic review and meta-analysis of published randomized controlled trials (RCTs) to examine the effects of NIV on the tracheal intubation rate and mortality compared to conventional oxygen therapy (COT: oxygen cannula, oxygen face mask, or venturi face mask) and/or HFNC in adult patients with AHRF.
Methods
This review was carried out according to the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) and the Patient, Intervention, Comparison, and Outcome (PICO) model was used. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42018087342). Institutional review board approval was not required because all study data had been previously published and this study did not include individual patient data.
Search strategy
Information sources
We searched PubMed, Embase, the Cochrane Library, ClinicalTrials.gov, CINAHL, and Web of Science using the following keywords (“noninvasive ventilation” OR “NIV” OR “noninvasive positive pressure ventilation” OR “NIPPV” OR “continuous positive airway pressure” OR “CPAP” or “bilevel positive airway pressure” OR “BiPAP”) AND (“acute lung injury” OR “ALI” OR “acute respiratory distress syndrome” OR “ARDS” OR “acute hypoxemic respiratory failure” OR “AHRF”. Studies from January 1995 to August 2019 were retrieved. In addition, references from retrieved papers were checked for additional studies. Data from the full published English paper were collected.
Eligibility criteria
Inclusion criteria were (1) trials conducted in adult age ≥ 18 years, with AHRF; (2) studies comparing NIV with COT and/or HFNC; (3) the outcomes included tracheal intubation rate, ICU mortality, or hospital mortality; (4) full text of the RCTs was available and published in English language; (5) for the results of a study published more than once, only those with the most complete and up-to-date information will be included in the analysis, and; (6) for RCTs with a mixed population of hypercapnic and nonhypercapnic patients, only data from the nonhypercapnic group were retrieved.
Exclusion criteria were (1) participants were children or adolescents (< 18 years); (2) majority of the participants were COPD or postoperative state, traumatic patients or postextubation state. We exclude these participants because the pathophysiology and indication and outcomes for NIV are different from patients with AHRF; (3) patients with hypercapnia (PaCO2 > 50 mm Hg); (4) the trial did not use COT or HFNC as a control; (5) palliative setting; (6) the study did not include extractable outcomes or mortality data.
Study selection criteria and procedures
To assess eligibity, two authors (P.A. and C.L.) independently reviewed the titles and abstracts generated by the literature search using the keyword terms specified above. Eligible studies were retrieved using the stated inclusion and exclusion criteria. Any discrepancies between the two authors were discussed among all four authors. The selection results from the duplicate review of articles were compared to ensure that all relevant articles were retrieved. The most complete and up-to-date information was included in the meta-analysis.
Data abstraction
A data collection form in Excel was used to gather all the information we need from selected studies. The form was discussed and approved by all investigators. Data were extracted independently in duplicate. The results of duplicate data collection were compared. Discrepancies in data collection between the two authors were resolved by discussion and consensus. The following data on the characteristics of the trial and its participants were collected: general information about the study (e.g. title, name of the trial, authors, year of publication), study characteristics (e.g. sample size, study design, randomization, blinding, duration of follow-up, loss of follow-up), intervention information (e.g. type of interface, duration and frequency of treatment for the intervention group and control group), participant demographics (e.g. age, gender, race/ethnicity, disease severity), study outcomes (tracheal intubation rate, ICU mortality, and hospital mortality), comorbid conditions, respiratory parameters, reasons for NIV failure, complications, and method of statistical adjustment. For categorical outcomes, reviewers extracted the number of intubations, ICU death, and hospital death, along with all-cause mortality, corresponding risk ratio, confidence intervals, and P values.
Assessment of study quality
Elements of the Cochrane Collaboration tool to assess risk of bias and PRISMA guidelines were used to examine the quality of the selected studies12. The elements used in this meta-analysis included random sequence generation and allocation concealment (selection bias), blinding of participants, personnel and outcome assessment (performance and detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). If one or more individual domains are assessed as having a high risk of bias, the trial is rated as having a high risk of bias. All domains must have been rated as having a low risk of bias for the overall risk of bias to be classified as low. In cases of unclear risk of bias or mixed assessments of low and unclear risk of bias, the overall score was classified as having an unclear risk of bias. Two authors (P.A. and C.L.) independently assessed the articles and the overall risk of bias was classified and disagreements were resolved by discussion and consensus with all four authors.
The principal summary measures
We examined the relationship between the use of NIV therapy in AHRF patients and the outcomes by first calculating the net effect size. Intubation rate, ICU mortality, and hospital mortality are considered as dichotomous data. We derived the pooled risk ratio (RR) for dichotomous outcomes with 95% confidence intervals (CIs). The statistical significance of the Alpha level was set at 0.05.
Data analysis
Individual RR and 95% CI for intubation rate, ICU mortality, and hospital mortality were included in the meta-analysis. Data for analysis were extracted directly and calculated from the text of each eligible article. As the studies may differ from each other in various ways and the goal of this meta-analysis is to extrapolate the result from this study population to others, the random effects model was used in the analysis. The DerSimonian and Laird random effects model was applied to calculate the pooled RR13. We evaluated the heterogeneity of effect size across studies with the Q statistic14, considering any P-value < 0.1 as evidence of heterogeneity. An I2 value of 25% to 49% is considered a low level of heterogeneity, 50% to 74% a moderate level, and 75% to 100% a high level15,16. Forest plots were constructed to demonstrate the RR and 95% CI for individual studies and the summary effect sizes. Statistical significance for z test was set at the P-value < 0.05. Heterogeneities were further explored with subgroup analysis, and sensitivity analysis. We aimed to further conduct subgroup analysis by immune status (nonimmunocompromised/immunocompromised), interface type (face mask/helmet), and type of oxygen support (COT/HFNC). Sensitivity analysis was performed to assess the robustness of our result using a leave-one-out method. Publication bias was assessed using the visual inspection of funnel plots, and Egger’s weight linear regression17. Statistical analyses were performed using STATA software version 14.0 (Stata Corp LP, College Station, TX).
We applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach in order to grade the strength of the evidence for our primary outcome (tracheal intubation rate). The assessment domains include risk of bias, inconsistency, imprecision, indirectness, and other factors. The strength of evidence is classified as high, moderate, low, or very low18. All authors individually evaluated the strength of the body of evidence with discrepancies resolved through consensus.
Results
Baseline characteristics of the included studies
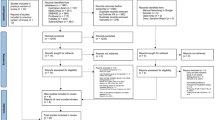
We initially identified 4266 articles. After the duplicate articles were removed, 2722 articles were screened for eligibility criteria. A total of 17 articles8,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34 were included for our systematic review and meta-analysis (Fig. 1).
Most studies compared NIV BiPAP with COT. Two studies use HFNC as control in their studies8,32. The primary outcome in most studies was the intubation rate. Only one study used intubation criteria as a primary outcome. One study used physiologic changes in PaO2/FiO2 as the primary outcome. One study34 had no intubation event or mortality in both groups, therefore it was not included in the forest plot. The baseline characteristics of the included studies are shown in Table1.
Quality assessment
The risk of biases in the overall studies are shown in Fig. 2. Most studies showed a low risk of random sequence generation and allocation concealment biases. However, the nature of the intervention (NIV) could not be blinded to patients or health care personnel. Therefore, there was a considerable risk of detection bias.
Primary outcome: tracheal intubation rate
Seventeen trials studied the differences between the intubation rate of the NIV and COT/HFNC groups. The intubation rate was lower in the NIV group compared to the COT/HFNC group (pooled RR 0.68, 95% CI 0.52–0.89, P = 0.005) (Fig. 3).
Secondary outcome: ICU mortality and hospital mortality
There were no significant differences between the NIV and COT/HFNC groups for ICU mortality (pooled RR 0.87, 95% CI 0.60–1.26, P = 0.45) (Fig. 4) or hospital mortality (pooled RR 0.71, 95% CI 0.51–1.00, P = 0.05) (Fig. 5).
Subgroup analysis
Subgroup analyses were performed to evaluate the causes of heterogeneity. The type of interface significantly caused heterogeneity in the tracheal intubation rate. The NIV application with helmet significantly reduced the intubation rate compared to the full face mask (test of heterogeneity between subgroup Q = 13.85, df = 1, P < 0.001) (Fig. 6). Subgroup analysis by immune status revealed that NIV used in immunocompromised patients led to a significant reduction in the intubation rate (test of heterogeneity between subgroup Q = 3.9, df = 1, P = 0.04) (Fig. S1). There were only two studies in the HFNC subgroup. Compared to HFNC, NIV did not result in a reduction in the intubation rate (Fig. 7).
Subgroup analysis according to interface type (facemask NIV or helmet NIV): intubation rate in acute hypoxemic respiratory failure patients randomized to noninvasive ventilation (NIV) versus conventional oxygen therapy/high-flow nasal cannula (HFNC). Boxes and horizontal lines represent point estimates and 95% confidence intervals, varying in size according to the weight in the analysis.
Subgroup analysis according to type of oxygen therapy [conventional oxygen therapy (COT) or high-flow nasal cannula (HFNC)]: intubation rate in acute hypoxemic respiratory failure patients randomized to NIV versus COT/HFNC. Boxes and horizontal lines represent point estimates and 95% confidence intervals, varying in size according to the weight in the analysis.
Sensitivity analysis
Sensitivity analysis revealed that after removing three RCTs that included some population of hypercapnic respiratory failure21,22,26, the primary outcome remained in the same direction (Fig. S2). NIV reduced the tracheal intubation rate compared to COT/HFNC therapy.
Publication bias
Based on visual inspection of the funnel plot (Fig. S3), there may be some asymmetry, and the Egger linear regression test suggested the existence of publication biases (P = 0.032) in the tracheal intubation rate.
We graded the overall strength of the evidence for the primary outcome (tracheal intubation rate) as low (Table S1).
Discussion
In our systematic review and meta-analysis of 17 randomized trials, including 1738 patients with medical illness and AHRF, NIV therapy significantly reduced the intubation rate compared to COT/HFNC. This result is consistent with previous meta-analyses35,36,37,38,39,40. Of the six previous reviews, three included patients with AHRF of various etiologies, one included only patients with pneumonia, and two included only immunocompromised patients. Our review focused on studies in patients with AHRF, defined as significant hypoxemia with clinical respiratory distress. We excluded patients with hypercapnic respiratory failure, mainly patients with COPD as the pathophysiology and outcome are very different from AHRF. Furthermore, the beneficial effects of NIV to reduce intubation and mortality in patients with COPD are well established. Similarly, we also excluded postoperative patients, traumatic patients and post-extubated patients. Therefore, our analysis included only patients with medical illness with AHRF for whom the role of NIV is most controversial. Patients in the studies included in our analysis predominantly presented with pneumonia, acute respiratory distress syndrome (ARDS), pulmonary edema, and AHRF in immunocompromised patients’.
We did not exclude patients with cardiogenic pulmonary edema as the etiology of AHRF is often unclear at presentation and the decision to start NIV is practically based on clinical respiratory distress together with significant hypoxemia and usually before the definitive diagnosis is known. In addition, cardiogenic pulmonary edema and ARDS may sometimes coexist41. Therefore, with respect to the application of NIV, we consider studies on these patients to be in the same category. This patient population poses a significant challenge within adult acute care settings, often leading to endotracheal intubation and invasive mechanical ventilation. From our analysis, compairing to COT/HFNC, NIV application may reduce the intubation rate in these patients. However, we graded the overall strength of the evidence for a reduction in the tracheal intubation rate as low since all included trials were unblinded due to the nature of the intervention (NIV), and therefore, all were considered to be at high risk of bias. In addition, there was moderate to high heterogeneity in this outcome analysis. Subgroup analysis to identify the source of heterogeneity revealed that NIV with helmet significantly reduced the intubation rate compared to full face mask. The helmet can effectively deliver a higher level of PEEP and the helmet neck seal allows a higher level of airway pressure delivery. This may correspond to a reduction in intubation rate in the helmet group6. Because of this significant difference in the outcome, the future studies regarding NIV should specifically define the type of interface used in the studies.
Among the 17 studies included in this analysis, nine studies reported ICU mortality and nine studies reported hospital mortality. Our analysis revealed that there were no significant difference between NIV and COT/HFNC for both ICU mortality and hospital mortality. Previous meta-analyses have yielded conflicting conclusions on the impact of NIV on mortality in patients with AHRF. Xu et al. showed that NIV significantly reduced hospital mortality35. This meta-analysis retrieved RCTs from inception to 2016 including six studies with 503 patients with AHRF excluding COPD and cardiogenic pulmonary edema. However, this analysis included some studies with nonmedical illness such as postoperative hypoxemia. Huang et al. analyzed immunocompromised patients with ARF, including five studies with 592 patients and reported that early use of NIV could reduce short-term mortality compared to COT36. In contrast, the analysis of Zayed et al. including seven studies with 664 immunocompromised patients, revealed that there were no significant differences between NIV and COT or NIV and HFNC regarding short-term mortality37. Ruzsics et al. analyzed patients with pneumonia-associated respiratory failure, including five studies with 121 patients and revealed that, compared to COT or invasive ventilation, NIV did not significantly reduce hospital mortality, especially if patients with COPD were excluded from the analysis38. Sakuraya et al. performed a network meta-analysis comparing the efficacy of noninvasive ventilation according to ventilation modes with HFNC, standard oxygen therapy (SOT), and IMV in adult patients with AHRF, excluding cardiopulmonary edema, acute exacerbation of COPD, hypercapnia, post-extubation, post-surgical status, trauma. Using SOT as a reference, CPAP was significantly associated with a lower risk of mortality. PSV and HFNC were not associated with a significantly lower risk of mortality39. Ferreyro et al. reported a network meta-analysis in which the efficacy of noninvasive respiratory management strategies was compared with that of SOT among adult patients with AHRF and found that both helmet NIV and facemask NIV were associated with a lower risk of mortality and intubation compared to SOT40. However, when excluding trials that included patients with COPD and/or congestive heart failure, face mask NIV was no longer associated with decreased mortality.
Put together, the effect of NIV on avoiding intubation in patients with AHRF seems to persist in all meta-analysis. However, the effect of NIV on mortality was inconsistent among different analyses. This may be explained by the difference between the patients included in the analyses. AHRF is a consequence of various lung diseases. Although early application of NIV may avoid intubation, the effect on mortality would depend on the balance between the beneficial effect of reducing complications associated with invasive mechanical ventilation, the reversibility and effectiveness of treatment for underlying causes of AHRF, and the well-recognized harmful effect of NIV, that is delayed endotracheal intubation. Furthermore, concerns have recently been raised about the safety of NIV for patients with AHRF based on possible associations with higher tidal volumes leading to perpetuating lung injury in patients with high respiratory effort and increased mortality in some studies4,5,6. Therefore, the effect of NIV on mortality is unpredictable and inconsistent between different studies.
Until recently, almost all studies on NIV compared it with oxygen delivered by COT. Recently, HFNC has been shown to offer several advantages compared to NIV, including promoting secretion drainage, reducing dead space and better tolerance7. Furthermore, in our subgroup analysis, NIV did not significantly reduce the intubation rate compared to HFNC. The use of HFNC might be diluting the benefits of NIV on the intubation rate in this analysis. However, there were only two studies evaluating HFNC in the subgroup analysis. Therefore, uncertainty remains about the effectiveness of this treatment. Further studies are required on the role of HFNC in AHRF compared to NIV.
The strength of the present study is the comprehensive systematic literature search from many databases according to the PRISMA guideline. Furthermore, we included only RCT to minimize internal biases of confounders as they usually occur in observational studies and the appraisal of internal validity was performed using the Cochrane Collaboration tool for assessing risk of bias and the PRISMA guidelines. Our analysis focuses only on patients with medical illness and AHRF and targets clinically important outcomes, including the intubation rate and mortality.
This study has some limitations. First, language restrictions may have contributed to incomplete inclusion of relevant studies. Second, the variation in baseline characteristics, severity of hypoxemia, severity of acute illness of the study population, and intervention protocols between trials may lead to a significant level of heterogeneity with insufficient data to explore relevant subgroup effects. Third, the nature of the intervention could not be blinded and may lead to different thresholds to provide endotracheal intubation, which was the primary outcome of this analysis. Fourth, because of the COVID-19 pandemic, our review and analysis was stopped in August 2019. Some studies regarding the effect of noninvasive respiratory support in patients with COVID-19 were not included in this analysis.
Conclusions
In patients with medical illness and AHRF, NIV application was associated with a lower risk of tracheal intubation compared to COT. NIV with helmet and HFNC are promising strategies to avoid tracheal intubation in this patient population and warrant further studies. NIV application had no effect on mortality.
Data availability
The data and material used for this meta-analysis were obtained from the articles in our list of references. The datasets used in this study are available from the corresponding author upon reasonable request.
Abbreviations
- AHRF:
-
Acute hypoxemic respiratory failure
- CAP:
-
Community-acquired pneumonia
- COT:
-
Conventional oxygen therapy
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- FiO2 :
-
Fraction of inspired oxygen
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- HFNC:
-
High-flow nasal cannula
- NIV:
-
Noninvasive ventilation
- PICO:
-
Patient, Intervention, Comparison, and Outcome
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-analysis
- RCT:
-
Randomized controlled trial
- RR:
-
Risk ratio
- SOT:
-
Standard oxygen therapy
References
Nava, S. & Hill, N. Non-invasive ventilation in acute respiratory failure. Lancet 374(9685), 250–259 (2009).
Rochwerg, B. et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 50(2), 1602426 (2017).
Brochard, L., Lefebvre, J. C., Cordioli, R. L., Akoumianaki, E. & Richard, J. C. Noninvasive ventilation for patients with hypoxemic acute respiratory failure. Semin. Respir. Crit. Care Med. 35(4), 492–500 (2014).
Carteaux, G. et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: Role of tidal volume. Crit. Care Med. 44(2), 282–290 (2016).
Kallet, R. H. & Diaz, J. V. The physiologic effects of noninvasive ventilation. Respir. Care 54(1), 102–115 (2009).
Patel, B. K., Wolfe, K. S., Pohlman, A. S., Hall, J. B. & Kress, J. P. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: A randomized clinical trial. JAMA 315(22), 2435–2441 (2016).
Frat, J. P. et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: An observational pilot study. Respir. Care 60(2), 170–178 (2015).
Frat, J. P. et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 372(23), 2185–2196 (2015).
Esteban, A. et al. Evolution of mechanical ventilation in response to clinical research. Am. J. Respir. Crit. Care Med. 177(2), 170–177 (2008).
Antonelli, M. et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit. Care Med. 35(1), 18–25 (2007).
Demoule, A. et al.; REVA Network (Research Network in Mechanical Ventilation). Changing use of noninvasive ventilation in critically ill patients: Trends over 15 years in francophone countries. Intensive Care Med. 42(1), 82–92 (2016).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials. 45(Pt A), 139–145 (2015).
Huedo-Medina, T. B., Sanchez-Meca, J., Marin-Martinez, F. & Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index?. Psychol. Methods 11(2), 193–206 (2006).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21(11), 1539–1558 (2002).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta- analyses. BMJ 327(7414), 557–560 (2003).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Guyatt, G. et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J. Clin. Epidemiol. 66(2), 151–157 (2013).
Antonelli, M. et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: A randomized trial. JAMA 283(2), 235–241 (2000).
Brambilla, A. M. et al. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 40(7), 942–949 (2014).
Wysocki, M., Tric, L., Wolff, M. A., Millet, H. & Herman, B. Noninvasive pressure support ventilation in patients with acute respiratory failure: A randomized comparison with conventional therapy. Chest 107(3), 761–768 (1995).
Confalonieri, M. et al. Acute respiratory failure in patients with severe community-acquired pneumonia—A prospective randomized evaluation of noninvasive ventilation. Am. J. Respir. Crit. Care Med. 160(5), 1585–1591 (1999).
Delclaux, C. et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: A randomized controlled trial. JAMA 284(18), 2352–2360 (2000).
Hilbert, G. et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N. Engl. J. Med. 344(7), 481–487 (2001).
Ferrer, M. et al. Noninvasive ventilation in severe hypoxemic respiratory failure: A randomized clinical trial. Am. J. Respir. Crit. Care Med. 168(12), 1438–1444 (2003).
Nava, S. et al. Noninvasive ventilation in cardiogenic pulmonary edema—A multicenter randomized trial. Am. J. Respir. Crit. Care Med. 168(12), 1432–1437 (2003).
Park, M. et al. Randomized, prospective trial of oxygen, continuous positive airway pressure, and bilevel positive airway pressure by face mask in acute cardiogenic pulmonary edema. Crit. Care Med. 32(12), 2407–2415 (2004).
Squadrone, V. et al. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med. 36(10), 1666–1674 (2010).
Ducros, L. et al. CPAP for acute cardiogenic pulmonary oedema from out-of-hospital to cardiac intensive care unit: A randomised multicentre study. Intensive Care Med. 37(9), 1501–1509 (2011).
Zhan, Q. et al. Early use of noninvasive positive pressure ventilation for acute lung injury: A multicenter randomized controlled trial. Crit. Care Med. 40(2), 455–460 (2012).
Lemiale, V. et al. Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: A randomized clinical trial. JAMA 314(16), 1711–1719 (2015).
Azevedo, J. R. et al. High flow nasal cannula oxygen (HFNC) versus non-invasive positive pressure ventilation (NIPPV) in acute hypoxemic respiratory failure. A pilot randomized controlled trial. Intensive Care Med. Exp. 3(Suppl 1), A166 (2015).
Wermke, M. et al. Respiratory failure in patients undergoing allogeneic hematopoietic SCT—A randomized trial on early non-invasive ventilation based on standard care hematology wards. Bone Marrow Transplant. 47(4), 574–580 (2012).
Cosentini, R. et al. Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community-acquired pneumonia: A randomized, controlled trial. Chest 138(1), 114–120 (2010).
Xu, X. P. et al. Noninvasive ventilation in acute hypoxemic nonhypercapnic respiratory failure: A systematic review and meta-analysis. Crit. Care Med. 45(7), e727–e733 (2017).
Huang, H. B., Xu, B., Liu, G. Y., Lin, J. D. & Du, B. Use of noninvasive ventilation in immunocompromised patients with acute respiratory failure: A systematic review and meta-analysis. Crit. Care. 21(1), 4 (2017).
Zayed, Y. et al. Noninvasive oxygenation strategies in immunocompromised patients with acute hypoxemic respiratory failure: A pairwise and network meta-analysis of randomized controlled trials. J. Intensive Care Med. 35(11), 1216–1225 (2020).
Ruzsics, I. et al. Noninvasive ventilation improves the outcome in patients with pneumonia-associated respiratory failure: Systematic review and meta-analysis. J. Infect. Public Health 15(3), 349–359 (2022).
Sakuraya, M., Okano, H., Masuyama, T., Kimata, S. & Hokari, S. Efficacy of non-invasive and invasive respiratory management strategies in adult patients with acute hypoxaemic respiratory failure: A systematic review and network meta-analysis. Crit. Care. 25(1), 414 (2021).
Ferreyro, B. L. et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: A systematic review and meta-analysis. JAMA 324(1), 57–67 (2020).
Ferguson, N. D. et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 38(10), 1573–1582 (2012).
Acknowledgements
The authors gratefully acknowledge Khemajira Karaketklang for fully involved in the statistical analysis, preparation of the manuscript and checked the manuscript.
Funding
This research received no specific grants from any funding agency in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
P.W., K.M. contributed to the conception and design of the review. P.A., C.L. screened the title and abstract for eligible criteria, performed the full-text review, extracted the data, and assessed the risk of bias. P.W., K.M., P.A., and C.L. performed data analysis and statistical analysis. P.W. prepared the draft of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aswanetmanee, P., Limsuwat, C., Maneechotesuwan, K. et al. Noninvasive ventilation in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 13, 8283 (2023). https://doi.org/10.1038/s41598-023-35323-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35323-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.