Abstract
Timing of endotracheal intubation in COVID-19 patients with acute respiratory distress syndrome (ARDS) remains controversial regarding its risk and benefit in patient outcomes. Our study aims to elucidate early versus late intubation outcomes among COVID-19 patients with ARDS. A protocol of this study is registered at the international prospective register of systematic reviews (PROSPERO) (CRD42021230272). We report our systematic review based on PRISMA and MOOSE guidelines. We searched the Cochrane Library, EBSCOhost, EMBASE, Grey Literature Report, OpenGrey, ProQuest, PubMed, and ScienceDirect from inception until 4 December 2021. Titles and abstracts were reviewed for their relevance. The risk of bias in each study was evaluated using the risk of bias in non-randomised studies-of interventions (ROBINS-I) guideline. Trial sequential analysis is done to elucidate firm evidence. We retrieved 20 observational studies that assessed an intervention (early vs. late intubation). Meta-analysis for in-hospital mortality reduction showed 119 fewer deaths per 1000 patients in early intubation. Early intubation reduces 2.81 days of ICU length of stay (LOS) and 2.12 days of ventilation duration. Benefits for mortality and ICU LOS reduction were based on studies with low to moderate risk of bias while ventilation duration was based on low disease burden setting. According to the contextualized approach, the benefit of mortality reduction showed a trivial effect, while ICU LOS and ventilation duration showed a small effect. GRADE certainty of evidence for mortality reduction in early intubation is moderate. The certainty of evidence for ICU length of stay, ventilation duration, ventilator-free days, and continuous renal replacement therapy are very low. This updated systematic review provided new evidence that early intubation might provide benefits in treating COVID-19 patients with ARDS. The benefits of early intubation appear to have an important but small effect based on contextualized approach for ICU LOS and ventilation duration. In reducing in-hospital mortality, the early intubation effect was present but only trivial based on contextualized approach. TSA showed that more studies are needed to elucidate firmer evidence.
Similar content being viewed by others
Introduction
High mortality from early reports in COVID-19 patients using mechanical ventilators raised concern that respiratory failure due to COVID-19 are different from other cause of ARDS1,2,3. Management of respiratory failure in COVID-19 patients are still being studied whether it should be similar with other ARDS common cause4. One of the most important aspect in treating respiratory failure is to decide the timing of intubation, either early or late4,5,6. This is crucial because late mechanical ventilation has been associated with worsened clinical outcome in ARDS7. By delaying invasive ventilation, patient may become susceptible to high respiratory drive which lead to self-induced lung injury (SILI)8.
A previous meta-analysis has shown that intubation timing may have no influence on mortality and morbidity of COVID-19 patients with ARDS and suggesting that a wait-and-see approach might be justified to reduce the administration of invasive ventilation9. There are some limitation that has been pointed out with this report especially regarding the definition of early intubation and other clinical variables that may be taken into consideration which could influence outcome such as respiratory profile and more importantly the severity of illness10,11. Other limitation that has been addressed by the authors including the heterogeneity findings, no trial sequential analysis, results that may be affected by confounding factors due to the nature of synthesizing observational studies, and potential bias on the subgroup analysis9. On the other hand, several new studies have emerged revealing lower mortality events in favor of early intubation group12,13,14,15.
In accordance with the consensus on updating systematic review16 our aim is to elucidate current evidence on outcome for early versus late intubation among COVID-19 patients with ARDS.
Methods
Protocol and registration
We reported our study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist17,18. A protocol of this study was registered at the International Prospective Register of Systematic Reviews (PROSPERO) with registration number: CRD4202123027219.
Data sources
We searched the Cochrane Library, EBSCOhost, EMBASE, ProQuest, PubMed and ScienceDirect from inception until 4 December 2021. Additionaly grey literature databases were also searched using Grey Literature Report and OpenGrey database. Manual searching was also done in Google Scholar.
Search strategy, study eligibility, and study selection
Using the MeSH terms and [All Field], we complemented search strategy by using combination of keywords as following: COVID-19, respiratory distress syndrome, intubation, timing, mortality, survival, ICU length of stay, ventilation duration, organ-failure free days, adverse events. Similar and relevant terminologies were used to broaden our search strategy.
Studies were reviewed and included based on: (1) population: Adult patients (> 18 years old) with a COVID-19 confirmed diagnosis using reverse-transcriptase polymerase chain reaction (RT-PCR) with ARDS according to Berlin Criteria20; (2) intervention: early intubation defined as intubated within 24–48 h of hospital/ICU admission/ARDS diagnosis; (3) comparison: late intubation defined as intubated 24–48 h after hospital/ICU admission/ARDS diagnosis; (4) primary outcome: in-hospital mortality and secondary outcome: ICU length of stay (LOS), ventilation duration, ventilator free days (VFD), continuous renal replacement therapy (CRRT), ICU free days, and adverse events. The type of study included was observational studies that assessed an intervention, in this case regarding early versus late intubation in COVID-19 patients. Case reports or case series were excluded if consisting less than 5 patients. Conference abstracts were included if sufficient information was provided. Studies on non-COVID-19 patients, children (< 18 years old), or pregnant females were excluded.
Two authors independently searched, screened and selected articles using Endnote X9. Disagreement (< 1% of individual assessments) were resolved by discussion with other authors.
Data collection
Using standardised table, two authors independently extracted data including: authors, publication year, country of origin, sample size, population characteristic (age, sex, commorbidity), eligibility criteria, follow-up duration, intubation timing, and outcome results along with their 95% confidence intervals (CIs), standard deviation (SD), or interquartile ranges (IQRs). We calculated mean and SDs for studies reporting median and IQRs using method as proposed by Wan et al.21 Authors of studies were contacted via email to request access for missing data.
Quality assessment
Two authors independently assessed the risk of bias using the Risk Of Bias In Non-randomised Studies-of Interventions (ROBINS-I) guideline22. Certainty of each outcome was evaluated using Grading of Recommendations Assessment, Development and Evaluation (GRADE) system with the GRADEpro Guideline Development Tool23,24. Certainty of assessment was based on consideration of risk of bias, inconsistency, indirectness and imprecision. Imprecision was evaluated based on absolute estimates using risk difference (RD) for in-hospital mortality and CRRT while using mean difference (MD) for ICU LOS, ventilation duration and VFD. Disagreement (< 1% of individual assessments) were resolved by discussion with other authors. A contextualized approach was adopted to define minimum benefit thresholds to be able to estimate imprecision25,26,27. Due to no data available on quantitative studies of patient values, the thresholds of small but important effects was chosen by consensus: 1% for in-hospital mortality, 1 day for ICU LOS, 1 day for ventilation duration, 1 day for VFD, and 1% for CRRT25,26,27.
Meta-analysis
Meta-analysis using RevMan 5.3 was conducted. For dichotomous outcomes, we expressed effect estimates by risk ratios (RR) with 95%CI. For continuous outcomes, MD with standard deviation (SD) was used. We combined means from two different groups in continuous variables based on StatsToDo formula (www.statstodo.com).
In order to detect heterogeneity statistically, we used Cochrane’s Q test (chi-squared test) and Higgins I2 statistics. We defined an I2 of 25–50% as moderate, 51–75% as substantial, I2 > 75% as considerable heterogeneity28,29. We performed random effects model (REM) using DerSimonian-Laird method regardless of the statistical heterogeneity because all studies might be conducted with variable clinical practices, patient characteristics, ICU admission criteria and therefore clinical heterogeneity were present30. A p value less than 0.05 denoted statistical significance.
Subgroup analysis
Subgroup analysis were performed according to: (1) year of publication (2020 vs. 2021); (2) timing of intubation cut-off (24 h vs. 48 h) which was further be categorized based on timing since: (a) ICU admission, (b) hospital admission or (c) ARDS onset based on available definition from each study; (3) burden of disease in the point of care where the study is conducted (high disease burden) versus (low disease burden). The information regarding the situation of disease burden during the time of each study was collected based on literature reports9,12,31,32,33,34,35.
Sensitivity analysis
We performed sensitivity analysis by performing: (1) leave-one-out meta analysis by excluding a single study to recalculate the pooled effect estimate and finding a study with exaggerated effect size that may distort overal result aiming to achieve I2 below 50% or 25% for meta-analysis with I2 more than 50 and 25%, respectively36; (2) exclusion of studies with serious to critical risk of bias; (3) exclusion of studies that did not report a comparable Sequential Organ Failure Assessment score (SOFA) score between groups; (4) exclusion of studies that did not report a comparable PaO2/FIO2 (PF) ratio.
Publication bias analysis
We used JASP Version 0.14.1 to evaluate publication bias37. We generated Begg’s Funnel plot when the number of included studies is at least 10 with no considerable heterogeneity29,38. This was further confirmed by additional Egger’s test and Begg and Mazumdar’s rank correlation test39,40. Correction of publication bias was based on Duval and Tweedie’s trim and fill method29,41.
Trial sequential analysis
Trial Sequential Analysis (TSA) was performed using Trial Sequential Analysis Software (version 0.9.5.10 Beta; Copenhagen Trial Unit, Copenhagen, Denmark)42. Adjustment of Z values threshold were done by using O’Brien–Fleming α-spending function to control the risk of a type-1 error and β-spending function and futility boundaries to control the risk of a type-2 error42. A two-sided 95% CI was used and diversity-adjusted estimated information size used α = 0.05 (two-sided) and β = 0.20 (80% power), with an anticipated estimated of: (1) 10 and 20% relative risk reduction (RRR) of mortality, (2) − 2.00 days mean difference in ICU LOS, (3) − 2.00 day mean difference in ventilation duration, (4) 2.00 days mean difference in VFD and (5) 10% and 20% RRR of CRRT, in which all estimation were model-variance based heterogeneity correction. The variance was calculated from data obtained from the trials included in this meta-analysis.
Results
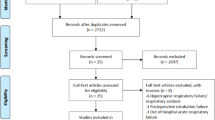
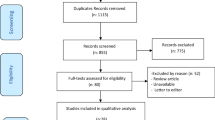
Our search strategy identified a total of 1887 studies (Supplementary Tables S1–S3). Results were imported into Endnote X9, and duplicates removed, leaving 1249 articles to be reviewed. We also collected results from manual searching in which 5 articles were retrieved. Abstracts were reviewed for relevance based on inclusion and exclusion criteria. After screening, 14 articles from database searching and 5 articles from manual searching were retained for full-review. We excluded 8 studies from database searching results and 3 studies from manual searching result with reasons that can be found in Fig. 1, leaving new 8 studies12,13,14,15,43,44,45,46 for updating previous systematic review9. We complemented 12 studies4,31,32,47,48,49,50,51,52,53,54,55 from previous systematic review9 for our final search results and thus a total of 20 studies were evaluated for this systematic review (See Fig. 1 for details of this process). All 20 studies (Supplementary Table S4) reported number of in-hospital mortality. There were 10 studies4,12,13,15,31,43,46,51,52,54 reported ICU LOS, 10 studies4,12,13,15,31,32,46,51,52,54 reported ventilation duration, 4 studies32,43,46,51 reported VFD, and 4 studies12,32,46,52 reported adverse events related to the use of continuous renal replacement therapy (CRRT).
Sixteen studies4,12,13,14,15,31,32,44,45,47,48,50,51,52,54,55 included are retrospective cohort, and four studies43,46,49,53 are prospective cohort. Country of origin of seven studies4,12,13,14,45,52,54 are United States, two studies31,55 are from Germany, two studies48,50 are from Italy, and each of one study is from South Korea51, India15, Greece32, Portugal44, France43, Chile46, Tunisia47, and there is one multinational study53 from Spain and Andorra, and another49 from France, Switzerland and Belgium. This systematic review included a total of 12,139 patients with age range from 30 to 89 years old. All patients included was hospitalized with SARS-CoV2 infection confirmed by using RT-PCR and diagnosed with ARDS in accordance with Berlin criteria. The definition of early and late intubation between studies were based on intubation timing cut-off 24 hours4,12,13,31,32,44,47,48,49,50,51,53,55 or 48 h14,15,43,45,46,52. Study by Pandya et al.54 have a slightly different categorization with 1.27 days for time distinction. Supplementary Table S4 summarize characteristic of included studies.
Primary outcome
In-hospital mortality
We evaluated all 20 studies for in-hospital mortality risk in COVID-19 patients with ARDS who were intubated early or late. In all 20 studies pooled together, there was no significant difference for in-hospital mortality between group (44.25% vs. 41.98%; RR 0.95, 95%CI 0.86–1.05, p = 0.29; I2 = 53%) (Supplementary Fig. S1).
Subgroup analysis for in-hospital mortality
Subgroup analysis based on publication year in 2020 and 2021 (Supplementary Fig. S2); intubation timing with 24-h and 48-h cut-off (Supplementary Fig. S3); high and low disease burden setting (Supplementary Fig. S4) showed a comparable in-hospital mortality between groups. Pooled analysis based on intubation timing within 24-h since hospital or ICU admission or ARDS onset, and 48-h since hospital or ICU admission or ARDS onset also showed comparable in-hospital mortality between groups (Fig. 2).
Subgroup Analysis According to Intubation Timing Categorized based on Timing since Hospital Admission, ICU Admission, or Acute Respiratory Distress Syndrome Onset for Risk of ICU-Mortality between Early Intubation Compared with Late Intubation Group. The solid squares denote the risk ratio, with the horizontal lines indicating the 95% confidence intervals and the diamond denotes the pooled effect size. ARDS, acute respiratory distress syndrome; Chi2 , chi-squared statistic; CI , confidence interval; df , degrees of freedom; I2 , I-squared heterogeneity statistic; ICU, intensive-care unit; IV , inverse variance; p , p value; SD , standard deviation; Z , Z statistic.
Sensitivity analysis for in-hospital mortality
Leave-one-out analysis showed no change for in-hospital mortality risk for pooled meta-analysis of all 20 studies (Supplementary Table S5), subgroup of publication year (Supplementary Table S6), intubation timing (Supplementary Table S7), and disease burden (Supplementary Table S8). Meta-analysis in studies with low to moderate risk of bias showed a significantly lower in-hospital mortality rate in early intubation group (36.43% vs. 49.62%; RR 0.76, 95%CI 0.60–0.97, p = 0.03; I2 = 49%) (Fig. 3). A further leave-one-out analysis excluding Vera et al.46 was performed and showed a borderline significant risk reduction (RR 0.83, 95%CI, 0.69–1.00; p = 0.05; I2 = 16%) (Supplementary Table S9). Meta-analysis in studies reporting a comparable SOFA score between groups showed no significant difference for in-hospital mortality rate between groups (Supplementary Fig. S5). A further leave-one-out analysis excluding Lee et al.51 change the result and showed a significantly lower risk of in-hospital mortality for early intubation group (38.89% vs. 57.76%; RR, 0.69, 95%CI 0.50–0.94, p = 0.02; I2 = 20%) (Supplementary Table S10). Meta-analysis done in studies reporting a comparable PaO2/FIO2 ratio between groups showed no significant difference (Supplementary Fig. S6). A further leave-one-out analysis excluding Vera et al. showed a similar result (Supplementary Table S11).
Sensitivity Analysis by Excluding Studies with Serious to Critical Risk of Bias for Risk of ICU-Mortality between Early Intubation Compared with Late Intubation Group. The solid squares denote the risk ratio, with the horizontal lines indicating the 95% confidence intervals and the diamond denotes the pooled effect size. Chi2 , chi-squared statistic; CI , confidence interval; df , degrees of freedom; I2 , I-squared heterogeneity statistic; IV , inverse variance; p , p value; SD , standard deviation; Z , Z statistic.
Trial sequential analysis for in-hospital mortality
All TSA on in-hospital mortality with an estimated risk reduction of 10% and 20% can be found in Supplementary Figs. S7–S30. TSA showed that more evidences are still needed to achieve a conclusive findings for analysis based on intubation timing cut-off 24-h since ARDS onset, intubation timing cut-off 48-h since hospital and ICU admission, low disease burden setting (for 10% RRR), studies with low to moderate risk of bias, studies reporting comparable SOFA or PaO2/FIO2 ratio between groups (for 10% RRR).
Secondary outcome
ICU length of stay
We evaluated 10 studies for ICU LOS in COVID-19 patients with ARDS who were intubated early or late. In all 10 studies pooled together, there was no significant difference for ICU LOS between early and late intubation group (MD -2.24, 95%CI − 4.55–0.07, p = 0.06; I2 = 72%) (Supplementary Fig. S31).
Subgroup analysis for ICU length of stay
Subgroup analysis based on publication year in 2020 and 2021 (Supplementary Fig. S32), intubation timing with 48-h cut-off (Supplementary Fig. S33), high disease burden setting (Supplementary Fig. S34) showed a comparable ICU LOS between group. Analysis based on intubation timing with 24 h cut-off showed a reduced ICU LOS for early intubation group (MD − 2.85, 95%CI − 5.64 to − 0.05, p = 0.05, I2 = 61%) (Supplementary Fig. S33). Analysis for studies done in low disease burden showed a reduced ICU LOS for early intubation group (MD − 5.64, 95%CI − 9.70 to − 1.58, p = 0.007, I2 = 72%) (Supplementary Fig. S34).
Sensitivity analysis for ICU length of stay
Leave-one-out analysis showed no change in ICU LOS for pooled meta-analysis of all 10 studies (Supplementary Table S12), subgroup of publication year (Supplementary Table S13), and high disease burden setting (Supplementary Table S14). When performed in subgroup of intubation timing (Supplementary Table S15) and low disease burden (Supplementary Table S14), results are change to not significant after exclusion of study with the most exaggerated effect. Meta-analysis done in studies with low to moderate risk of bias showed a reduced ICU LOS in early intubation group (MD − 2.81, 95%CI − 5.42 to − 0.20, p = 0.03; I2 = 70%) (Fig. 4). A further leave-one-out analysis excluding Vera et al.46 was performed and change result to not significant (Supplementary Table S16). Meta-analysis in studies reporting a comparable SOFA score between groups showed no significant difference (Supplementary Fig. S35). A further leave-one-out analysis excluding Lee et al.51 showed a comparable result (Supplementary Table S17). Meta-analysis in studies reporting a comparable PaO2/FIO2 ratio between groups showed no significant difference (Supplementary Fig. S36). A further leave-one-out analysis excluding Vera et al.46 showed a comparable result (Supplementary Table S18).
Sensitivity Analysis by Excluding Studies with Serious to Critical Risk of Bias for ICU Length of Stay between Early Intubation Compared with Late Intubation Group. The solid squares denote the mean difference, with the horizontal lines indicating the 95% confidence intervals and the diamond denotes the pooled effect size. Chi2 , chi-squared statistic; CI , confidence interval; df , degrees of freedom; I2 , I-squared heterogeneity statistic; IV , inverse variance; p , p value; SD , standard deviation; Z , Z statistic.
Trial sequential analysis for ICU length of stay
All TSA on ICU LOS with an estimated mean difference reduction of − 2.00 days can be found in Supplementary Figs. S37–S44. TSA showed that more evidences are still needed to achieve a conclusive findings for analysis based on intubation timing cut-off 24-h and 48-h, low disease burden setting, studies with low to moderate risk of bias, studies reporting comparable SOFA score between groups and comparable PaO2/FIO2 ratio between groups.
Ventilation duration
We evaluated 10 studies for ventilation duration in COVID-19 patients with ARDS who were intubated early or late. In all 10 studies pooled together, there was no significant difference for ventilation duration between early and late intubation group (MD − 0.33, 95%CI − 2.14–1.49, p = 0.73). Moderate heterogeneity was detected (I2 = 59%) (Supplementary Fig. S45).
Subgroup analysis for ventilation duration
Subgroup analysis based on publication year in 2020 and 2021 (Supplementary Fig. S46); intubation timing with 24-h and 48-h cut-off (Supplementary Fig. S47); and high disease burden setting (Fig. 5) showed a comparable ventilation duration between group. For studies done in low disease burden showed a reduced ventilation duration for early intubation (MD − 2.12, 95%CI − 3.86 to − 0.38, p = 0.02, I2 = 0%) (Fig. 5).
Subgroup Analysis According to Disease Burden for Ventilation Duration between Early Intubation Compared with Late Intubation Group. The solid squares denote the mean difference, with the horizontal lines indicating the 95% confidence intervals and the diamond denotes the pooled effect size. Chi2 , chi-squared statistic; CI , confidence interval; df , degrees of freedom; I2 , I-squared heterogeneity statistic; IV , inverse variance; p , p value; SD , standard deviation; Z , Z statistic.
Sensitivity analysis for ventilation duration
Leave-one-out analysis showed no change for in-hospital mortality risk for pooled meta-analysis of all 10 studies (Supplementary Table S19), subgroup of publication year (Supplementary Table S20), and intubation timing (Supplementary Table S21). When performed in studies with high disease burden setting, result for ventilation duration change and become significant after exclusion of study by Pandya et al.54 with increase ventilation duration for early intubation versus late intubation group (MD 1.58, 95%CI 0.21–2.95, p = 0.02; I2 = 0%) (Supplementary Table S22). Meta-analysis in studies with low to moderate risk of bias showed no different ventilation duration between groups (Supplementary Fig. S48). A further leave-one-out analysis excluding Pandya et al.54 was performed and showed no significant difference between groups (Supplementary Table S23). Meta-analysis in studies reporting a comparable SOFA score between groups also showed no significant difference (Supplementary Fig. S49). A further leave-one-out analysis excluding Lee et al.51 showed a similar result (Supplementary Table S24). Meta-analysis in studies reporting a comparable PaO2/FIO2 ratio between groups showed no significant difference (Supplementary Fig. S50). A further leave-one-out analysis excluding Pandya et al.54 showed a similar result (Supplementary Table S25).
Trial sequential analysis for ventilation duration
All TSA on ventilation duration with an estimated mean difference reduction of − 2.00 days can be found in Supplementary Figs. S51–S58. TSA showed a firm evidence that there was an increase ventilation duration for early intubation for studies with high disease burden setting. TSA showed that more evidences are still needed to achieve a conclusive findings for analysis based on intubation timing cut-off 24-h and 48-h, low disease burden setting, studies with low to moderate risk of bias, studies reporting comparable SOFA score between groups.
Ventilator-free days
We evaluated 4 studies for VFD in COVID-19 patients with ARDS who were intubated early or late. In all studies pooled together, there was no significant difference between group (MD − 0.84, 95%CI − 4.80–3.12, p = 0.68; I2 = 75%) (Supplementary Fig. S59). Subgroup analysis is not performed due to limited number of studies. Leave-one-out analysis showed no change in VFD for pooled meta-analysis of all 4 studies (Supplementary Table S26). TSA showed that more studies are necessary to confirm evidence (Supplementary Fig. S60).
Continuous renal replacement therapy
We evaluated 4 studies for CRRT in COVID-19 patients with ARDS who were intubated early or late. In all 4 studies pooled together, there was no significant difference for CRRT between early and late intubation group (MD 0.65, 95%CI 0.27–1.55, p = 0.33; I2 = 75%) (Supplementary Fig. S61). Leave-one-out analysis showed stable results (Supplementary Table S27). TSA showed that more studies are necessary to confirm the evidence (Supplementary Fig. S62–S63).
ICU free days
ICU-free days was only reported by Siempos et al.32 and result showed that there was no significant difference between early and late intubation with a median of 0 day (0–16 days) for early intubation and 0 day (0–4 days) for late intubation. Currently, there has been no study evaluate organ-failure free days between early and late intubation.
Other adverse events
Based on Lee et al.51 number of events for acute kidney injury, acute cardiac injury, catheter–related blood stream infection, bleeding, cardiopulmonary–cerebral resuscitation was not different between early and late intubation. Lee et al.51 and Siempos et al.32 did not find a significant difference on number of events for septic shock between early and late intubation. Lee et al.51 and Ferraz et al.44 did not find a significant difference on number of events for ventilator associated pneumonia.
Quality assessment, quality of evidence and publication bias
Quality assessment using ROBINS-I shows all studies’ risk of bias result range from moderate to critical. Complete reasoning can be found in Supplementary Table S28. GRADE certainty of evidences are judged to be moderate for mortality reduction and very low for ICU LOS, ventilation duration, VFD, and CRRT (Supplementary Table S29). Additionaly, Supplementary Figs. S64–S68 showed the minimum benefit threshold of in-hospital mortality, ICU LOS, ventilation duration, VFD, and CRRT between early and late intubation that used to evaluate imprecision. We did not downgrade the level of evidence for imprecision of in-hospital mortality because the pooled effect is between the minimum benefit threshold and null effect showing that early intubation has a trivial effect in reducing mortality [Supplementary Fig. S64]. We downgraded one level of evidence for imprecision of ICU LOS because the pooled effect crossed the minimum benefit threshold but the upper confidence interval reached the area of trivial effect. Thus, early intubation probably reduces ICU LOS [Supplementary Fig. S65]. We downgraded one level of evidence for imprecision of ventilation duration because the pooled effect crossed the minimum benefit threshold but the upper confidence interval fell on the area of trivial effect. Thus, early intubation probably reduces ventilation duration [Supplementary Fig. S66]. We downgraded two level of evidence for imprecision of VFD because the point estimate of pooled effect is on the area of trivial effect while the confidence interval crossed the minimum benefit and harm threshold. Thus, early intubation may have trivial or no effect in VFD [Supplementary Fig. S67]. We did not downgrade the level of evidence for imprecision of CRRT because the pooled effect is between the minimum benefit and harm threshold. Thus, early intubation has a trivial or no effect for risk in using CRRT [Supplementary Fig. S68].
A funnel plot with trim and fill analysis is generated for in-hospital mortality (Supplementary Fig. S69), ICU LOS (Supplementary Fig. S70), and ventilation duration (Supplementary Fig. S71). Egger’s test showed no publication bias for in-hospital mortality (Z = − 1.679, p = 0.093) and ventilation duration (Z = − 1.130, p = 0.259) but significant result was found in ICU LOS (Z = − 2.167, p = 0.030). Rank correlation test showed no publication bias for in-hospital mortality (Tau = − 0.274, p = 0.098), ICU LOS (Tau = − 0.289, p = 0.291), and ventilation duration (Tau = 0.067, p = 0.862). Overall, a minor publication bias may exist.
Discussion
We found that early intubation is related to 24% relative risk reduction of in-hospital mortality when adjusted with only studies with low to moderate risk of bias. However, the result become borderline significant after a leave-one-out analysis was performed. On the other hand, our result also suggests a 31% lower risk of in-hospital mortality after leave-one-out analysis was performed in studies reporting comparable SOFA score. Although SOFA adjustment method showed a lower heterogeneity (I2 = 20% vs. I2 = 49%), the number of included studies are however smaller (4 studies vs. 9 studies) when compared with analysis based on study quality. TSA showed that more studies are needed to make a firm evidence for both analysis. In early intubation, there are 119 fewer death per 1000 patients based on studies with low to moderate risk of bias. According to the contextualized approach, this effect is considered to be trivial.
In our analysis, early intubation was also related to 2.85 days (6 studies) or 5.64 days (4 studies) reduction of ICU LOS based on intubation timing cut-off 24 h or when studies done in low disease burden setting, respectively. However, both results reveal substantial heterogeneity and a leave-one-out analysis performed on both adjustments showed unstable result. Studies with low to moderate risk of bias showed a 2.81 days reduction of ICU LOS. Although the number of studies is larger (8 studies), heterogeneity is still substantial while a leave-one-out analysis showed unstable result. According to the contextualized approach, this effect is considered to be small.
Our analysis in ventilation duration showed an interesting result revealing 1.58 days increase of ventilation duration for early intubation in high disease burden setting. In early intubation, there are 2.12 days reduction of ventilation duration based on studies in low disease burden setting. According to the contextualized approach, this effect is considered to be small. Our analysis showed no benefit or harm of VFD, CRRT, ICU-free days or other adverse events between groups. Based on analysis, there is a minor publication bias for in-hospital mortality, ICU-LOS and ventilation duration outcome.
We addressed some important limitations provided by previous systematic review9 and other reports10,11. We found that clinical parameters (SOFA score), level of disease burden, and study quality are important to be adjusted for elucidating benefit or harm of intubation timing. We also included TSA in our analysis to control risk of type-1 and type-2 error42. All positive findings supporting early intubation still required more studies for a conclusive meta-analysis. Our TSA show firm evidence for increase ventilation duration when early intubation done in high disease burden setting. By adding up 8 new studies12,13,14,15,43,44,45,46 and modifying subgroup and sensitivity analysis, current meta-analysis revealed different results compared to the previous study9 but in favor of prior international guidelines56,57,58,59. We did not perform sensitivity analysis based on a prior trial of high flow nasal cannula or non-invasive ventilation because previous report suggested that this additional definition might classify the same subject into either early or late intubation group and thereby have a vastly different implication10.
Previous recommendation from experts suggested that early intubation and invasive ventilation is in favor to prevent too vigorous patient’s respiratory efforts that may trigger SILI and affect short- and long-term clinical outcome6. Work of breathing remain to be one of the decision basis in judging whether to intubate early or late6. Factors such as tidal volume, respiratory rate, minute ventilation, and worsening respiratory status may indicate early intubation6,60. Additionally, more objective measurement to indicate intubation timing can be calculated in pneumonia patients with ARDS by using ratio of oxygen saturation (ROX) index61. One study62 validate this tool based on admission day. A ROX less than 25.26, 21.34 or 11.71 at day 1, 2 or 3 of admission, respectively, are significantly associated with intubation62. Currently, there are only 2 studies13,53 included in our analysis which report ROX index between groups and thus, further pooled analysis is limited. Future studies may evaluate outcomes by incorporating variables in work of breathing along with ROX index to elucidate risk and benefit for early intubation.
Our systematic review has limitations because all studies are observational. GRADE certainty of evidence is currently very low for early intubation benefit in ICU LOS, ventilation duration, VFD, and CRRT. Due to the nature of observational study, subjective factors including clinician, patient and family preferences along with ventilator availability, institutional culture, and the fact that more severe patients might get earlier intubation, were unable to be controlled by the authors. However, our analysis has included a subgroup based on study quality. A prospective, randomized trials with a large sample would be crucial in the future to address unmeasured confounders and confirmed the results. Other limitation is that our analysis for VFD and CRRT have considerable heterogeneity and this might be due to heterogeneity from population characteristics and clinical practices.
Conclusion
This updated systematic review provided new evidence that early intubation might provide benefits in treating COVID-19 patients with ARDS. The benefits of early intubation appear to have an important but small effect based on contextualized approach with 2.81 days shorter of ICU LOS, and 2.12 days shorter of ventilation duration. In reducing in-hospital mortality, the early intubation effect was present but only trivial based on contextualized approach with 119 fewer deaths per 1000 patients. TSA showed that more studies are needed to elucidate firmer evidence.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- APACHE:
-
Acute physiology and chronic health evaluation
- ARDS:
-
Acute respiratory distress syndrome
- CI:
-
Confidence interval risk ratios (RR)
- COVID-19:
-
Coronavirus disease 2019
- CRRT:
-
Continuous renal replacement therapy
- FEM:
-
Fixed effect model
- GCS:
-
Glasgow coma scale
- MD:
-
Mean difference
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PROSPERO:
-
Prospective register of systematic reviews
- RT-PCR:
-
Reverse-transcriptase polymerase chain reaction
- HR:
-
Hazard ratios
- LOS:
-
Length of stay
- MV:
-
Mechanical ventilator
- OR:
-
Odd ratios
- PF:
-
PaO2:FIO2
- REM:
-
Random effect model
- RR:
-
Risk ratios
- RRR:
-
Relative risk reduction
- SILI:
-
Self-induced lung injury
- SOFA:
-
Sequential organ failure assessment
- TSA:
-
Trial sequential analysis
- VFD:
-
Ventilator free days
References
Gattinoni, L. et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes?. Intens. Care Med. 46, 1099–1102 (2020).
Arentz, M. et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA 323, 1612–1614 (2020).
Bhatraju, P. K. et al. Covid-19 in critically ill patients in the Seattle region—case series. N. Engl. J. Med. 382, 2012–2022 (2020).
Hernandez-Romieu, A. C. et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: A single-center cohort study. Crit. Care Med. https://doi.org/10.1097/CCM.0000000000004600 (2020).
Pierson, D. J. Indications for mechanical ventilation in adults with acute respiratory failure. Respir. Care 47, 245–249 (2002).
Cabrini, L. et al. Early versus late tracheal intubation in COVID-19 patients: A pro-con debate also considering heart-lung interactions. Minerva Cardioangiol. https://doi.org/10.23736/S0026-4725.20.05356-6 (2020).
Kangelaris, K. N. et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit. Care Med. 44, 120–129 (2016).
Brochard, L., Slutsky, A. & Pesenti, A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am. J. Respir. Crit. Care Med. 195, 438–442 (2017).
Papoutsi, E. et al. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: A systematic review and meta-analysis of non-randomized cohort studies. Crit. Care 25, 1–9 (2021).
Thomson, D. A. & Calligaro, G. L. Timing of intubation in COVID-19: Not just location, location, location?. Crit. Care 25, 1–2 (2021).
Tsolaki, V. & Zakynthinos, G. E. Timing of intubation in Covid-19 ARDS: What “time” really matters?. Crit. Care 25, 1–2 (2021).
Bavishi, A. A., Mylvaganam, R. J., Agarwal, R., Avery, R. J. & Cuttica, M. J. Timing of intubation in coronavirus disease 2019: A study of ventilator mechanics, imaging, findings, and outcomes. Crit. Care Explor. 3, e0415 (2021).
Fayed, M. et al. Effect of intubation timing on the outcome of patients with severe respiratory distress secondary to COVID-19 pneumonia. Cureus 13 (2021).
Hyman, J. B. et al. Timing of intubation and in-hospital mortality in patients with coronavirus disease 2019. Crit. Care Explor. 2, e0254 (2020).
Zirpe, K. G. et al. Timing of invasive mechanical ventilation and mortality among patients with severe COVID-19-associated acute respiratory distress syndrome (CARDS). Indian J. Crit. Care Med. 25, 493–498 (2021).
Garner, P. et al. When and how to update systematic reviews: Consensus and checklist. bmj 354 (2016).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, T. P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement 6 (2009).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting Meta-analysis of observational. Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 (2000).
Ridjab, D. A., Ivan, I., Budiman, F. & Juzar, D. A. Outcome in early vs late intubation among COVID19 patients with acute respiratory distress syndrome: A systematic review. PROSPERO https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021230272 (2021).
Ferguson, N. D. et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intens. Care Med. 38, 1573–1582 (2012).
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 1–13 (2014).
Sterne, J. A. et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, 4–10 (2016).
Guyatt, G. H. et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926 (2008).
McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool (2015).
Hultcrantz, M. et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 87, 4–13 (2017).
Zeng, L. et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J. Clin. Epidemiol. 137, 163–175 (2021).
Zeng, L. et al. GRADE Guidance 34: Update on rating imprecision using a minimally contextualized approach. J. Clin. Epidemiol. https://doi.org/10.1016/j.jclinepi.2022.07.014 (2022).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Higgins, J. P. T. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated February 2021) (Cochrane, 2021).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 7, 177–188 (1986).
Karagiannidis, C. et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir. Med. 8, 853–862 (2020).
Siempos, I. I. et al. Effect of early vs. delayed or no intubation on clinical outcomes of patients with COVID-19: An observational study. Front. Med. 7, 1–6 (2020).
Dighe, A. et al. Response to COVID-19 in South Korea and implications for lifting stringent interventions. BMC Med. 18, 1–12 (2020).
Shaaban, A. N., Peleteiro, B. & Martins, M. R. O. COVID-19: What is next for Portugal?. Front. Public Heal. 8, 1–8 (2020).
Joshi, A., Mewani, A. H., Arora, S. & Grover, A. India’s COVID-19 Burdens, 2020. Front. Public Heal. 9, 1–15 (2021).
Patsopoulos, N. A., Evangelou, E. & Ioannidis, J. P. A. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 37, 1148–1157 (2008).
JASP Team. JASP (Version 0.16.1) (2022).
Terrin, N., Schmid, C. H., Lau, J. & Olkin, I. Adjusting for publication bias in the presence of heterogeneity. Stat. Med. 22, 2113–2126 (2003).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Duval, S. & Tweedie, R. Trim and fill: A simple funnel-plot-based method. Biometrics 56, 455–463 (2000).
Thorlund, K. et al. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit Centre Clin. Interv. Res. Copenhagen Denmark 1, 1–115 (2011).
Dupuis, C. et al. Association between early invasive mechanical ventilation and day-60 mortality in acute hypoxemic respiratory failure related to coronavirus disease-2019 pneumonia. Crit. Care Explor. 3, e0329 (2021).
Ferraz, M. et al. COVID-19: Is early mechanical ventilation the way forward?. Intens. Care Med. Exp. 9, 177 (2021).
Parish, A. J. et al. Early intubation and increased coronavirus disease 2019 mortality: A propensity score-matched retrospective cohort study. Crit. Care Explor. 3, e0452 (2021).
Vera, M. et al. Intubation timing as determinant of outcome in patients with acute respiratory distress syndrome by SARS-CoV-2 infection. J. Crit. Care 65, 164–169 (2021).
Saida, I. B. et al. Very severe covid-19 in the critically ill in tunisia. Pan Afr. Med. J. 35, 1–12 (2020).
Zuccon, W. et al. Intensive care for seriously ill patients affected by novel coronavirus sars-CoV–2: Experience of the Crema Hospital, Italy. Am. J. Emerg. Med. 45, 156–161 (2021).
Schmidt, M. et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intens. Care Med. 47, 60–73 (2021).
Grasselli, G. et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 180, 1345–1355 (2020).
Lee, Y. H. et al. Clinical significance of timing of intubation in critically ill patients with COVID-19: A multi-center retrospective study. J. Clin. Med. 9, 2847 (2020).
Matta, A. et al. Timing of intubation and its implications on outcomes in critically ill patients with coronavirus disease 2019 infection. Crit. care Explor. 2, e0262 (2020).
Mellado-Artigas, R. et al. High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit. Care 25, 1–10 (2021).
Pandya, A. et al. Ventilatory Mechanics in early vs late intubation in a cohort of coronavirus disease 2019 patients with ARDS: A single center’s experience. Chest 159, 653–656 (2021).
Roedl, K. et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: A multicentric study in Germany. Aust. Crit. Care 34, 167–175 (2021).
Zuo, M. et al. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin. Med. Sci. J. 35, 105–109 (2020).
Cook, T. M. et al. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetist. Anaesthesia 75, 785–799 (2020).
Brown, C. A. III., Mosier, J. M., Carlson, J. N. & Gibbs, M. A. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J. Am. Coll. Emerg. Physicians Open 1, 80 (2020).
Brewster, D. J. et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med. J. Aust. 212, 472–481 (2020).
Alhazzani, W. et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intens. Care Med. 46, 854–887 (2020).
Roca, O. et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am. J. Respir. Crit. Care Med. 199, 1368–1376 (2019).
Suliman, L. A., Abdelgawad, T. T., Farrag, N. S. & Abdelwahab, H. W. Validity of rox index in prediction of risk of intubation in patients with covid-19 pneumonia. Adv. Respir. Med. 89, 1–7 (2021).
Author information
Authors and Affiliations
Contributions
Conceptualization: D.R., I.I., F.B., D.J. Data curation: D.R., I.I., F.B. Formal analysis: D.R., I.I., F.B. Investigation: D.R., I.I., F.B., D.J. Methodology: D.R., I.I., F.B. Project Administration: D.R., I.I. Resources: D.R., I.I., F.B. Supervision: D.R., D.J. Validation: D.R., D.J. Visualization: D.R., I.I., F.B. Software: D.R., I.I., F.B. Writing—original draft: D.R., I.I., F.B. Writing—review & editing: D.R., I.I., F.B., D.J.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ridjab, D.A., Ivan, I., Budiman, F. et al. Outcome in early vs late intubation among COVID-19 patients with acute respiratory distress syndrome: an updated systematic review and meta-analysis. Sci Rep 12, 21588 (2022). https://doi.org/10.1038/s41598-022-26234-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26234-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.