Abstract
The experience of pain has been dissociated into two interwoven aspects: a sensory-discriminative aspect and an affective-motivational aspect. We aimed to explore which of the pain descriptors is more deeply rooted in the human brain. Participants were asked to evaluate applied cold pain. The majority of the trials showed distinct ratings: some were rated higher for unpleasantness and others for intensity. We compared the relationship between functional data recorded from 7 T MRI with unpleasantness and intensity ratings and revealed a stronger relationship between cortical data and unpleasantness ratings. The present study underlines the importance of the emotional-affective aspects of pain-related cortical processes in the brain. The findings corroborate previous studies showing a higher sensitivity to pain unpleasantness compared to ratings of pain intensity. For the processing of pain in healthy subjects, this effect may reflect the more direct and intuitive evaluation of emotional aspects of the pain system, which is to prevent harm and to preserve the physical integrity of the body.
Similar content being viewed by others
Introduction
The experience of pain has been dissociated into sensory-discriminative and affective-motivational aspects1,2, which are widely assessed as subjective ratings of pain intensity and pain unpleasantness3,4,5. Both descriptors of pain share a conceptual core variance and are therefore often highly correlated: very intense pain is associated with higher levels of unpleasantness6,7. By contrast, previous research also emphasised the disparity of both pain descriptors by revealing a diverging evaluation depending on the type of pain, for example health-related pain conditions are associated with higher pain unpleasantness ratings2. Differential ratings of intensity and unpleasantness have been revealed in dependence on the type of experimentally-applied pain8. Likewise, the experience of pain unpleasantness is considered to exhibit a particularly strong influence on well-being9,10, as it has been found to be tightly connected to pain catastrophising9.
Studies exploring the cortical underpinnings of pain unpleasantness are scarce and non-specific as they implicitly include the shared variance with pain intensity 11,12. The conceptual and empirical proximity of both aspects has made an investigation on the differences between the cortical underpinnings of pain intensity and pain unpleasantness challenging. The only attempt to experimentally dissociate pain intensity and pain unpleasantness processing has been conducted in a series of early PET studies. Using hypnosis, a specific modulation of pain unpleasantness was reported in the anterior cingulate cortex (ACC) but not for the somatosensory cortices (SI and SII) and the insular cortex (IC)13. This clear effect was shadowed by a subsequent study7, where the authors were not able to hypnotically disentangle intensity and unpleasantness. The ACC activation was slightly shifted compared to the previous study and despite (unintended) differences in unpleasantness in response to the hypnotic modulation of pain, there was no effect for the ACC. The shift of activity in the ACC region between studies, the absence of information on the sample size for one study13, and the absence of an ACC effect in the follow-up study leave some questions regarding distinct cortical processing of pain intensity and pain affect in this region.
Nonetheless, despite a high correlation between both subjective pain aspects, it is indeed possible to dissociate the cortical underpinnings of the encoding of pain intensity and pain unpleasantness in a within-subject design without the need for any potentially unreliable modulatory intervention. To this end, we aimed to investigate the brain regions that differentially process pain intensity and pain unpleasantness. As a major advantage, by avoiding undesirable session or sample variability, both aspects are explored in relation to the very same underlying cortical data; each trial has been evaluated for either descriptor of the subjective experience of pain. The majority of the trials indeed differed and enabled us to elucidate which aspect of pain perception is more deeply rooted in the human brain.
Materials and methods
Subjects
20 healthy subjects (16 female/4 male, age 27 ± 5 years; mean ± standard deviation) were included in the study. All subjects gave written informed consent, none reported any history of chronic pain.
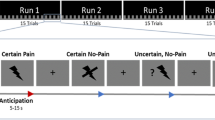
The data were taken from our previous study and the experimental procedure has been described in detail in our previous publications14,15. The previous studies explored the cortical underpinnings of cognitive interventions to attenuate pain: the experiment consisted of four conditions across four separate blocks, where each block comprised 12 trials from the same condition. In all conditions and trials, the subjects received cold pain stimuli on the dorsum of their left hand delivered by a thermode (Pathway II; Medoc Ltd, Israel). The first condition of the experiment was always the unmodulated pain condition. The subsequent three conditions were counterbalanced blocks of pain attenuation: (A) an attentional shift, (B) an imaginal strategy, and (C) a non-imaginal reinterpretation. After each trial, the subjects were prompted to rate pain intensity and pain unpleasantness within 10 s (Fig. 1). The scale ranged between 0 and 100 in steps of 5 points. The endpoints of the scale were determined as no pain (0) and the maximum pain the subjects were willing to tolerate (100). There were a total of 48 trials during the fMRI recording. The 40 s of painful stimulation were preceded by a rest period of 10 s at 38 °C thermode temperature. The different conditions and their modulation of pain across trials are not relevant here as we are exploring the within-trial differences between two descriptors of pain in the same trial. Our approach avoids a distinct modulating intervention for intensity or unpleasantness, which may not always work as intended7. In addition, a distinct intervention for either descriptor of pain may introduce undesirable effects of variability due to recordings from different sessions or samples. Oscillating pain stimulation was used to prevent habituation or sensitisation16,17,18.
Data acquisition
Imaging data were acquired on a 7 T Siemens MRI scanner using parallel image acquisition (GRAPPA, factor = 2). In order to cover the whole brain, each of the 1768 functional echo-planar imaging (EPI) volumes comprised 34 axial slices of 2 mm thickness and 2 × 2 mm in-plane resolution with a 1 mm gap between slices. The repetition time (TR) was 1.96 s, the echo time (TE) was 25 ms (flip angle 90°), the field of view (FOV) was 220 × 220 mm, and the matrix size was 110 × 110 pixels. A T1-weighted structural image (isotropic 1 mm3 voxel) was acquired for the registration of the EPI to the MNI (Montreal Neurological Institute) template.
Imaging analysis – preprocessing
The data were pre-processed and analysed with FSL19 and Matlab (version R2020a, The Mathworks, USA). The preprocessing consisted of brain extraction, high-pass filtering with a frequency cutoff of 1/90 Hz, a correction for head motion during scanning, spatial normalisation to the MNI template, and spatial smoothing (6 mm FWHM). The data were further semi-automatically cleaned of artefacts with MELODIC20. Beta coefficients representing the magnitude of cortical activity for each trial (modulated and unmodulated) were computed in FEAT15.
Image analysis—extraction of regions of interest data
The time series of functional volumes were converted to MNI space and subsequently projected to surface space by using the “Connectome Workbench” package. We used a template that allowed us to project from 3D standard MNI space to 2D surface space. Regions of interest (ROIs) were defined by subdividing the cortical surface into 180 regions per hemisphere21. Six further regions (5 bilateral) that are important for the processing of pain, such as the PAG, the thalamus and the amygdala, were also included. The ROIs were based on the Oxford Atlas, implemented in FSL.
Image analysis—computation of single trial functional connectivity scores
The time courses for all voxels of cortical activity for a specific region of the Glasser Atlas, e.g. the anterior insula, were extracted. We computed principal component analyses (PCA) separately for each ROI and subject and selected the first component (Matlab, The MathWorks, Inc., USA). The plateau phase of the last ~ 30 s of painful stimulation (15 data points), derived from the FEAT design matrix, has been extracted from each region and trial for each subject and condition. Outliers were removed from the data by using the Grubbs’ test (Grubbs, 1950). These 15 data points determined the connectivity for a brain region for a given trial. Kendall’s tau correlation coefficients (τ) were computed for each trial and for each of the 371 ROIs with the remaining 370 ROIs. The single trial correlation coefficients were Fisher Z-transformed and fed into group-level statistical analysis.
Statistical analysis—comparison between intensity and unpleasantness
Differences between pain intensity and pain unpleasantness occurred in 75% of the trials. Using Linear Mixed Effects models22,23, we aimed to differentiate the relationship between the two aspects of pain ratings and cortical activity. The statistical model is expressed in Wilkinson notation24, the included fixed effect of interest (rating_type:fmri) describes the magnitudes of the population common intercept and the population common slopes for the relationship between cortical data and the single-trial differences in pain perception. The added random effect (i.e. 1|subject) models the specific intercept differences for each subject (e.g. subject-specific differences in fMRI beta coefficients):
In this formula, rating concatenates the single trial pain ratings (unpleasantness, intensity) and fmri relates to the single trial estimate of the BOLD response, which is identical for the corresponding ratings of intensity and unpleasantness (see example in Supplementary Material). Consequently, for each voxel, the statistics include all single-trial pain intensity ratings and the respective cortical data, as well as all single-trial unpleasantness ratings and the same cortical data. Each rating entry was assigned its corresponding single-trial grouping code (rating_type, nominal [− 1 1]) and BOLD estimate (fmri). Please note our more suitable modelling in Supplementary Material, where we—by generating a gradient vector—put more weight on trials that had greater single-trial rating differences.
Please note that a separate mapping for pain intensity coding and pain unpleasantness coding is not possible. First, there is no separate unpleasantness or intensity condition. Both pain descriptors are referring to the very same trials. Second, a potential computation of pain intensity encoding or unpleasantness encoding would be confounded by the 4 experimental pain attenuation conditions, which exhibit different levels of effectiveness.
Due to its relative character, we can not know whether a potential negative difference is due to a weaker positive relationship or a stronger negative relationship. Therefore, the results can only be evaluated in light of the general map of pain processing (activation, deactivation). Consequently, we created a 3D map consisting of the group result of the analysis in FEAT by contrasting the pain trials with the no-pain baseline period (cluster corrected, p < 0.05). The LME analysis was restricted to the significant voxels within this map. As delineated above, the direction of the contrast (activations, deactivations) determines the interpretation of the direction of effect for the differential encoding of pain intensity and pain unpleasantness.
To correct for multiple comparisons, we applied a randomisation approach. Behavioural data were shuffled and the entire analysis was repeated 5000 times within the boundaries of the 3D brain mask. The highest absolute t-values of each repetition across the whole volume were extracted. This procedure resulted in right-skewed distribution for each condition. Based on these distributions, the statistical thresholds were determined using the “palm_datapval” function publicly available in PALM25.
Statistical analysis—mapping of pain processing
In order to interpret the relative findings between pain intensity and pain unpleasantness it was required to assess whether cortical processes increase or decrease in response to pain. For the BOLD data, we contrasted in FEAT the phases of pain experience (all 4 conditions pooled) compared to the baseline period. The map closely resembles the neurologic signature for pain map26.
For connectivity data, we also utilised the information from the design matrix and compared the pooled connectivity of all pain phases with the connectivity in baseline periods. The majority of the pain trials (97%) and the baseline periods (98%) showed a positive τ at single-trial level. Similar to the interpretation of the BOLD data, the results of these analyses are relevant insofar as we can determine whether a lower connectivity for unpleasantness can be interpreted as weaker coupling (in case we observe general increase of connectivity during pain compared to the baseline) or as stronger anticorrelation (in case we observe general decrease of connectivity during pain compared to the baseline). For BOLD and connectivity analyses, we will mask the comparison results (intensity vs. unpleasantness) with the findings from the pain mapping (pain vs. baseline) using a liberal threshold (abs(t) > 2).
Ethics approval and consent to participate
The study was approved by the Medical Sciences Interdivisional Research Ethics Committee of the University of Oxford (MSD-IDREC-C1-2014–157) and conducted in conformity with the Declaration of Helsinki.
Results
Behavioural data
The single-trial pain ratings indicate that the applied cold pain is particularly suited for distinguishing the cortical underpinnings of pain intensity and pain unpleasantness. Unlike previous research8, we did not find any systematic rating difference between both descriptors (paired t-test, p < 0.05,34 ± 14 for pain intensity and 31 ± 17 for pain unpleasantness). Although highly correlated (r = 0.86, p < 0.01, Fig. 2), the majority of the trials exhibited a distinct rating for intensity and unpleasantness (75%, Fig. 2). Only 25% of the trials were rated identically (e.g. 25/100 for intensity and 25/100 for unpleasantness). The trial-by-trial differences in pain ratings can be utilised to elucidate whether the cortical activity is more closely related to pain intensity or to pain unpleasantness.
Description of pain ratings. The left side (A) shows the correlation between pain intensity [I] and pain unpleasantness [U]. The correlation coefficient is r = 0.86. Nevertheless, the ratings for both aspects were identical only for a minority of the trials. The colour-coded points indicate the frequency of the occurrence of each single-trial combination of intensity and unpleasantness ratings. Near-identical ratings of intensity and unpleasantness occur more often than more distant ratings. The histogram (B) shows the difference between pain intensity ratings and pain unpleasantness ratings; most trials (75%) exhibited different ratings. For some trials, pain intensity was rated higher (negative Δ), for others pain unpleasantness was rated higher (positive Δ).
Imaging data - positive BOLD effect
The results indicate a stronger positive relationship for pain unpleasantness in regions that are known to exhibit higher activity in response to experimental pain: the bilateral insula and frontal opercular cortex, pre- and postcentral regions, as well as in frontal regions (p < 0.05, PALM corrected). These positive effects occur exclusively in brain regions that are activated in response to pain in the contrast of the unmodulated pain trials compared to the baseline (pale colours in Fig. 3).
Differences between pain unpleasantness encoding and pain intensity encoding—BOLD. Our results must be interpreted with respect to the overall activation or deactivation of pain stimuli relative to baseline periods. This contrast is represented using pale and transparent colours, with a threshold of abs(t) > 2. The distinction between unpleasantness and intensity is overlaid using opaque colours. Our findings reveal that brain areas activated by pain stimulation (represented by pale red/yellow) exhibit a stronger positive correlation between unpleasantness and brain activity compared to the correlation between intensity and brain activity (opaque red/yellow). Conversely, regions that are deactivated in response to pain (represented by pale blue) exhibit a stronger negative correlation between pain unpleasantness and brain activity compared to pain intensity and brain activity (represented by opaque blue overlays).
Imaging data - negative BOLD effects
Additionally, we found a more positive relationship for pain intensity for cuneal and precuneal regions, the occipital cortex, as well as angular and frontal regions (p < 0.05, PALM corrected). These negative results occur exclusively in brain regions that are suppressed in response to pain in the contrast of the unmodulated pain trials compared to the baseline (pale colours in Fig. 3). Therefore, they reflect a stronger negative relationship between pain ratings and brain activity for unpleasantness (Fig. 3, Table 1).
Imaging data - positive connectivity effects
We did not find any positive effects for two reasons. The comparison for pain trials vs. baseline period exhibited exclusively lower connectivity scores for the pain periods, indicating a cortical decoupling during pain (see Fig. 4).
Differences between pain unpleasantness encoding and pain intensity encoding—connectivity. Similar to the analysis of the BOLD findings, our interpretation of connectivity results is reliant upon the contrast between pain stimulation and baseline phases. The confusion matrix (A) corresponds to the pale/transparent overlay in Fig. 3, in which we computed pain-related processes across all trials and conditions relative to baseline periods with no pain. For connectivity, we identified a generalised disruption of connectivity during pain trials in comparison to baseline periods (t-tests). The comparison of pain intensity and unpleasantness ratings indicated that the disruption of connectivity was more pronounced for unpleasantness ratings (LME) (B). The 3D map in (D) illustrates the brain regions that exhibit a significantly stronger effect for unpleasantness compared to intensity. The threshold for presentation was set at n = 20. The circular plot (E) depicts the contributing brain regions that exhibit a significant effect for unpleasantness compared to intensity.
Imaging data - negative connectivity effects
Throughout all pairs of cortical connections, we found a more positive relationship for pain intensity and connectivity than for pain unpleasantness and cortical connectivity (p < 0.05, PALM corrected). As cortical processes disconnect during pain processing, the contrast between intensity and connectivity implies a stronger disconnection effect for unpleasantness (Fig. 4, Supplementary Spreadsheet 1).
Taken together, the findings across BOLD and connectivity analyses exclusively indicate a stronger effect for unpleasantness. Areas that are activated through pain stimulation show a stronger positive relationship for unpleasantness. In a similar vein, stronger negative relationships in deactivated areas. In addition, unpleasantness ratings are related to a stronger cortical decoupling during pain processing. Consequently, the interpretation of the effects for activity and connectivity is identical: for all brain regions and cortical connections, cortical processes are always tighter connected to unpleasantness ratings. Please note that the differences are in a gradual fashion; due to the fact that both aspects of pain evaluation are highly correlated, we can not assume distinct processes for intensity and unpleasantness.
Discussion
In the present study, we investigated the cortical underpinnings of pain intensity and pain unpleasantness. Despite each aspect sharing the same fundamental core, we took advantage of the sufficient variability, which allowed us to investigate cortical functions related to the subjective experience of pain in a within-subject design at a single-trial level. We show that the emotional-affective aspect of pain is more deeply rooted in the human brain: throughout the brain, we revealed a stronger relationship for the pain-related cortical processes regarding the affective aspect compared to the sensory-discriminative aspect. No brain region showed a stronger relationship to pain intensity. As the processing of long-lasting pain is predominantly represented through disrupted connectivity, as shown here and in our previous work14,27, the current findings also point to a stronger disruption-related effect for pain unpleasantness than for pain intensity.
Regions that increased activity in response to pain had a more positive effect for unpleasantness, and regions and connections that exhibited decreased connectivity showed a more negative effect for unpleasantness. Consequently, unpleasantness ratings may reflect the more direct and intuitive processing of emotions in the human brain rather than the more complex and “minded” evaluation of pain intensity. Due to the methodological differences of previous work and their inherent weaknesses, the present study is not comparable to previous imaging studies13,28. However, our results underline other research indicating the preferential processing of pain-related emotions in the human brain29,30. It has been suggested that the feeling of unpleasantness belongs to the initial part of the noxious sensation and occurs before any conscious and cognitive processing of the sensation31. Therefore, the stronger effect of pain unpleasantness may better reflect the biological function of the pain system to prevent harm and to preserve the physical integrity of the body32.
Indeed, there is a large framework of research that supports the notion that emotional processes are tightly associated with pain processing in the brain. Higher pain affect has been found to increase autonomic responses, such as skin conductance33,34, which in turn were found to encode pain-related cortical responses35,36. Potentially emotion-related (“less minded”) autonomic responses were found to be more tightly related to cortical activity than ratings of pain intensity36.
Our study complements previous findings by Zeidan and colleagues who shed light on individual abilities to modulate pain intensity and pain unpleasantness through meditation28. The better-performing participants were able to make use of specific subunits of the pain modulation system. The participants who exhibited a better performance in the attenuation of pain unpleasantness modulated the activity of the orbitofrontal cortex28. This region has been suggested to serve as a major hub of emotional modulation37. In a similar vein, the hub that modulates the ability to attenuate pain intensity through meditation could be located in the anterior insula28. Depending on the pain attenuation strategy, we would assume the existence of further cortical hubs that are differently utilised by individual subjects and which alter pain intensity and pain unpleasantness14,15.
Limitations
The present study employed prolonged cold pain stimulation, a type of stimulation that has not been extensively studied but was aimed to approximate the long-lasting pain that individuals can experience in everyday life. However, most studies have utilised short and transient painful heat, which—in our view—has less ecological validity. Consequently, the decreased connectivity for prolonged pain stimulation periods may not generalise to other experimental pain conditions. Indeed, unlike our findings with brain-wide disconnection in response to painful stimulation, other studies that investigate pain-related connectivity in response to brief pain have reported increased connectivity during pain perception38,39.
Furthermore, our experimental design was intended to control for the different pain levels in the four experimental conditions: both highly correlated pain descriptors are associated with the same trial, characterised by the same brain activity and connectivity. The levels of pain perception in the four conditions are believed to be accounted for by the first fixed-effect factor (factor: fMRI), and the second fixed-effect factor is thought to explain the specific differential effect (factor rating_type:fMRI) of pain encoding between the two pain descriptors. However, we cannot rule out the possibility of a slight difference in pain encoding for each experimental condition (unconditioned pain, cognitive reappraisal, imagination, distraction) between the two pain descriptors.
Conclusions
The present study shows that pain unpleasantness is more tightly related to cortical processing than the cognitive evaluation of pain intensity. These findings generalise to all brain regions that exhibit pain-related responses, irrespective of whether they increase or decrease their activity. However, the stronger relationship to the affective aspect does not imply that the assessment of pain intensity might be obsolete. Previous research has shown the usefulness of the assessment of intensity, which is more sensitive to change for certain pain conditions than unpleasantness2,8,40. Future studies need to shed further light on either aspect of pain evaluation under specific experimental (e.g. different types of applied pain) or disease-related conditions (episodic or chronic pain). However, across all conditions, we hypothesise temporal differences in the cascade of pain-related cortical processes: the cortical processes of pain unpleasantness evaluation are processed faster and may occur before the cognitive (“minded”) cortical processes of pain intensity evaluation.
Data availability
The data are available at the Open Science Framework (https://osf.io/tbc2u/). No custom code has been developed for this study.
References
Melzack R, Casey KL. 1968. Sensory, motivational, and central control determinants of pain: a new conceptual model in pain In: Kenshalo DR, editor. Springfield, Ill. pp. 423–443.
Price, D. D., Harkins, S. W. & Baker, C. Sensory-affective relationships among different types of clinical and experimental pain. Pain 28, 297–307. https://doi.org/10.1016/0304-3959(87)90065-0 (1987).
Kunz, M., Lautenbacher, S., LeBlanc, N. & Rainville, P. Are both the sensory and the affective dimensions of pain encoded in the face?. Pain 153, 350–358. https://doi.org/10.1016/j.pain.2011.10.027 (2012).
Perlman, D. M., Salomons, T. V., Davidson, R. J. & Lutz, A. Differential effects on pain intensity and unpleasantness of two meditation practices. Emotion 10, 65–71. https://doi.org/10.1037/a0018440 (2010).
Rainville, P., Carrier, B., Hofbauer, R. K., Bushnell, C. M. & Duncan, G. H. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 82, 159–171. https://doi.org/10.1016/S0304-3959(99)00048-2 (1999).
Coghill, R. C., Sang, C. N., Maisog, J. M. & Iadarola, M. J. Pain intensity processing within the human brain: A bilateral, distributed mechanism. J. Neurophysiol. 82, 1934–1943. https://doi.org/10.1152/jn.1999.82.4.1934 (1999).
Hofbauer, R. K., Rainville, P., Duncan, G. H. & Bushnell, M. C. Cortical representation of the sensory dimension of pain. J. Neurophysiol 86, 402–411. https://doi.org/10.1152/jn.2001.86.1.402 (2001).
Rainville, P., Feine, J. S., Bushnell, M. C. & Duncan, G. H. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot. Res. 9, 265–277. https://doi.org/10.3109/08990229209144776 (1992).
Gagnon-Dolbec, A., Fortier, M. & Cormier, S. Pain intensity and pain unpleasantness in triathletes: A study examining their associations with pain catastrophizing and pain expectations. Psychol Sport Exerc https://doi.org/10.1016/j.psychsport.2021.101928 (2021).
Shriver, A. The unpleasantness of pain for humans and other animals. Philosophy. Pain https://doi.org/10.4324/9781351115865-8 (2018).
Schreckenberger, M. et al. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology 64, 1175–1183. https://doi.org/10.1212/01.WNL.0000156353.17305.52 (2005).
Zunhammer, M., Geis, S., Busch, V., Eichhammer, P. & Greenlee, M. W. Pain modulation by intranasal oxytocin and emotional picture viewing — a randomized double-blind fMRI study. Sci. Rep. https://doi.org/10.1038/srep31606 (2016).
Rainville, P., Duncan, G. H., Price, D. D., Carrier, B. & Bushnell, M. C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971 (1997).
Schulz, E. et al. Ultra-high-field imaging reveals increased whole brain connectivity underpins cognitive strategies that attenuate pain. Elife https://doi.org/10.7554/eLife.55028 (2020).
Schulz, E., Stankewitz, A., Witkovský, V., Winkler, A. M. & Tracey, I. Strategy-dependent modulation of cortical pain circuits for the attenuation of pain. Cortex https://doi.org/10.1016/j.cortex.2018.12.014 (2019).
Erpelding, N. & Davis, K. D. Neural underpinnings of behavioural strategies that prioritize either cognitive task performance or pain. Pain 154, 2060–2071. https://doi.org/10.1016/j.pain.2013.06.030 (2013).
Lautenbacher, S., Roscher, S. & Strian, F. Tonic pain evoked by pulsating heat: temporal summation mechanisms and perceptual qualities. Somatosens Mot. Res. 12, 59–70 (1995).
Stankewitz, A. et al. Pain sensitisers exhibit grey matter changes after repetitive pain exposure: A longitudinal voxel-based morphometry study. Pain 154, 1732–1737. https://doi.org/10.1016/j.pain.2013.05.019 (2013).
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015 (2012).
Griffanti, L. et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage 95, 232–247. https://doi.org/10.1016/j.neuroimage.2014.03.034 (2014).
Glasser, M. F. et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. https://doi.org/10.1038/nature18933 (2016).
Michail, G., Dresel, C., Witkovský, V., Stankewitz, A. & Schulz, E. Neuronal oscillations in various frequency bands differ between pain and touch. Front. Hum. Neurosci. 10, 182. https://doi.org/10.3389/fnhum.2016.00182 (2016).
Witkovský, V. Estimation, testing, and prediction regions of the fixed and random effects by solving the Henderson’s mixed model equations. Meas. Sci. Rev. 12, 234–248 (2012).
Wilkinson, G. N. & Rogers, C. E. Symbolic description of factorial models for analysis of variance. J. R. Stat. Soc. Ser. C Appl. Stat. 22, 392–399. https://doi.org/10.2307/2346786 (1973).
Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M. & Nichols, T. E. Permutation inference for the general linear model. Neuroimage 92, 381–397. https://doi.org/10.1016/j.neuroimage.2014.01.060 (2014).
Wager, T. D. et al. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 368, 1388–1397. https://doi.org/10.1056/NEJMoa1204471 (2013).
Mayr, A. et al. Individually unique dynamics of cortical connectivity reflect the ongoing intensity of chronic pain. Pain https://doi.org/10.1097/j.pain.0000000000002594 (2022).
Zeidan, F. et al. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci. 31, 5540–5548. https://doi.org/10.1523/JNEUROSCI.5791-10.2011 (2011).
Baliki, M. N. et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J. Neurosci. 26, 12165–12173. https://doi.org/10.1523/JNEUROSCI.3576-06.2006 (2006).
Zhou, F. et al. Empathic pain evoked by sensory and emotional-communicative cues share common and process-specific neural representations. Elife https://doi.org/10.7554/eLife.56929 (2020).
Kong, J. et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum. Brain Mapp 27, 715–721. https://doi.org/10.1002/hbm.20213 (2006).
Wiech, K. & Tracey, I. Pain, decisions, and actions: a motivational perspective. Front. Neurosci. 7, 46. https://doi.org/10.3389/fnins.2013.00046 (2013).
Geuter, S., Gamer, M., Onat, S. & Büchel, C. Parametric trial-by-trial prediction of pain by easily available physiological measures. Pain 155, 994–1001. https://doi.org/10.1016/j.pain.2014.02.005 (2014).
Loggia, M. L., Juneau, M. & Bushnell, C. M. Autonomic responses to heat pain: Heart rate, skin conductance, and their relation to verbal ratings and stimulus intensity. Pain 152, 592–598. https://doi.org/10.1016/j.pain.2010.11.032 (2011).
Mobascher, A. et al. Fluctuations in electrodermal activity reveal variations in single trial brain responses to painful laser stimuli–a fMRI/EEG study. Neuroimage 44, 1081–1092. https://doi.org/10.1016/j.neuroimage.2008.09.004 (2009).
Nickel, M. M. et al. Autonomic responses to tonic pain are more closely related to stimulus intensity than to pain intensity. Pain 158, 2129–2136. https://doi.org/10.1097/j.pain.0000000000001010 (2017).
Rolls, E. T. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia 128, 14–43. https://doi.org/10.1016/j.neuropsychologia.2017.09.021 (2019).
Ploner, M., Lee, M. C., Wiech, K., Bingel, U. & Tracey, I. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex 21, 719–726. https://doi.org/10.1093/cercor/bhq146 (2011).
de Oliveira Franco, Á. et al. Hyper-connectivity between the left motor cortex and prefrontal cortex is associated with the severity of dysfunction of the descending pain modulatory system in fibromyalgia. PLoS One 17, e0247629. https://doi.org/10.1371/journal.pone.0247629 (2022).
Wade, J. B., Dougherty, L. M., Archer, R. C. & Price, D. D. Assessing the stages of pain processing: A multivariate analytical approach. Pain 68, 157–167. https://doi.org/10.1016/S0304-3959(96)03162-4 (1996).
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was funded by the “Deutsche Forschungsgemeinschaft” (DFG, SCHU 2879/1–2)”.
Author information
Authors and Affiliations
Contributions
A.S. and E.S. contributed to the conception and design of the study, acquisition and analysis of the data and drafting the manuscript and figures. A.M. contributed to the analysis of the data and drafting the manuscript. S.I. contributed to drafting the manuscript. V.W. contributed to the analysis of the data and drafting the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stankewitz, A., Mayr, A., Irving, S. et al. Pain and the emotional brain: pain-related cortical processes are better reflected by affective evaluation than by cognitive evaluation. Sci Rep 13, 8273 (2023). https://doi.org/10.1038/s41598-023-35294-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35294-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.