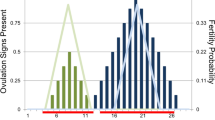
Abstract
Many species exhibit seasonal patterns of breeding. Although humans can shield themselves from many season-related stressors, they appear to exhibit seasonal patterns of investment in reproductive function nonetheless, with levels of sex steroid hormones being highest during the spring and summer months. The current research builds on this work, examining the relationship between day length and ovarian function in two large samples of women using data from the Natural Cycles birth control application in each Sweden and the United States. We hypothesized that longer days would predict higher ovulation rates and sexual motivation. Results revealed that increasing day length duration predicts increased ovulation rate and sexual behavior, even while controlling for other relevant factors. Results suggest that day length may contribute to observed variance in women’s ovarian function and sexual desire.
Similar content being viewed by others
Introduction
In many habitats, environmental conditions vary considerably across seasons. These shifting conditions can present distinct survival and reproductive challenges to the organisms that inhabit them1. Winter months are characterized by lower temperatures, reductions in food availability, and increased risk for certain infectious diseases relative to summer months2,3,4,5. Such stressors demand investment in somatic maintenance, which often comes at the cost of investment in reproduction, particularly when energetic resources are limited6,7,8. Accordingly, many species exhibit breeding patterns that allow for the most costly expenditures in reproduction—such as those incurred from birthing and lactation—to occur during the summer months when exposure to environmental stressors is low9,10,11,12,13. Thus, for species with short (< 6 months; eg., goats and sheep14) or long (≥ 12 months; e.g., horses15) gestation periods, reproductive activity is highest in the summer months to allow conception and birthing to occur during months when an organism’s internal and external resources are abundant and favorable for gestation, birth, and lactation.
Although seasonal variability in reproductive function is well-established across sexes in a variety of non-human species—including rodents16,17, domestic cats and dogs18,19, and ring-tail lemurs20—much less is known about the impact of seasonal changes on human reproductive rhythms. Some initial evidence supports that human reproductive rhythms may be subject to seasonal variation. For example, research finds that levels of the sex hormone testosterone and investment in mate attraction and sexual behavior21,22,23,24 each peak in the spring and summer months. Others find that human conceptions peak in the spring and summer months when days are longer and reach a low point during the winter months when days are shorter25,26. These patterns are reasoned to occur in response to the changing environmental conditions in the spring and summer months, which are more favorable for embryonic development26 and implantation27. Indeed, these patterns are found to be robust to gradations in latitude25,28,29 and persist even after controlling for seasonal differences in sexual behavior30. Further, although the strength of seasonal influences on reproductive behavior varies across geographies31, populations32, and time33, they continue to be observed despite contraception allowing individuals to exert more control over reproductive timing based on the demands of the school calendar e.g.4, cyclical farming obligations e.g.9 and seasonal migratory employment e.g.34.
In the following, we seek to build on this prior work, examining whether human females exhibit seasonality in their reproductive function. Specifically, we used data collected from two large samples of women using the Natural Cycles fertility tracking application in each Sweden and the United States (U.S.) to examine the relationship between photoperiod—a reliably-occurring seasonal modulator of reproductive activity6,25,30—and (a) ovulation rates and (b) sexual motivation. We predicted that photoperiod would be positively related to women’s ovulation rate, with women ovulating more frequently when the days are longer. Additionally, we predicted that these patterns would be accompanied by complementary changes in women’s sexual motivation and behavior, with these outcomes also increasing when days are longer. Results seek to provide insight into human female reproductive function that have implications each for theory, clinical practice, and healthcare planning.
Unlike many species, contemporary humans are able to protect themselves from many of the energetic and immunological stressors associated with the fall and winter months. Such protections are a relatively new fixture in human evolutionary history, however. One of the legacies of these exposures is that many human biological systems—such as those that govern energy expenditure35, hormone release36,37,38,39, and immune function12—vary seasonally, similar to what is observed in seasonal breeders. For example, research finds powerful effects of photoperiod on multiple facets of immunological function, such as peripheral blood mononuclear cell proliferation12 which each peak in the winter months, when exposures to harsh environmental conditions would have historically been high38,40,41.
The season-based patterns in immune function and bodily repair are the opposite of the patterns observed for sexual function and reproduction, which peak in the spring and summer months. Despite the growing body of evidence suggesting seasonal patterns in testosterone production and conception rates, little is known about the impact of seasonality on human female ovarian function. Do women exhibit seasonal patterns of ovulation, similar to what is observed in other species of seasonally breeding organisms? Although most healthy, reproductive-aged women ovulate with a relatively high degree of regularity, research indicates that between 3.4% and 18.6% of these women’s cycles are anovulatory42,43. To date, factors that have been identified as contributors to this variability are almost exclusively person-based, such as differences in women’s obesity status44, history of anovulatory events42, fiber intake45, energy availability22,37,46, exercise intensity47, and exposure to stress29,48. Little attention has yet been given to the possibility that variability in human ovulation rates may also be accounted for by the changing seasons, with ovulation rates being higher as the days get longer.
The current study was designed to examine this possibility. Specifically, we use data collected from two large samples of women using the Natural Cycles fertility tracking application from each Sweden and the United States (U.S.). We limit our focus to Sweden and the U.S. because these are the two geographic samples for which we have regional information, which we then use to estimate photoperiod. The final sample included approximately 65,240 women with 353,411 reported cycles from the United States and 10,940 women with 72,498 reported cycles from Sweden. We predicted that longer days would correspond to a greater frequency of ovulatory cycles. Further, consistent with the idea that greater investment in female ovarian function in this context emerges in response to a larger prioritization of mating and reproductive goals during the spring and summer months, we predicted that (a) photoperiod would also predict increased sexual desire and behavior and (b) all patterns would persist while controlling for relevant biological factors (e.g., women’s menstrual cycle length), as well as secondary environmental factors (e.g., temperature) (Table 1).
Results
Target model
Ovulation rate
In both samples (U.S. and Sweden), day length significantly predicted ovulation rate across multiple indicators of ovulation (Table 2). In the U.S. sample, results revealed that there was a significant positive relationship between day length and ovulation across all four measures of ovulation: Natural Cycles ovulation (p ≤ 0.001), Coverline ovulation (p ≤ 0.001), 3-over-6 ovulation (p ≤ 0.001), and LH tests (p ≤ 0.01), such that, women exhibited increased rates of ovulation as days grew longer. Three of these positive relationships hold with significance in the Swedish sample (Natural Cycles, p ≤ 0.001; Coverline, p ≤ 0.001; 3-over-6, p ≤ 0.001). Women in the Swedish sample instead exhibited a significant negative relationship between day length and ovulation as measured using LH tests (p ≤ 0.05).
Logged libido and sexual behavior
In addition to examining the relationship between day length and ovulation rate, we also assessed whether day length was associated with corresponding effects on women’s logged libido and sexual behavior frequency (see Table 2). For these analyses, we utilize a generalized Gaussian mixed-effects model, as logged libido and logged sexual behavior are continuous, cycle-specific variables. Results showed that across both geographic samples, day length was a significant positive predictor of sexual behavior (p ≤ 0.001). Studying the relationship between day length and logged libido, we found a significant positive association in the U.S. sample (p ≤ 0.001) and a nonsignificant relationship in the Swedish sample (p > 0.10).
Follow-up models
Do the results of the target models hold when controlling for temperature?
When controlling for the daily temperature of each individuals’ central logged location, the relationship between day length and ovulation rate persisted (see Table 3). That is, day length remained a significant positive predictor of ovulation rate across the three temperature-based measures of ovulation (i.e., Natural Cycles algorithm, Coverline, and 3-over-6; ps ≤ 0.009). While controlling for daily temperature, day length instead is not a significant predictor of LH test measured ovulation rate (U.S., p = 0.084, and Sweden, p = 0.066), however the trend of these results is consistent with results of the other ovulation rate measures, with longer days being associated with increased rates of ovulation.
Similarly, we examined the impact of day length on logged libido and sexual behavior in the US sample and found that day length remained a significant positive predictor of both logged sexual behavior frequency and libido (p ≤ 0.001). However, these relationships became nonsignificant in the Swedish sample (ps ≥ 0.40; see Table 3).
Do the results hold when we restrict our results to participants likely to be fertile (ages 18–45)
Further restricting analyses to women between 18 and 45 years old, the positive association between day length and ovulation rate remained significant across the three basal body temperature measures of ovulation rate (i.e., Natural Cycles algorithm, Coverline, and 3-Over-6) in both U.S. and Sweden samples (ps ≤ 0.01) (Table 4). As with the prior alternative models, day length was not significantly associated with LH test measured ovulation rate in the Sweden samples, (p = 0.12) and a significant positive predictor in the US sample (p = 0.02), when restricting analyses to women between 18 and 45 years old. In the U.S. sample, day length remained a significant positive predictor of both logged sexual behavior (p ≤ 0.001) and libido (p ≤ 0.001). As with the earlier analyses, we found both relationships to be nonsignificant in the Swedish sample.
Influence of equinox on ovulation rate
We find R2 scores of < 0.05 when we apply peak fitting approaches, and that the difference between ovulation rates for days with 12 h are not significantly different from ovulation rates for days with approximately equivalent day length and night length (day length measured to be between 11 and 13 h), and all other days (ps > 0.12). When considering logged libido and logged sex as dependent variables, we find similar results in each geographical cohort (ps > 0.4 in both analyses). Therefore, the results suggest that there are no significant effects of spring or autumn equinoxes on the relationship between day length and ovulation rate, logged libido, and sexual behavior.
Robustness to range normalization
GPBoost relies on gradient descent to identify model parameters. Gradient descent is known to be sensitive to the scale of different covariates. Here, we sought to replicate the earlier experiments on range-standardized data while standardizing the measure of day length to values between 0 and 48, corresponding to units of half hours. This closely reflects the ranges of the other covariates: age (17–64), BMI (13–56), and cycle length (15–40).
Although the model fit is not significantly different (as measured by log likelihood on a held-out test set), the observed pattern of results differed somewhat (Tables 5, 6, 7). Specifically, all reported results remained significant in the US cohort (with the exception of LH test results, discussed below), but we found fewer significant relationships between day length and ovulation in the Swedish cohort.
Within the Swedish cohort, using these models yielded no relationship between day length and ovulation measured using Coverline or the Natural Cycles algorithm (ps > 0.10). When controlling for temperature, we found that no measure of ovulation—barring 3-over-6 ovulation (p ≤ 0.001)—was significantly related to day length (ps > 0.30), and day length was no longer related to logged sex or libido (ps > 0.40) (Table 6). Restricting the sample to those users most likely to be fertile (i.e., users between the ages of 18 and 45) revealed a positive significant relationship between day length and each measure of ovulation (ps ≤ 0.005) with the exception of the LH test measure (similar to what was observed in the original model) but no relationship between day length and logged sex or logged libido (ps > 0.10) (Table 7).
As noted, the original results from the US sample replicated with the exception of the LH test results in the model that controlled for temperature. The results of this model again found a significant relationship between day length and ovulation as indicated by LH test results (p ≤ 0.01); however, the relationship between these variables became negative, with increased day length being associated with decreased rates of ovulation as indicated by a positive LH test. Potential explanations for these patterns are discussed below.
Discussion
Many species exhibit seasonal patterns of breeding, with mating effort being highest in the summer months, when resource availability is high and exposure to environmental stressors is low. For example, reproductive activity is highest in the summer for long day breeding species, such as those with short or very long gestation period that breed and birth offspring in the summer. Conversely, species with moderate gestation periods (6–8 months) exhibit increased gonadal function during the fall months so that later stages of gestation and more energetically costly functions, such as lactation, then occur during the summer. Despite the growing body of evidence suggesting seasonal patterns in each testosterone production and conception rates, little is known about the impact of seasonality on human female ovarian function. The results of the present study provide some of the first evidence to date demonstrating that ovarian function may also vary seasonally.
Specifically, the majority of our statistical models found support for the hypothesis that months with longer days were associated with higher rates of ovulation, even when controlling for several individual and personal and environmental factors associated with ovulation in human females (e.g., age, BMI, cycle length) or day length (ambient temperature). Further, the majority our statistical models found support for the relationship between day length and ovulation being one that generalized (a) across samples from two geographically distinct locales (the U.S. and Sweden), particularly when restricting the analyses to include only those users who are likely to be fertile, and (b) across three different ovulation indexes (i.e., ovulation as labeled by the Natural Cycles ovulation detection algorithm, the 3-Over-6 method, or the Coverline method). Lastly, we found no evidence of equinox effects on the relationship between day length and ovulation rate in either sample.
While many of our findings did generalize across both geographic samples, there were some discrepant results that emerged when we attempted to replicate the results using a range-standardized measure of photoperiod. In particular, we found that many of the relationships between day length and women’s ovulation, sexual behavior, and sexual motivation did not reach significance in the Swedish sample when the data was range-standardized (although it is worth noting that the range-standardized results did replicate when run only on the likely-fertile users).
Although unanticipated, there are a variety of factors that could have contributed to this pattern. First, given that the pattern replicated in the Swedish sample when the sample was restricted to women who were most likely to be fertile, it is possible that the relationship between photoperiod and ovulation rate is most evident in younger women due to the greater between-cycle variability that emerges as women age. Second, it is possible that these results may have failed to emerge in response to the relatively narrow range of possible daylight hours between individual Swedish women during a given time of year due to Sweden’s relatively small land mass combined with the relatively broad range of possible daylight hours within Swedish women across seasons due to Sweden’s relatively large distance from the equator (which grants them far fewer daylight hours in the winter and far more daylight hours in the summer than what is observed in the mainland U.S.). These differences in between- and within- individual variability in day length, particularly when combined with the much-smaller Swedish sample size, may have contributed to the failure of the range standardized models to yield the expect results from the full Swedish sample, as the estimated effects were based on both between and within women effects.
It is additionally possible that the discrepant results between the U.S. and Swedish samples could instead be due to cultural differences interacting with photoperiod effects to impact ovulation rates. For example, research finds that Swedish individuals, on average, maintain a higher quality of life than U.S. individuals49. Therefore, it could be that some of the factors contributing to quality of life among Swedish individuals buffer the effects of environmental stressors on women’s fertility. Future research would benefit from examining this possibility.
It is also important to address the discrepancies that arose between models that used LH tests as the marker of ovulation. While our unstandardized models found a positive relationship between day length and the frequency of positive LH tests among U.S. women, the standardized model—as well as both models run using this ovulation diagnostic conducted on the data from the Swedish sample—found the opposite. These contrasting LH results may have emerged in response to LH tests’—despite being a popular method of predicting impending ovulation—high rate of false negatives8,23.
It is also possible that these contrasting results could reflect levels of LH being inversely related to the probability of successful ovulation across (but not within) cycles. Much research finds that levels of pituitary releasing hormones (of which LH is one) are often negatively related to receptor density and efficiency50,51,52. If the seasonal changes in ovulation rate are mediated by changes in the efficiency of signaling between LH and ovarian LH receptors (with less efficient signaling occurring in the winter months) this could correspond to both a) increased pituitary release of LH and b) lower rates of successful ovulation. Given that LH tests are often not sensitive enough to reliably detect pre-ovulatory levels of LH, women may be most likely to have a positive LH test during cycles in which the LH-ovarian communication pathway is least likely to result in successful ovulation. This interpretation is consistent with past work demonstrating that high levels of LH are associated with disrupted ovulation in some contexts53. Future research would benefit from examining this possibility. Regardless, the LH test measured ovulation results should be interpreted with caution, especially considering the reduced sample size of cycles that had available LH test results.
In addition to the relationship between day length and ovulation rate, we also found evidence of links between day length and women’s self-reported sexual motivation and logged sexual behavior. Women reported having higher libido and logged more sexual behavior on longer days than shorter ones. As with ovulation rate, the sexual motivation and behavior effects in U.S. women remained significant while controlling for variations in relevant individual and environmental factors. However, changes in day length were unrelated to changes in libido among Swedish women. As the libido-specific analyses were restricted to women’s cycles that included at least one logged entry for libido—that is, we exclude cycles which appear to have a libido of 0 due to an absence of logs, rather than logged low libido—the sample sizes for these analyses were reduced to 9,739 cycles (3,854 participants) from Sweden and 68,417 cycles (27,175 participants) from the U.S. This reduced sample size provides one possible explanation of the lack of a significant relationship between day length and logged libido in the Swedish sample. It is also possible that cross-cultural differences in sexual attitudes and behaviors may have contributed to these patterns.
Overall, despite some notable between-location differences, the observed relationships between day length and ovulation rate, sexual motivation, and sexual behavior in the present study are generally consistent with much research in the evolutionary biological sciences that suggest an important role of physical environmental conditions—including day length—in influencing how organisms regulate investment in reproductive activity38,54 and adapt trade-offs in somatic maintenance versus reproduction55,56. Together, these results suggest that human physiological systems—including the functioning of women’s HPG axes—may be seasonally sensitive. That these patterns continue to persist despite the numerous modifications to our environment to minimize the experienced impact of seasonal changes (e.g., temperature-controlled homes, artificial light sources, etc.), is a testament to their embeddedness in our biology. Taking these differences into account could play an important role in shaping the future of contraception, fertility planning, and fertility treatments, as the success of each of these outcomes is likely to be touched by the observed seasonal patterns in reproductive function.
Along with differences in between-location relationships, there are several limitations of the present study that should be considered. First, there are theoretical limitations. Specifically, we are unable to determine whether the observed relationship between day length and ovulation rate has emerged in response to greater investment in reproductive function during the summer months or decreased investment in reproductive function during the winter months. Given past research finds increased investment in immunological activity and concomitant reductions in total levels of the sex hormone testosterone in men during shorter days12, the effects observed in the current results may be (a) driven by adaptations designed to promote reproduction in the spring and summer months or (b) a byproduct of increased investment in bodily maintenance and repair during the fall and winter months. Future work would benefit from identifying the mechanisms that underlie these seasonal patterns to better understand what, if any, adaptive function they might serve.
Additionally, although the present study was able to rule out ambient temperature as an alternative driver of the observed relationship between day length and female reproductive function, temperature is but one of many possible environmental factors that vary alongside seasonal shifts in biological and behavioral functioning. Future research would therefore also benefit from collecting a broader range of environmental measures that have been implicated in human seasonality research, such as vacations and time-off of work. Lastly, the present research does not experimentally assess the biological mechanisms driving fluctuations in ovulation rate as a function of day length, which provides an important area for future research. One such biological mechanism that could present a promising direction for future research is the role of pineal melatonin. Pineal melatonin secretion is inversely proportional to day length duration1,57, and is implicated in the seasonal reproductive functioning of several non-human animals21,58.
Conclusions
Through examination of longitudinal data from two large samples across two countries, the present research finds that human females’ ovarian function may be related to day length, with longer days being associated with higher ovulation rates. The present results also found complementary shifts in women’s sexual motivation and behavior, with women logging higher libido and more sexual behavior during times in the year with longer days relative to shorter. These results are consistent with a growing body of work demonstrating powerful seasonal patterns in human biological systems, many of which can have implications for everything ranging from healthcare to population planning.
Method
Natural cycles health application
Data were obtained from users of Natural Cycles, an FDA cleared birth control app. The app uses a proprietary statistical algorithm that estimates the probability of conception on a given day. Users are recommended to take their temperature 5 times per week before getting out of bed first thing after waking up. They input this temperature into the app before it gives a daily fertility status. There are three modes in the app, NC° Birth Control, NC° Plan Pregnancy, and NC° Follow Pregnancy, allowing users to choose the most suitable option for their current intentions. Most women start using the app in NC° Birth Control, that is, to prevent a pregnancy by avoiding unprotected intercourse during days which the app calculates to be fertile days. Users may subsequently change their intentions and are instructed to register this by switching to NC° Plan Pregnancy, in which the app serves as an aid to the timing of intercourse to achieve conception.
The core of accurately detecting ovulation is the same for both products, but there are differences in the risk profile. For NC° Birth Control, the algorithm is optimized to have a very low risk of wrongly attributing a green (safe) day in the fertile window, while for NC° Plan Pregnancy, it is optimized to better isolate the fertile window and the most fertile days. The same colors are displayed (red for fertile and green for not fertile); however, in NC° Birth Control a day is either strictly red or green, whereas in NC° Plan Pregnancy a color scale is used during the fertile window.
The Natural Cycles algorithm identifies ovulation retrospectively based on the first day of menstruation and basal body temperatures, which may be supplemented by positive urinary LH tests. Basal body temperatures are recorded each morning using a thermometer sensitive to the hundredth place, and with measures excluded if the user reports any illness, alcohol intake, or changes in sleep that might influence basal temperatures. To reduce the risk of misidentifying ovulations, the algorithm reports ovulation by rising basal body temperature only if the average temperature from three consecutive calendar days is greater than the woman’s follicular phase average and her baseline average across all data entries, as well as consistent with her luteal phase average. If no temperature rise is observed and the data quality and quantity is deemed sufficient, the cycle is flagged as anovulatory.
Participants
Participants included an initial sample of 293,224 women between the ages of 18 and 63 years old with approximately 1.7 million logged cycles on the Natural Cycles application. Participants provided informed consent for the use of their data in research during registration. The Texas Christian University Institutional Review Board has waived the need for ethical approval in this study (IRB-2022-459). All experiments were performed in accordance with the relevant guidelines and regulations. Participants in this initial sample included women who logged at least one menstrual cycle and declared no ovulation-disrupting preconditions (i.e., PCOS, endometriosis, menopause, or thyroid-related conditions). We excluded data from cycles that were greater than 40 days in length and cycles in which women logged fewer than 10 basal body temperature measurements. For participants who reported a miscarriage, pregnancy, or taking an emergency contraceptive, we excluded the cycle in which the event was recorded and the cycle immediately following. If a pregnancy is reported, we excluded the two cycles immediately following the pregnancy.
Next, we isolated data from the two largest geographical cohorts with regional information present in the sample, which included women living in each Sweden and the United States. These cohorts served as our data analytic sample. The final sample included approximately 65,240 women with 353,411 reported cycles from the United States and 10,940 women with 72,498 reported cycles from Sweden.
From each of these samples, data from women were excluded if they lacked sufficient geographical specificity to assess day length. This included women from the following regions: AP (Armed Forces Pacific), AE (Armed Forces Europe), MH (Marshall Islands), AA (Armed Forces America), or lack of region recognition (FM and BY). This exclusionary criterion only affected women from the U.S. cohort, leading to the removal of 9 participants’ data. After this exclusion, The US sample included 65,231 women reporting 353,351 cycles. We also exclude cycles for which we have no temperature data, to ensure consistency in the cohorts used for each model. This leaves 61,696 participants and 326,137 cycles in the US data and 8,678 participants and 56,936 cycles in the Swedish data. Sample characteristics can be found in Table 1. The maximum number of cycles reported by a single participant is 13 and the minimum is 1.
Measures
Measures of ovulation rate
Ovulation rate was assessed using basal body temperature (BBT) based ovulation detection algorithms (confirmatory measures of ovulation) and luteinizing hormone (LH) tests. Basal body temperature increases slightly—typically less than a 1/2 degree F (0.3 C)—when ovulation occurs. Ovulation is confirmed using BBT when the slightly higher temperature remains steady for three days or more. In this way, BBT-based measures are confirmatory, as they label a cycle as ovulatory based on physiological changes that occur in the body as a consequence of ovulation. LH tests, on the other hand, are anticipatory, as these tests measures levels of luteinizing hormone in the urine, which only reach detectable levels prior to ovulation, when pituitary release of LH surges to prompt ovarian release of the now mature ovum.
To measure participants’ ovulation rate, we used 4 measures, including two established basal body temperature-based ovulation prediction algorithms (the Coverline and 3-over-6 methods), one using a proprietary basal body temperature-based ovulation prediction algorithm (the Natural Cycles ovulation detection algorithm), and a final measure assessing ovulation based on hormonal measurements (LH tests). The Coverline method59 operates by examining the first 10 days of a participant’s menstrual cycle. The ‘coverline’ is then calculated by taking the maximum basal body temperature measurement from the first 10 days, adding to it 0.15 degrees (Fahrenheit), and then identifying if any subsequent temperature exceeds this threshold. If so, the cycle is declared ovulatory. The 3-over-6 method60 declares a cycle as ovulatory based on the presence of three consecutive basal body temperature measurements that exceed the maximum of the preceding six temperature measurements during the same cycle. We also used the Natural Cycles ovulation label and LH test results (when available) as robustness checks. The Natural Cycles proprietary algorithm makes use of prior logged data from the Natural Cycles application to determine whether ovulation occurred. LH tests detect a spike in luteinizing hormone, which precedes ovulation and were recorded for 31% of available cycles.
Day length
Day length was operationalized as the time elapsed between sunrise and sunset. These data were imported from the Suntime library61 based on participants’ geographical coordinates. Geographic coordinates for day length measurement were assessed using the central latitude and longitude of a participant’s reported county (Sweden) or state (United States). For example, all participants in the state of California were assigned the geographic coordinates (latitude = 33.944197°, longitude = -118.402446°), participants in the state of Massachusetts were assigned the geographic coordinates (latitude = 42.4072°, longitude = 71.3824°), and so on. By assuming each participant is at the state or county’s center, we introduce a day length estimation error of up to 25 min. Note that this corresponds studying the impact of absolute day length, rather than relative day length (i.e. days growing longer or shorter).
Daytime temperature
While day length is a powerful predictor of upcoming seasonal changes for a wide variety of species62,63,64, including humans (e.g. 12,), it is possible that it is not the preeminent seasonally-variable cue that modulates women’s ovarian function (e.g. 34,). For example, given that temperature extremes can initiate physiological stress responses (see65 for review), which can disrupt ovulation66, it is possible that the predicted relationship between day length and ovulation rate may occur as a byproduct of each of these variables being linked to temperature. To test for this alternative account of the predicted results, we used the Meteostat library67 to import data on location-specific temperature for each participant using the same latitude and longitude estimate described above. Temperatures were imported for the start date for each reported cycle. We exclude cycles where Meteostat does not offer the temperature (this occurs for ~ 10% of the cycles).
Logged libido and logged sex
On each cycle day, participants have the opportunity to log their libido and sexual behavior within the app. Options for libido are: low (1), medium (2), or high (3). Options for logging sexual behavior are: had unprotected sex, had protected sex, or had no sex.
All logged sexual behaviors (“had sex” and “had unprotected sex”) were first collapsed into a single variable “had sex”. Next, on days in which participants did not log libido or sexual behavior, the missing data was assigned a score of 0. To calculate each cycle’s average libido and sexual behavior, we then dropped all days with a score of zero to ensure that our estimates of libido and / or sexual behavior were not artificially low for those participants who did not log frequently. We then took the average of each reported libido and sexual behavior score from the data logged across the remaining days. For example, if a participant has logged sexual activity on 3 of 4 daily logs in a given cycle, this cycle would receive a sexual activity score of 0.75.
Data analysis plan
Prior to analyzing the data, we examined each covariate (age, body mass index [BMI], cycle length, and day length) for normality and found each to be approximately normal and need no transformation. A large majority of participants’ daily logs did not record libido or sexual activity (96% and 82% respectively). Consequently, imputation was not feasible and cycle aggregate measures were computed for each behavioral measure, instead.
It is rare for a participant to log BBT during each day of the data collection period. Since most temperature-based ovulation classifiers require sequences of recorded temperatures, we estimate missing BBT measurements using data imputation. To do this, we will use an approach called cubic spline-fitting, which allows estimation of a continuous curve from a set of points. Cubic spline-fitting is an appropriate interpolation method as, (1) it is robust to uncertain measurements (logged BBT can be irregular due to the time of measurement and participant behavior, including diet, exercise, and sleep) and (2) this approach demonstrates lower error than other polynomial-based fitting procedures. Further, this approach has also been supported by recent literature examining BBT imputation (see e.g. 63,). Concisely, cubic spline-fitting fits a set of piecewise cubic functions to the observed data to produce a twice-differentiable estimate of the true function (in this case, a function that maps cycle day to temperature). The method estimates the piecewise cubic-function through a system of equations derived from the differentiability constraints (for example, that the second derivatives at boundary points between adjacent pieces of the function are equivalent). Differentiability constraints are important to ensuring that the curve is smooth through all observed measurements.
We will use generalized logistic mixed effects models to test our predictions. This model was chosen to adjust for the multilevel nature of the data (i.e., cycles nested within participants), and to account for the binary nature of the outcome variable (i.e., did or did not ovulate at each cycle). Random intercepts will be added per participant to control for the nested data structure. We considered random slopes per participant and found that the model failed to converge due to missing data. Model parameters will be found using the GPBoost software68 and we will report beta coefficients, p-values, and standard errors to demonstrate the robustness of the analysis.
In each analysis, we will adjust for three covariates with established relationships to ovulation: age, BMI, and cycle length. Follow-up analyses will be performed to identify whether the relationship between day length and each ovulation, logged libido, and sexual behavior persist while controlling for seasonal differences in ambient temperature. We then restrict our focus to participants aged 18–45 years old, who are most likely fertile, to examine the stability of our findings. Additionally, we will examine the robustness of our findings to covariate normalization by including day length in units of half-hours, where the covariate mimics the ranges of BMI (up to 56), age (up to 63), and cycle length (up to 40 days). Finally, because others have found evidence that human conception rates peak near the spring equinox45, we will test for the presence of equinox effects on each ovulation, logged libido, and sexual behavior. Each of the above analyses will be considered with respect to all 4 methods of ovulation rate measurement: the Natural Cycles method, the Coverline method, the 3-over-6 method, and LH tests.
Data availability
Data that support this study are available from the corresponding author (D.S.) subject to data access approval from Natural Cycles. Due to the sensitivity of the underlying data, the data cannot be made public.
References
Walton, J. C., Weil, Z. M. & Nelson, R. J. Influence of photoperiod on hormones, behavior, and immune function. Front. Neuroendocrinol. 32(3), 303. https://doi.org/10.1016/j.yfrne.2010.12.003 (2011).
Demas, G. E. & Nelson, R. J. Photoperiod, ambient temperature, and food availability interact to affect reproductive and immune function in adult male deer mice (Peromyscus maniculatus). J. Biol. Rhythms 13(3), 253–262. https://doi.org/10.1177/074873098129000093 (1998).
Enders, L. S. & Nunney, L. Seasonal stress drives predictable changes in inbreeding depression in field-tested captive populations of Drosophila melanogaster. Proc. R. Soc. B: Biol. Sci. 279(1743), 3756–3764. https://doi.org/10.1098/rspb.2012.1018 (2012).
Martinez, M. E. The calendar of epidemics: Seasonal cycles of infectious diseases. PLoS Pathog. 14(11), e1007327. https://doi.org/10.1371/journal.ppat.1007327 (2018).
Wunder, B. A. 5. Morphophysiological Indicators of the Energy State of Small Mammals. In 5. Morphophysiological Indicators of the Energy State of Small Mammals, 83–104. Cornell (University Press, 2019). https://doi.org/10.7591/9781501737978-006
Dawson, A., King, V. M., Bentley, G. E. & Ball, G. F. Photoperiodic control of seasonality in birds. J. Biol. Rhythms 16(4), 365–380. https://doi.org/10.1177/074873001129002079 (2001).
Foster, R. G. & Roenneberg, T. Human responses to the geophysical daily. Annual Lunar Cycles. Curr. Biol. 18(17), R784–R794. https://doi.org/10.1016/j.cub.2008.07.003 (2008).
McGovern, P. G. et al. Absence of secretory endometrium after false-positive home urine luteinizing hormone testing. Fertil. Steril. 82(5), 1273–1277. https://doi.org/10.1016/j.fertnstert.2004.03.070 (2004).
Bijlsma, R. & Loeschcke, V. Environmental stress, adaptation and evolution: An overview. J. Evol. Biol. 18(4), 744–749. https://doi.org/10.1111/j.1420-9101.2005.00962.x (2005).
Bronson, F. H. Seasonal variation in human reproduction: Environmental factors. Q. Rev. Biol. 70(2), 141–164. https://doi.org/10.1086/418980 (1995).
Demas, G. E., Drazen, D. L. & Nelson, R. J. Reductions in total body fat decrease humoral immunity. Proc. R. Soc. B: Biol. Sci. 270(1518), 905. https://doi.org/10.1098/rspb.2003.2341 (2003).
Gassen, J. et al. Day length predicts investment in human immune function: Shorter days yield greater investment. Psychoneuroendocrinology 107, 141–147. https://doi.org/10.1016/j.psyneuen.2019.05.011 (2019).
Heideman, P. D. & Bronson, F. H. A pseudoseasonal reproductive strategy in a tropical rodent, Peromyscus nudipes. J. Reprod. Fertil. 95(1), 57–67. https://doi.org/10.1530/jrf.0.0950057 (1992).
Rosa, H. J. D. & Bryant, M. J. Seasonality of reproduction in sheep. Small Rumin. Res. 48(3), 155–171. https://doi.org/10.1016/S0921-4488(03)00038-5 (2003).
Aurich, C. Reproductive cycles of horses. Anim. Reprod. Sci. 124(3), 220–228. https://doi.org/10.1016/j.anireprosci.2011.02.005 (2011).
Dantas, M. R. T., Souza-Junior, J. B. F., Castelo, T. D. S., Lago, A. E. D. A. & Silva, A. R. Understanding how environmental factors influence reproductive aspects of wild myomorphic and hystricomorphic rodents. Anim. Reprod. 18, 1 (2021).
Kenagy, G. J. & Barnes, B. M. Seasonal reproductive patterns in four coexisting rodent species from the Cascade Mountains, Washington. J. Mammal. 69(2), 274–292 (1988).
Blottner, S. & Jewgenow, K. Moderate seasonality in testis function of domestic cat. Reprod. Domest. Anim. 42(5), 536–540 (2007).
Gavrilovic, B. B., Andersson, K. & Forsberg, C. L. Reproductive patterns in the domestic dog—A retrospective study of the Drever breed. Theriogenology 70(5), 783–794 (2008).
Horn, R. N. V. Primate breeding season: Photoperiodic regulation in captive Lemur catta. Folia Primatol. 24(2–3), 203–220. https://doi.org/10.1159/000155690 (1975).
Casao, A. et al. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod. Biol. Endocrinol. 8(1), 59. https://doi.org/10.1186/1477-7827-8-59 (2010).
Ellison, P. T., Panter-Brick, C., Lipson, S. F. & O’Rourke, M. T. The ecological context of human ovarian function. Hum. Reprod. 8(12), 2248–2258. https://doi.org/10.1093/oxfordjournals.humrep.a138015 (1993).
Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: A committee opinion. Fertil. Steril. 103(6), e44–e50 (2015).
Roenneberg, T., & Aschoff, J. (1990b). Annual Rhythm of Human Reproduction: II. Environmental Correlations—Till Roenneberg, Jürgen Aschoff, 1990. https://doi.org/10.1177/074873049000500304
Nelson, R. J. Seasonal immune function and sickness responses. Trends Immunol. 25(4), 187–192. https://doi.org/10.1016/j.it.2004.02.001 (2004).
Nicholls, T. J., Follett, B. K. & Robinson, J. E. A photoperiodic response in gonadectomized Japanese quail exposed to a single long day. J. Endocrinol. 97(1), 121–126. https://doi.org/10.1677/joe.0.0970121 (1983).
Stanton, S. J., Mullette-Gillman, O. A. & Huettel, S. A. Seasonal variation of salivary testosterone in men, normally cycling women, and women using hormonal contraceptives. Physiol. Behav. 104(5), 804–808. https://doi.org/10.1016/j.physbeh.2011.07.009 (2011).
Dabbs, J. M. Age and seasonal variation in serum testosterone concentration among men. Chronobiol. Int. 7(3), 245–249. https://doi.org/10.3109/07420529009056982 (1990).
Kala, M. & Nivsarkar, M. Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen. Comp. Endocrinol. 225, 117–124. https://doi.org/10.1016/j.ygcen.2015.09.010 (2016).
Nakane, Y. & Yoshimura, T. Photoperiodic Regulation of Reproduction in Vertebrates. Ann. Rev. Anim. Biosci. 7(1), 173–194. https://doi.org/10.1146/annurev-animal-020518-115216 (2019).
Wesselink, A. K. et al. Seasonal patterns in fecundability in North America and Denmark: a preconception cohort study. Hum. Reprod. 35(3), 565–572 (2020).
Lam, D. A. & Miron, J. A. Seasonality of births in human populations. Soc. Biol. 38(1–2), 51–78 (1991).
Dahlberg, J. & Andersson, G. Changing seasonal variation in births by sociodemographic factors: A population-based register study. Hum. Reprod. Open 4, 1. https://doi.org/10.1093/hropen/hoy015 (2018).
Grobman, A. B. The effect of soil temperatures on emergence from hibernation of Terrapene Carolina. and T ornata. Am. Midland Nat. 124(2), 366–371. https://doi.org/10.2307/2426186 (1990).
Panter-Brick, C., Lotstein, D. S. & Ellison, P. T. Seasonality of reproductive function and weight loss in rural Nepali women. Hum. Reprod. (Oxford, England) 8(5), 684–690. https://doi.org/10.1093/oxfordjournals.humrep.a138120 (1993).
Bjørnerem, Å., Straume, B., Øian, P. & Berntsen, G. K. R. Seasonal variation of estradiol, follicle stimulating hormone, and dehydroepiandrosterone sulfate in women and men. J. Clin. Endocrinol. Metab. 91(10), 3798–3802. https://doi.org/10.1210/jc.2006-0866 (2006).
Jasienska, G. & Ellison, P. T. Energetic factors and seasonal changes in ovarian function in women from rural Poland. Am. J. Hum. Biol. 16(5), 563–580. https://doi.org/10.1002/ajhb.20063 (2004).
Kauppila, A., Kivelä, A., Pakarinen, A. & Vakkuri, O. Inverse seasonal relationship between melatonin and ovarian activity in humans in a region with a strong seasonal contrast in luminosity. J. Clin. Endocrinol. Metab. 65(5), 823–828. https://doi.org/10.1210/jcem-65-5-823 (1987).
Tendler, A., Bar, A., Mendelsohn-Cohen, N., Karin, O., Korem Kohanim, Y., Maimon, L., Milo, T., Raz, M., Mayo, A., Tanay, A., & Alon, U. (2021). Hormone seasonality in medical.
Dopico, X. C. et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 6, 7000. https://doi.org/10.1038/ncomms8000 (2015).
Srinivasan, V. et al. Immunomodulation by melatonin: Its significance for seasonally occurring diseases. NeuroImmunoModulation 15(2), 93–101. https://doi.org/10.1159/000148191 (2008).
Hambridge, H. L. et al. The influence of sporadic anovulation on hormone levels in ovulatory cycles. Hum. Reprod. 28(6), 1687–1694. https://doi.org/10.1093/humrep/det090 (2013).
Lynch, K. E. et al. Assessment of anovulation in eumenorrheic women: Comparison of ovulation detection algorithms. Fertil. Steril. 102(2), 511-518.e2. https://doi.org/10.1016/j.fertnstert.2014.04.035 (2014).
Dağ, Z. Ö. & Dilbaz, B. Impact of obesity on infertility in women. J. Turk. German Gynecol. Assoc. 16(2), 111–117. https://doi.org/10.5152/jtgga.2015.15232 (2015).
Gaskins, A. J., Mumford, S. L., Zhang, C., Wactawski-Wende, J., Hovey, K. M., Whitcomb, B. W., Howards, P. P., Perkins, N. J., Yeung, E., Schisterman, E. F., & BioCycle Study Group. (2009). Effect of daily fiber intake on reproductive function: The BioCycle Study. Am. J. Clin. Nutrit. 90(4), 1061–1069.https://doi.org/10.3945/ajcn.2009.27990
Ellison, P. T. Energetics, Reproductive Ecology, and Human Evolution. https://dash.harvard.edu/handle/1/2643116 (2008).
Hakimi, O. & Cameron, L. C. Effect of exercise on ovulation: a systematic review. Sports Med. 47(8), 1555–1567 (2017).
Cameron, J. L. Reproductive dysfunction in primates, behaviorally induced. In Encyclopedia of Stress (ed. Fink, G.) 366–372 (Academic Press, 2000).
The Economist Intelligence Unit. Quality of Life Index. https://www.economist.com/media/pdf/quality_of_life.pdf (2005).
Crabbe, P. et al. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: Contribution of the androgen receptor polyglutamine tract polymorphism. J. Clin. Endocrinol. Metab. 92(9), 3604–3610. https://doi.org/10.1210/jc.2007-0117 (2007).
Rao, C. V. & Lei, Z. M. Consequences of targeted inactivation of LH receptors. Mol. Cell. Endocrinol. 187(1–2), 57–67. https://doi.org/10.1016/s0303-7207(01)00694-3 (2002).
van Rossum, E. F. C., & Lamberts, S. W. J. (2011). Glucocorticoid Resistance. In M. D. Bronstein (Ed.), Cushing’s Syndrome: Pathophysiology, Diagnosis and Treatment (pp. 235–248). Humana Press. https://doi.org/10.1007/978-1-60327-449-4_19
Shoham, Z., Jacobs, H. S. & Insler, V. Luteinizing hormone: Its role, mechanism of action, and detrimental effects when hypersecreted during the follicular phase. Fertil. Steril. 59(6), 1153–1161. https://doi.org/10.1016/s0015-0282(16)55968-8 (1993).
Roenneberg, T., & Aschoff, J. Annual Rhythm of Human Reproduction: I. Biology, Sociology, or Both?—Till Roenneberg, Jürgen Aschof, 1990. https://doi.org/10.1177/074873049000500303 (1990).
Ellison, P. T. Energetics and reproductive effort. Am. J. Hum. Biol. 15(3), 342–351. https://doi.org/10.1002/ajhb.10152 (2003).
Reiches, M. W. et al. Pooled energy budget and human life history. Am. J. Hum. Biol. 21(4), 421–429. https://doi.org/10.1002/ajhb.20906 (2009).
Underwood, H. & Hyde, L. L. The effect of daylength on the pineal melatonin rhythm of the lizard Anolis carolinensis. Comp. Biochem. Physiol. A Physiol. 94(1), 53–56. https://doi.org/10.1016/0300-9629(89)90783-4 (1989).
Bittman, E. L., Dempsey, R. J. & Karsch, F. J. Pineal melatonin secretion drives the reproductive response to Daylength in the Ewe*. Endocrinology 113(6), 2276–2283. https://doi.org/10.1210/endo-113-6-2276 (1983).
Su, H. W., Yi, Y. C., Wei, T. Y., Chang, T. C. & Cheng, C. M. Detection of ovulation, a review of currently available methods. Bioeng. Transl. Med. 2(3), 238–246 (2017).
Dunlop, A. L., Schultz, R. & Frank, E. Interpretation of the BBT chart: using the “Gap” technique compared to the Coverline technique. Contraception 71(3), 188–192 (2005).
Stopa, K., Kobyshev, A., Matthias, B. H. SunTime, GitHub Repository. https://github.com/SatAgro/suntime (2019).
Hoffmann, K. Photoperiod, Pineal, Melatonin and Reproduction in Hamsters. In J. A. Kappers & P. Pévet (Eds.) Progress in Brain Research Vol. 52, 397–415 (Elsevier, 1979). https://doi.org/10.1016/S0079-6123(08)62946-5
Ortavant, R. et al. Seasonality of reproduction in sheep and its control by photoperiod. Aust. J. Biol. Sci. 41(1), 69–86. https://doi.org/10.1071/bi9880069 (1988).
Sundararaj, B. I. & Vasal, S. Photoperiod and temperature control in the regulation of reproduction in the female Catfish Heteropneustes fossilis. J. Fish. Res. Board Can. 33(4), 959–973. https://doi.org/10.1139/f76-123 (1976).
Kenney, W. L. A review of comparative responses of men and women to heat stress. Environ. Res. 37(1), 1–11. https://doi.org/10.1016/0013-9351(85)90044-1 (1985).
López-Gatius, F. & Hunter, R. H. F. Local cooling of the ovary and its implications for heat stress effects on reproduction. Theriogenology 149, 98–103. https://doi.org/10.1016/j.theriogenology.2020.03.029 (2020).
Lamprecht, C. S. Meteostat Python [Computer software].
Sigrist, F. Gaussian Process Boosting. [Cs, Stat]. http://arxiv.org/abs/2004.02653 (2021).
Ayeni, O. Seasonal variation of births in rural southwestern Nigeria. Int. J. Epidemiol. 15(1), 91–94. https://doi.org/10.1093/ije/15.1.91 (1986).
Björnsson, B. T., Halldórsson, Ó., Haux, C., Norberg, B. & Brown, C. L. Photoperiod control of sexual maturation of the Atlantic halibut (Hippoglossus hippoglossus): Plasma thyroid hormone and calcium levels. Aquaculture 166(1), 117–140. https://doi.org/10.1016/S0044-8486(98)00276-2 (1998).
James, W. H. Seasonal variation in human births. J. Biosoc. Sci. 22(1), 113–119. https://doi.org/10.1017/S0021932000018423 (1990).
Martinez-Bakker, M., Bakker, K. M., King, A. A. & Rohani, P. Human birth seasonality: Latitudinal gradient and interplay with childhood disease dynamics. Proc. R. Soc. B: Biol. Sci. 281(1783), 20132438. https://doi.org/10.1098/rspb.2013.2438 (2014).
Odlen, I. (2019). Basal body temperature curves and fitting with periodic smoothing splines. Master’s Theses in Mathematica Sciences. http://lup.lub.lu.se/student-papers/record/8973067
Petersen, D. J. & Alexander, G. R. Seasonal variation in adolescent conceptions, induced abortions, and late initiation of prenatal care. Public Health Rep. 107(6), 701 (1992).
Rizzi, E. L. & Dalla-Zuanna, G. The seasonality of conception. Demography 44(4), 705–728. https://doi.org/10.1353/dem.2007.0040 (2007).
Rojansky, N. et al. Seasonal variability in fertilization and embryo quality rates in women undergoing IVF. Fertil. Steril. 74(3), 476–481. https://doi.org/10.1016/S0015-0282(00)00669-5 (2000).
van Anders, S. M., Hampson, E. & Watson, N. V. Seasonality, waist-to-hip ratio, and salivary testosterone. Psychoneuroendocrinology 31(7), 895–899. https://doi.org/10.1016/j.psyneuen.2006.03.002 (2006).
Wehr, T. A. Melatonin and seasonal rhythms. J. Biol. Rhythms 12(6), 518–527. https://doi.org/10.1177/074873049701200605 (1997).
Whittier, J. M., & Crews, D. Seasonal Reproduction: Patterns and Control. In D. O. Norris & R. E. Jones (Eds.) Hormones and Reproduction in Fishes, Amphibians, and Reptiles, 385–409. (Springer, 1987). https://doi.org/10.1007/978-1-4613-1869-9_13.
Wood, S., Quinn, A., Troupe, S., Kingsland, C. & Lewis-Jones, I. Seasonal variation in assisted conception cycles and the influence of photoperiodism on outcome in in vitro fertilization cycles. Hum. Fertil. 9(4), 223–229. https://doi.org/10.1080/14647270600806557 (2006).
Author information
Authors and Affiliations
Contributions
D.S. cleaned the data, ran the analyses, reviewed the results, wrote the Methods section, and edited the draft. M.E. reviewed results, wrote the Discussion section, and edited the draft. J.G. provided feedback on the experimental set-up, reviewed results, and edited the draft. A.L., J.P., and E.B. provided underlying data, reviewed results, and edited the draft. S.H. proposed the question, guided experimental analyses, reviewed the results, and edited the draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shanmugam, D., Espinosa, M., Gassen, J. et al. A multi-site study of the relationship between photoperiod and ovulation rate using Natural Cycles data. Sci Rep 13, 8379 (2023). https://doi.org/10.1038/s41598-023-34940-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34940-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.