Abstract
The metabolic profile of cancerous cells is shifted to meet the cellular demand required for proliferation and growth. Here we show the features of cancer metabolic profiles using peripheral blood of healthy control subjects (n = 78) and lung adenocarcinoma (LUAD) patients (n = 64). Among 121 detected metabolites, diagnosis of LUAD is based on arginine, lysophosphatidylcholine-acyl (Lyso.PC.a) C16:0, and PC-diacyl (PC.aa) C38:3. Network analysis revealed that network heterogeneity, diameter, and shortest path were decreased in LUAD. On the contrary, these parameters were increased in advanced-stage compared to early-stage LUAD. Clustering coefficient, network density, and average degree were increased in LUAD compared to the healthy control, whereas these topologic parameters were decreased in advanced-stage compared to early-stage LUAD. Public LUAD data verified that the genes encoding enzymes for arginine (NOS, ARG, AZIN) and for Lyso.PC and PC (CHK, PCYT, LPCAT) were related with overall survival. Further studies are required to verify these results with larger samples and other histologic types of lung cancer.
Similar content being viewed by others
Introduction
Lung cancer is one of the malignancies with the highest mortality and is second in incidence and fourth in prevalence worldwide. The global mortality and incidence of lung cancer were 18.0 and 22.4 per 100,000, respectively, in 20201. Around 230,000 patients are newly diagnosed with lung cancer annually, and 130,000 died from lung cancer in the United States in 20212,3,4. Although deaths per 100,000 are decreasing, lung cancer shows the highest death rate among all cancers2,3,4.
Metabolomics has supported genomics and proteomics to provide information regarding cancer metabolic processes that are related to tumor formation and/or proliferative potential5,6. These biomarker metabolites can enhance our understanding of tumor biology and have potential for applied use in early diagnosis. Among them, acylcarnithines, amino acids, biogenic amines, lysophosphatidylcholines (LPCs), phosphatidylcholine (PC), and sphingolipids can be extracted from tissue, blood, serum, or body fluids. Arginine is an amino acid that is used for creatine and urea biosynthesis. In the kidney, proline, glutamate, and glutamine can be converted to citrulline and with aspartate can be synthesized to agininosuccinate and then to arginine by agininosuccinate lyase via the urea cycle7,8,9. Arginine is required for tumor cell proliferation; however, it is also required for normal lymphocytes, which could act as a double-edged sword in cancer treatment7.
LPC and PC are precursors of membrane lipids related with plasma membrane remodeling in various cancers10. Lung cancer cells dysregulate lipid metabolism, the effects of which are reflected in the peripheral blood8,9. LPC is a lipid biomolecule derived from PC by phospholipase A2 or from free cholesterol and fatty acids by lecithin cholesterol acyltransferase11,12. Cancer cells convert LPC to produce various PCs via LPC acyltransferase 1 (LPCAT1), and the PCs are utilized for plasma membrane remodeling13. Decreased LPC and increased PC concentration are found in cancer tissue and in the peripheral blood of cancer patients13,14.
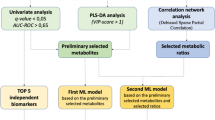
To study the metabolic nature of lung cancer using peripheral blood, metabolites from healthy control and LUAD patient samples were compared. A statistical model was constructed based on these metabolites for early diagnosis of LUAD. Network analysis was performed to analyze these biomarkers among healthy controls and early and advanced LUAD patients.
Materials and methods
Patients and controls
This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital. After informed consent, samples were collected from Seoul St. Mary’s Hospital Biobank, The Catholic University of Korea, Seoul, Republic of Korea. All the methods were performed in accordance with the relevant guidelines and regulations. Plasma samples from the peripheral blood of healthy patients (n = 80) and LUAD cancer patients (n = 65) were analyzed in the study. All LUAD patients underwent surgical intervention, and the diagnosis was confirmed by a pathologist during routine diagnostic procedures15,16. All patients were classified according to the World Health Organization definitions and according to the seventh edition of the tumor-node-metastasis (TNM) classification of the American Joint Committee on Cancer Staging Manual17. The TNM stages of the LUAD patients are presented (Supplementary Table 1). Blood samples from LUAD patients were collected on the day of surgery. The healthy control samples were those from routine health checkup patients. After measurements, the samples that passed quality control (QC) included 64 and 78 samples for healthy and lung patient samples, respectively.
Targeted metabolomic analysis and sample preparation
The AbsoluteIDQ® p180 (Biocrates Life Sciences AG, Innsbruck, Austria) kit is a MS-based assay for quantification of target metabolites in body fluids using stable isotope–labeled standards. The kit allows targeted analysis of 188 metabolites, covering a wide range of analytes and metabolic pathways in one targeted assay. Amino acids (21) and biogenic amines (21) are determined in the LC–MS mode; acylcarnitines (40), LPCs (14), PCs (76), sphingomyelins (15), and the sum of hexoses (1) were analyzed via flow injection analysis (FIA; Supplementary Table 2).
Metabolite measurement and sample preparation from a total of 145 plasma samples from healthy and LUAD patients were carried out according to the manufacturer’s protocol. Briefly, 10 μL of internal standard (amino acids and biogenic amines) followed by 10 μL of sample (calibrator, QC, and plasma sample) were added to the filter inserts (which contained several internal standards corresponding to glycerophospholipids, sphingomyelins, acylcarnitine, and hexoses) of the 96-well plate and dried for 30 min under a nitrogen stream. Then, 50 μL of a 5% phenylisothiocyanate (PITC) solution was added to derivatize amino acids and biogenic amines and then incubated at room temperature for 1 h. After incubation, the metabolites and internal standards were extracted using 5 mM ammonium acetate in methanol (300 µL) into the lower 96-well plate for MS analysis. One-half of the eluate was measured by liquid chromatography (LC) LC–MS/MS, and the other half was determined by FIA-MS/MS.
Liquid chromatography and mass spectrometry
Analyses for amino acids and biogenic amines were performed using a Zorbax Eclipse XDBC18 column (3 mm × 100 mm, 3.5 µm, Agilent Technologies) at a flow rate of 0.5 ml/min with water:acetonitrile containing 0.2% formic acid in the LC gradient condition. Analyses for glycerophospholipids, sphingolipids, acylcarnitines, and hexoses were performed at a flow rate of 0.03 ml/min with elution solvent in FIA gradient conditions. Multiple reaction monitoring modes of the tandem mass spectrometer (API 4000, AB Sciex) in positive electrospray ionization (ESI) were used for quantification of target metabolites. However, only hexose was analyzed in negative ESI mode.
Data management and analysis
Quantification was carried out using internal standards and a calibration curve18. Briefly, after pre-processing (peak integration and concentration determination from calibration curves) with Analyst 1.5.1 software (AB Sciex, Darmstadt, Germany), data were uploaded into Biocrates MetIDQ software. The concentrations of metabolites determined by FIA were directly calculated in MetIDQ. Specifically, the concentrations of derivative metabolites consisting of amino acids and biogenic amines were quantified precisely with a seven-point calibration curve and internal standards, while the remaining metabolites corresponding to glycerophospholipids, sphingolipids, acylcarnitines, and hexoses were calculated semi-quantitatively based on specific stable isotope internal standard intensities. The final metabolite concentration was determined with inter- and intra-plate normalization using the median of three QC2 (from Biocrates Inc.) replicate injections of each plate compared to the target value.
Network analysis
Network topologic parameters were defined as follows19,20,21,22,23,24,25. Node (N) indicates each metabolite, and link (L) indicates correlation between the nodes and plotted by a line. The average clustering coefficient (<C>) was defined as the friend’s friend, which was calculated as follows: Ci = 2Li/ki(ki-1), where Ci represents the clustering coefficient, Li represents the number of lines between neighbors of ki, and ki represents the node i with degree ki. Finally, <C> = 1/N \(\sum_{i=1}^{N}Ci\). If Ci = 0, none of the node i neighbors are linked. If Ci = 0.5, there is a 50% chance that the node i neighbors are linked. Network density was calculated as follows: density (d) = L/Lmax, where L indicates the number of links, and Lmax indicates the maximum probable links among the nodes. Increased density implies that there are more correlations between molecules. Network heterogeneity was calculated as follows: Heterogeneity = √variance(k)/mean(k), which implies that network heterogeneity is variance of the degree of a node divided by the mean degree number. Network centralization (degree centralization) shows the importance of certain nodes with high connection (degree) compared to other nodes, which was calculated as follows: \(C(j)=\sum_{j=1}^{n}A_{ij}\), where A indicates adjacency matrix and n indicates the total number of nodes. Shortest path or average shortest path was calculated as follows: <l> = \(2{\sum }_{ij}lij\)/N(N-1), where \(l\) indicates the shortest path between two nodes (i, j). Average degree (<k>) was calculated as follows: <k> = 2L/N = dN(N-1)/N. These calculations are performed using a Netanalyzer that was attached to Cytoscape26,27,28. Assortativity and modularity were calculated using the igraph package29. Assortativity coefficient indicates the association of nodes with similar weights. Modularity indicates the tendency of nodes to cluster and to form groups or clusters19,20.
Public dataset analysis
To verify the tested results in this study, the LUAD dataset was used. The Genomic Data Commons (GDC) The Cancer Genome Atlas (TCGA) LUAD dataset from the National Cancer Institute was downloaded from the University of Santa Cruz (UCSC) Xena Browser platform (https://xenabrowser.net)30,31. Primary tumors were selected, followed by deletion of duplicate patient samples. Survival analysis of these expression datasets was conducted via the Kaplan Meier method using the R package. The arginine biosynthesis pathway was plotted with modification based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (hsa00220) and on the work of Morris et al.31,32. The PC and LPC pathways were plotted with modification based on the respective KEGG pathway (hsa00564) and on the work of Lesko et al.31,32,33. Arginine, citrulline, ornithine, PC, and LPC concentrations from this study were interpolated using these pathways.
Statistical analysis
Significance among the mean values for healthy and LUAD samples was measured using Mann Whitney U tests with unequal variance and Student’s t-tests for equal variance. Many of the metabolites demonstrated collinearity; therefore, the least absolute shrinkage and selection operator (LASSO) regression analysis was performed for selecting diagnostic factors using the glmnet R package34,35. To select variables, multivariate logistic regression analysis was performed using the glm R package36. In addition, network analysis was performed based on the correlation matrix by Pearson’s correlation analysis. Correlation coefficient and p-values were calculated using the Hmisc R package37. Correlation coefficients greater than or equal to 0.8 with statistical significance were selected. Unweighted, undirected networks were constructed with these selected nodes, and network topological studies were plotted using Cytoscape26,27,28. From public datasets, Kaplan Meier analysis was performed using the survival38 R package and plotted using the survminer R package39,40,41. For receiver operating characteristics curve analysis, the pROC R package was used42. Data were analyzed using dplyr and plotted using ggplot241. All the statistical analysis was performed using the R program43.
Results
Measured metabolites
Among the 188 targeted metabolites, 121 including acyl carnitines, amino acids, biogenic amines, glycerophospholipids, and sphingolipids were measured and detected. The mean and standard deviation of these metabolites are listed in Fig. 1 (Supplementary Table 3). Most of the metabolites showed statistical significance, which implies a metabolic difference between the healthy control and LUAD patients. Among the 64 LUAD patients, 44 were diagnosed with early LUAD, whereas 20 were diagnosed with advanced LUAD (Supplementary Table 4). Among the measured metabolites, PC and LPC were decreased in advanced LUAD compared to early LUAD. TNM stage and overall survival analysis revealed that early-stage LUAD patients, which included T1 or T2 status, showed 97.7% overall survival. On the contrary, advanced LUAD, which included half of T2 and most of T3 and T4 status and stage 2 or greater, showed relatively unfavorable overall survival (Supplementary Table 5).
Regression and analysis
As there were many variables and some showed collinearity, LASSO regression was performed, and independent variables related to LUAD diagnosis were investigated (Table 1). Optimal lambda parameter was screened in the LASSO model using the minimum criterion for tenfold cross validation. The left and right dashed lines indicate lambda and standard error of 1ambda values of the minimized mean square error (Supplementary Fig. 1A). LASSO regression penalizes variables and converges values of unimportant variables to zero, which was plotted in variable profiles (Supplementary Fig. 1B). LASSO regression analysis revealed that the tenfold cross-validated lambda was 0.0158. The regression analysis resulted in 18 of 121 variables that showed statistical significance, including C0, C10:1, arginine (Arg), aspartate (Asp), citrulline (Cit), glutamine (Gln), glutamate (Glu), ornithine (Orn), phenylalanine (Phe), serine (Ser), total DMA (dimethylarginine), lysophosphatidylcholine (lyso.PC) acyl C16:0 (lyso.PC.a.C:16), phosphatidylcholine acyl-alkyl (PC.aa) C34:3 (PC.aa.C34:4), PC.aa.C38:3, PC.aa.C40:5, PC.aa.C42:0, PC.aa.C42:5, and PC.aa.C38:1 (Table 1). To construct a diagnostic model, multivariate logistic regression analysis was performed using the backward stepwise method among these variables (Table 1). As some variables showed collinearity, the variable with the highest OR was eliminated. Finally, arginine, lyso.PC.a.C16:0, and PC.aa.C38:3 were included in the model.
ROC curve analysis
ROC curve analysis was performed for all variables in LUAD diagnosis. Area under the receiver operation curve (AUROC), cut off values, sensitivity, specificity, positive predictive value (ppv), and negative predictive value (npv) were calculated (Supplementary Table 6). Amino acids showed higher AUROC followed by acylamines and PC for diagnosis of LUAD. LPCs showed relatively lower AUROC compared to other metabolites. Arg, Gln, Cit, and Asn showed the highest AUROCs of 0.981, 0.942, 0.915, and 0.854, respectively. For metabolites selected by regression analysis, arginine, lyso.PC.a.C16:0, and PC.aa.C38:3 resulted in AUROCs of 0.981, 0.697, and 0.715, respectively (Supplementary Fig. 2).
Network analysis
An unweighted, undirected network was constructed based on the Pearson’s correlation matrix from the healthy control and LUAD groups (Fig. 2). Among them, a correlation coefficient greater than or equal to 0.8 was selected. Network construction was performed for the healthy controls and resulted in parameters related to nodes and links (Fig. 3A,B). The hub node was PC.ae.C34.1 with 41 degrees, followed by SM.C16.1 with 40 degrees and PC.ae.C38.3 with 39 degrees (Supplementary Tables 7, 8). For LUAD, the hub node was PC.ae.38.3 with 43 degrees, followed by PC.ae.C36.1, PC.ae.C42.3, and PC.ae.C42.2 all with 40 degrees (Supplementary Tables 9, 10, Fig. 3B). Network topologic parameters revealed slightly more nodes and links in the healthy control group compared to the LUAD group (Table 2). Clustering coefficient and network density were increased in the LUAD network, which implies correlation between molecules. However, network heterogeneity was lower, which denotes a smaller variance of degree in the cancer network. Diameter or shortest path of the LUAD network was shorter than that of the healthy control, which indicates that metabolites are more correlated. Average degree was higher in the LUAD network, which implies correlated metabolites.
Early and advanced LUAD also were analyzed. For early LUAD, the hub nodes were PC.ae.C38.3 and PC.ae.C42.3 with 45 degrees (Supplementary Tables 11, 12). For advanced LUAD, the hub node was PC.ae.C38.6 with 23 degrees, followed by PC.ae.C38.5 and PC.ae.C32.1 with 21 degrees (Supplementary Tables 13, 14). Network parameters revealed decreased clustering coefficient, network density, and average degree in advanced-stage LUAD (Fig. 4A,B). However, network heterogeneity and shortest path were increased in advanced-stage LUAD, which implies a weaker correlation between molecules than in early-stage LUAD (Table 2).
Public data analysis
From Xena Browser, 877 samples from the GDC TCGA LUAD were downloaded. Among them, 597 primary tumor samples were selected, of which 73 were deleted due to null data. A total of 511 samples with survival data was selected; after deletion of 11 duplicate samples, 500 unique patient samples were analyzed via Kaplan Meier analysis. Arginine and the PC and LPC pathways were plotted with modifications28,29,30, and concentration data from this study were interpolated. Arginine, citrulline, and PC levels were increased in cancer patients, whereas lysoPC was decreased. Gene expression was also selected for Kaplan Meier analysis (Fig. 5), and the results showed that AZIN2, NOS1, and ARG that encode enzymes related to arginine biosynthesis were significantly upregulated in the LUAD group. Low gene expression was related with favorable overall survival rate. Arginine and citrulline were increased and ornithine was decreased in LUAD in this study. NOS, ARG, and AZIN2 are expected to be decreased in LUAD, which would increase arginine level. For the PC and LPC pathways, LPCAT and PCYT1 were significantly upregulated in the Kaplan Meier analysis (Supplementary Fig. 3).
Discussion
Treatment of lung cancer could benefit from early diagnosis followed by surgical and pharmaceutical treatment. Early diagnosis has been achieved using low-dose computed tomography (CT) and was shown to decrease mortality by 20% compared to a control group3,4. Screening by low-dose CT is the only approved method for early diagnosis, but this can be a burden to the patient undergoing radiological testing. Thus, diagnosis of cancer using blood biomarkers might be more suitable.
Metabolic profiles could provide valuable information for early LUAD diagnosis. Metabolic shifts are noted in cancer cells and are a part of the metabolic adaptation required to meet high demand for energy production required for proliferation in cancer cells44. Most of the metabolic profiles showed differences between healthy controls and LUAD samples. Among the tested 188 metabolites, three were selected for early LUAD diagnosis, arginine, Lyso.PC.a.C16:0, and PC.aa.C38:3.
Arginine is required for biological systems including cell growth and immune response modulation. Arginine deprivation is related to caspase-dependent apoptosis, caspase-independent apoptosis, caspase-independent autophagic cell death, and necroptosis in various tumors6. As regulatory T cell or myeloid-derived suppressor cells suppress the immune response and metabolize arginine through arginase-I, arginine supplementation boosted chemotherapy efficacy45,46. As arginine is a precursor of nitric oxide (NO), NO could have anti-tumor effects due to p53 accumulation, which could be a possible mechanism in LUAD45,46. In this study, arginine was increased in the plasma of LUAD patients. Increased arginine might trigger the formation of NO that could result in vascular proliferation and tumor growth47,48. As increased NO expression is related with unfavorable prognosis, increased arginine could also be related to unfavorable prognosis, although this requires further study.
Unexpectedly, Lyso.PC.a.C16:0 was decreased in LUAD samples. LPC is decreased in cells due to the activity of LPCAT, which is an LPC acyltransferase that catalyzes the conversion of LPC to PC. Previously, LPCAT has been reported to be increased in cancer patients; therefore, LPC might be decreased in cancer patients49,50,51. In this study, except for one LPC or lysoPC.a.C28:1, every LPC was decreased in LUAD samples. Furthermore, Lyso.PC.a.C16:0 was included in the prognostic model, implying that LPC could be a crucial factor affecting cancer pathobiology49,50,51. Normally, LPCs are components of surfactants made by alveolar type II cells in the lung, and decreased LPC in lung cancer patients implies either consumption by cancer cells or conversion by LPCAT. PCs are used for membrane construction, and PC metabolism is reprogrammed for proliferation in cancer cells30,52. In this study, most of the measured PCs were increased in LUAD patients, and PC.aa.C38:3 was increased in the diagnostic model.
Network analysis can analyze complex or multi-dimensional data without losing or omitting many data points19,25,53. Network heterogeneity reflects the degree distribution of nodes, and increased heterogeneity implies the presence of a hub node. Biological genetic networks tend to be heterogeneous due to the presence of a hub gene that is related to many other genes. In this study, heterogeneity was lower in LUAD compared to the normal healthy control group, indicating decreased presence of a hub node. The small world property involves shorter paths between nodes and small parameter diameter. Shorter paths between nodes implies a fast response against external stimuli and adaptation to the external environment51,53, as observed in LUAD. Assortativity indicates relationships between hub and hub nodes, whereas dissortativity implies relationship between hub and non-hub nodes. In this study, there was no significant difference between the groups in assortativity. Modularity implies functional aspects of a network and was similar between the compared groups.
In conclusion, the metabolic profiles of healthy control and LUAD patients were compared. Diagnosis of LUAD was achieved using arginine, Lyso.PC.a.C16:0, and PC.aa.C38:3 biomarkers. Network analysis revealed that heterogeneity, diameter, and shortest path were decreased in LUAD. On the contrary, these parameters were increased in advanced-stage compared to early-stage LUAD. Clustering coefficient, network density, and average degree were increased in LUAD compared to the normal healthy controls, whereas these topologic parameters were decreased in advanced LUAD compared to early LUAD. Further studies are required to verify these results with larger samples and other histologic types of lung cancer.
Data availability
All the available data generated or analyzed during this study are included in this published article and its supplementary information files.
References
World Health Organization. Globocan 2020 https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (2020).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021).
US Preventive Services Task Force. Screening for lung cancer: US preventive services task force recommendation statement. JAMA 325, 962–970 (2021).
de Koning, H. J. et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 382, 503–513 (2020).
Bamji-Stocke, S., van Berkel, V., Miller, D. M. & Frieboes, H. B. A review of metabolism-associated biomarkers in lung cancer diagnosis and treatment. Metabolomics 14, 81 (2018).
Albaugh, V. L., Pinzon-Guzman, C. & Barbul, A. Arginine-Dual roles as an onconutrient and immunonutrient. J. Surg. Oncol. 115, 273–280 (2017).
Chen, C. L., Hsu, S. C., Ann, D. K., Yen, Y. & Kung, H. J. Arginine signaling and cancer metabolism. Cancers 13, 3541 (2021).
Kim, E. A. et al. Lysophosphatidylcholine induces endothelial cell injury by nitric oxide production through oxidative stress. J. Matern. Fetal Neonatal Med. 22, 325–331 (2009).
Hollie, N. I. et al. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochim. Biophys. Acta 1841, 888–895 (2014).
Zalba, S. & Ten Hagen, T. L. M. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 52, 48–57 (2017).
Zhao, Z. et al. Plasma lysophosphatidylcholine levels: Potential biomarkers for colorectal cancer. J. Clin. Oncol. 25, 2696–2701 (2007).
Law, S. H. et al. An updated review of lysophosphatidylcholine metabolism in human diseases. Int. J. Mol. Sci. 20, 1149 (2019).
Kühn, T. et al. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 14, 13 (2016).
Bi, J. et al. Oncogene amplification in growth factor signaling pathways renders cancers dependent on membrane lipid remodeling. Cell Metab. 30, 525-538.e528 (2019).
Jeon, D. S. et al. Five-year overall survival and prognostic factors in patients with lung cancer: Results from the Korean Association of Lung Cancer Registry (KALC-R) 2015. Cancer Res. Treat. https://doi.org/10.4143/crt.2022.264 (2022).
Ko, Y. H. et al. Risk factors for primary lung cancer among never-smoking women in South Korea: A retrospective nationwide population-based cohort study. Korean J. Intern. Med. 35, 692–702 (2020).
Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 17, 1471–1474 (2010).
Ramsay, S. L. et al. Apparatus and method for analyzing a metabolite profile. US patent 8,265,877 (2012).
Barabási, A. L., Gulbahce, N. & Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 12, 56–68 (2011).
Barabasi, A. Network Science (Cambridge University Press, 2016).
Jekarl, D. W., Kim, K. S., Lee, S., Kim, M. & Kim, Y. Cytokine and molecular networks in sepsis cases: A network biology approach. Eur. Cytokine Netw. 29, 103–111 (2018).
Kim, K. S. et al. Immune gene expression networks in sepsis: A network biology approach. PLoS ONE 16, e0247669 (2021).
Barabási, A. L. & Oltvai, Z. N. Network biology: Understanding the cell’s functional organization. Nat. Rev. Genet. 5, 101–113 (2004).
Pavlopoulos, G. A. et al. Using graph theory to analyze biological networks. BioData Min. 4, 10 (2011).
Newman, M. E. J. Networks: An Introduction (Oxford University Press, 2010).
Saito, R. et al. A travel guide to Cytoscape plugins. Nat. Methods 9, 1069–1076 (2012).
NetworkAnalyzer https://med.bioinf.mpi-inf.mpg.de/netanalyzer/index.php (2017).
Carlin, D. E., Demchak, B., Pratt, D., Sage, E. & Ideker, T. Network propagation in the cytoscape cyberinfrastructure. PLoS Comput. Biol. 13, e1005598 (2017).
Csardi, G. & Nepusz, T. The igraph software package for complex network research. InterJournal Complex Syst. 1695, 1–9 (2006).
Goldman, M. J. et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38, 675–678 (2020).
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550 (2014).
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y. & Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45, D353–D361 (2017).
Morris, S. M. Jr. Arginine: Beyond protein. Am. J. Clin. Nutr. 83, 508S-512S (2006).
Lesko, J. et al. Phospholipid dynamics in ex vivo lung cancer and normal lung explants. Exp. Mol. Med. 53, 81–90 (2021).
Dormann, C. F. et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46 (2013).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
Marschner, I. C. glm2: Fitting generalized linear models with convergence problems. R J. 3, 12–15 (2011).
Harrell, F. E. Hmisc: Harrell miscellaneous https://cran.r-project.org/package=Hmisc2022 (2022).
Therneau, T. M. & Grambsch, P. M. Modeling Survival Data: Extending the Cox Model (Springer, 2020).
Kassambara, A., Kosinski, M. & Biecek, P. survminer: drawing survival curves using ggplot2 https://CRAN.R-project.org/package=survminer (2021).
Grolemund, G. & Wickham, H. R for Data Science: Import, Tidy, Transform, Visualize, and Model Data (O’Reilly, 2017).
Robin, X. et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 12, 77 (2011).
R Developoment Core Team. R: A language and environment for statistical computing http://cran.r-project.org/ (2017).
Cheng, C. T. et al. Arginine starvation kills tumor cells through aspartate exhaustion and mitochondrial dysfunction. Commun. Biol. 1, 178 (2018).
Satoh, Y. et al. Supplementation of l-arginine boosts the therapeutic efficacy of anticancer chemoimmunotherapy. Cancer Sci. 111, 2248–2258 (2020).
Bronte, V. & Zanovello, P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5, 641–654 (2005).
Xu, W., Liu, L. Z., Loizidou, M., Ahmed, M. & Charles, I. G. The role of nitric oxide in cancer. Cell Res. 12, 311–320 (2002).
Zhou, H., Li, J., Chen, Z., Chen, Y. & Ye, S. Nitric oxide in occurrence, progress and therapy of lung Cancer: A systemic review and meta-analysis. BMC Cancer 21, 678 (2021).
Chen, X., Hyatt, B. A., Mucenski, M. L., Mason, R. J. & Shannon, J. M. Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc. Natl. Acad. Sci. USA 103, 11724–11729 (2006).
Klupczynska, A. et al. Serum lipidome screening in patients with stage I non-small cell lung cancer. Clin. Exp. Med. 19, 505–513 (2019).
Dong, J. et al. Lysophosphatidylcholine profiling of plasma: Discrimination of isomers and discovery of lung cancer biomarkers. Metabolomics 6, 478–488 (2010).
Ide, Y. et al. Human breast cancer tissues contain abundant phosphatidylcholine(36∶1) with high stearoyl-CoA desaturase-1 expression. PLoS ONE 8, e61204 (2013).
Jekarl, D. W. et al. Complex interaction networks of cytokines after transarterial chemotherapy in patients with hepatocellular carcinoma. PLoS ONE 14, e0224318 (2019).
Acknowledgements
We thank Bill Lim (Omics biotech) for excellent technical support.
Funding
The authors wish to acknowledge financial support from the Catholic Medical Center Research Foundation in the 2021 program year. This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2021R1F1A1046464).
Author information
Authors and Affiliations
Contributions
K.S.K. and D.W.J. designed the study, analyzed data and drafted the main manuscript. S.W.M., M.H.M., K.Y.H., S.J.K., and Y.K.K. participated in patient management and sample collection. K.Y.K. experimented and analyzed data. E.O. and Y.K. provided resources. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, K.S., Moon, S.W., Moon, M.H. et al. Metabolic profiles of lung adenocarcinoma via peripheral blood and diagnostic model construction. Sci Rep 13, 7304 (2023). https://doi.org/10.1038/s41598-023-34575-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34575-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.







