Abstract
Adjuvant trastuzumab in HER2+ breast cancer reduces recurrence and mortality, and has been the standard treatment since 2006. The objective was to analyze health outcomes in the real world. Observational, retrospective study of patients with HER2+ breast cancer, stages I–III, treated with adjuvant trastuzumab in the past 15 years in only one center and for the first time in Spain. Survival was analyzed according to the number of cycles and cardiotoxicity. Two hundred and seventy-five HER2positive patients (18.60%) out of 1479 received adjuvant (73%) or neoadjuvant/adjuvant (26%) trastuzumab, concomitantly (90%) or sequentially (10%) with chemotherapy. The probability of overall and disease-free survival (OS and DFS) at 5 years was 0.93 (95% CI 0.89–0.96), and 0.88 (95% CI 0.83–0.92). The number of cases with a significant and asymptomatic decrease in ventricular ejection fraction and heart failure were 54 (19.64%) and 12 (4.36%), respectively. Sixty-eight patients (24.70%) received 16 or fewer cycles, especially those older than 65 (OR 0.371, 95% CI 0.152–0.903; p = 0.029) and with cardiotoxicity (OR 15.02, 95% CI 7.437–30.335; p < 0.001). The risk of cardiotoxicity was associated with having received radiotherapy (OR 0.0362, 95% CI 0.139–0.938; p = 0.037). Arterial hypertension (HR 0.361, 95% CI 0.151–0.863, p = 0.022), neoadjuvant treatment (HR 0.314, 95% CI 0.132–0.750, p = 0.009) and cardiotoxicity (HR 2.755, 95% CI 1.235–6.143, p = 0.013) maintained significant association with OS. Only neoadjuvant treatment maintained a significant association with DFS (HR 0.437, 95% CI 0.213–0.899, p = 0.024). The effectiveness of neoadjuvant and adjuvant trastuzumab can be considered comparable to those of clinical trials. In the real world, factors such as age, hypertension, radiotherapy, neoadjuvant treatment, and cardiotoxicity should be taken into consideration to optimize outcomes.
Similar content being viewed by others
Introduction
HER2 (Human epidermal growth factor receptor 2) is a member of the family of four transmembrane receptor tyrosine kinases that regulate cell growth, survival, and differentiation through multiple signal transduction pathways1. Of all patients with breast carcinoma, the HER2-positive subtype overexpresses this receptor, it accounts for 20% of cases2,3 and is associated with a worse prognosis4.
Trastuzumab, a monoclonal antibody directed against the HER2 protein extracellular domain, is active in patients with HER2-positive breast cancer5. Targeted therapy with 1 year of trastuzumab in combination with chemotherapy has been shown to significantly improve disease-free survival (DFS) and overall survival (OS) in HER2-positive early stage breast cancer6,7,8. Several trials have investigated shorter durations of adjuvant trastuzumab, between 9 weeks and 6 months, with varying results9,10,11,12,13. Systematic review of these trials finds that, compared with 1 year, the shorter duration of adjuvant trastuzumab is associated with significantly worse DFS and OS, despite the more favorable cardiotoxicity profile14.
The cardiac toxicity associated with trastuzumab is primarily heart failure and is a significant adverse effect because it prevents patients from receiving the full treatment and ultimately affects outcomes and survival15. It ranges from asymptomatic decrease in left ventricular ejection fraction (LVEF) to overt congestive heart failure, and is often reversible15. It is important to assess baseline risk factors in all patients and regular monitoring of LVEF using echocardiography or isotopic ventriculography during the year of adjuvant treatment16.
This study analyzed the outcomes of women with HER2-type breast carcinoma who received adjuvant trastuzumab since its approval by the European Medicines Agency (https://www.ema.europa.eu/en/medicines/human/EPAR/herceptin#assessment-history-section) and by the Spanish Agency of Medicines and Medical Devices (Agencia Española de Medicamentos y Productos Sanitarios) (https://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/2006/home.htm) in 2006, to test whether the health outcomes, in terms of survival and toxicity, are similar to those obtained in the clinical trials of reference. In addition, the study compared the survival of patients who have completed 12 months of trastuzumab with that of patients who have received it for less time. There are no similar publications in Spain with detailed information on the cardiac toxicity and efficacy of adjuvant trastuzumab in real-world situations and with a prolonged follow-up of 15 years.
Results
Patient flow
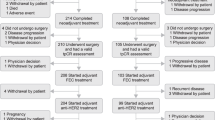
A total of 1479 medical histories of women diagnosed with breast carcinoma were reviewed, of which 298 (20.15%) were HER2-positive (Fig. 1).
Patient characteristics
Table 1 shows the characteristics of the patients studied.
The median number of three-weekly cycles of trastuzumab administered was 17 (range: 1–23). Regarding trastuzumab discontinuation, 68 patients (24.70%) received 16 or fewer cycles, and 205 (74.50%) received 17 or more cycles. The most frequent causes of trastuzumab discontinuation were asymptomatic LVEF decline (54, 19.64%) and heart failure (12, 4.36%).
In the 72 patients treated with neoadjuvant systemic therapy, pathologic complete response in the breast was obtained in 39 patients (54.17%), and in the axilla in 52 patients (72.22%). Thirty-five patients achieved complete pathologic response (48.61%).
Association between variables and the number of trastuzumab cycles
In the bivariate analysis the dependent variable “number of trastuzumab cycles” was statistically associated with age, with the way of administering chemotherapy and trastuzumab (concomitant or sequential) and with cardiotoxicity (Table 2). In the multivariate analysis, age and cardiotoxicity remained statistically significant (Table 2). For the number of trastuzumab cycles, only age (OR 0.371, 95% CI 0.152–0.903; p = 0.029) and cardiotoxicity (OR 15.02, 95% CI 7.437–30.335; p < 0.001) remain in the final multivariate model as definitively significant.
Association between variables and cardiotoxicity
Of all the variables none was significantly associated with cardiotoxicity in the bivariate analysis, and only the way of administering chemotherapy and trastuzumab (concomitant or sequential) and radiotherapy treatment, approached statistical significance in the multivariate analysis (Table 3). For the cardiotoxicity as a dependent variable only radiotherapy treatment showed statistical significance in the multivariate model (OR 0.0362, 95% CI 0.139–0.938; p = 0.037).
Follow-up of patients and events
The median follow-up of the patients was 71 months (range: 4–194). In this period, 49 patients (17.90%) presented events that are summarized in Table 4.
One patient died from heart failure in the Intensive Care Unit. She had developed cardiogenic shock during the period they were receiving adjuvant trastuzumab. Another patient died from ischemic heart disease several years after adjuvant trastuzumab had been discontinued.
Survival analysis
The probability of surviving after 5 years for the 275 patients was 0.93 (95% CI 0.89–0.96), at 10 years it was 0.88 (95% CI 0.84–0.93) and at 15 years 0.81 (95% CI 0.72–0.90). Five-year DFS was 0.88 (95% CI 0.83–0.92), 10-year DFS was 0.80 (95% CI 0.74–0.86), and 15-year DFS was 0.61 (95% CI 0.48–0.74). Figure 2 shows the OS and DFS curves (A, B). The median OS and DFS have not been reached.
Tables 5 and 6 present the associations observed in the bivariate and multivariate analysis between the different variables and OS and DFS.
In the multivariate model, hypertension (HR 0.361, 95% CI 0.151–0.863, p = 0.022), neoadjuvant treatment (HR 0.314, 95% CI 0.132–0.750, p = 0.009), and cardiotoxicity (HR 2.755, 95% CI 1.235–6.143, p = 0.013) maintained significant association with OS. Only the neoadjuvant treatment maintained a significant association with DFS (HR 0.437, 95% CI 0.213–0.899, p = 0.024).
Discussion
The results presented confirm the effectiveness of trastuzumab in the real world, with OS and DFS that can be considered comparable to those of clinical trials6,7,8. In the Early Breast Cancer Trialists’' Collaborative group (EBCTCG) meta-analysis17, with a median follow-up of 10.7 years, 26.60% of patients had recurrence of HER2 breast cancer and 19.70% died. For our patients, with a shorter follow-up, 6 years, 11.34% had recurrence of breast cancer and 9.44% died. The real-world effectiveness of trastuzumab is also demonstrated when it was administered with neoadjuvant intent, with pathologic complete response in 48.61% of cases, better or similar to that obtained in trials involving chemotherapy and trastuzumab18,19,20.
The way of measuring cardiac toxicity, which was different for each study, means that the results are not entirely comparable. Some 18.6% of participants in the treatment arm with doxorubicin and cyclophosphamide followed by docetaxel plus trastuzumab, in the BCIRG 006 trial (follow-up 65 months)8, 14% in the chemotherapy plus trastuzumab treatment arm of the NSABP-31 trial (follow-up 24 months)21, and 7.08% in the chemotherapy arm followed by 1 year of trastuzumab in the HERA trial (follow-up 12 months)6,22, had significant decreases in LVEF. The percentage of grade 3 and 4 heart failure was 4.5% in a meta-analysis of five trastuzumab trials23. Our cardiotoxicity results were an asymptomatic decline in LVEF in 19.64% and heart failure in 4.36%. Cardiotoxicity was the main reason for discontinuing trastuzumab, with 24.70% receiving 16 or fewer cycles.
In our study, only having received radiotherapy was associated with an increased risk of cardiotoxicity, without detecting, as in other studies15, any association with age, arterial hypertension or obesity. The risk of cardiotoxicity associated with radiation received by the heart, and administration of potentially cardiotoxic trastuzumab is unclear. It is important to highlight that in an analysis of 1503 patients treated with adjuvant radiation in the randomized NCCTG N9831 trial, no increased cardiotoxicity was observed, even in patients treated with concomitant trastuzumab and radiotherapy22.
In an attempt to identify the variables associated with a lower number of trastuzumab cycles—being over 65 years of age, and suffering from cardiotoxicity showed significant odds ratios in the multivariable analysis, so we can confirm the association between not completing the 12 months of trastuzumab and age, and cardiotoxicity. Of the women studied, 17.8% were older than 65 years. In real-world studies, these older patients specifically are not well represented in clinical trials, and patients who are poorly represented experience worse outcomes than those who are well represented23.
One of the consequences of trastuzumab cardiotoxicity is the obligation to discontinue treatment, causing patients to be unable to receive all 17–18 cycles in the 12 months of treatment. In our real world, although patients received a median of 17 cycles, the 68 (24.70%) who received 16 or fewer cycles did not have a worse OS than those who received more than 16 cycles. These results are at odds with the systematic review of trials on de-escalation in the duration of adjuvant trastuzumab therapy14, where a shorter duration of adjuvant trastuzumab is associated with significantly worse DFS and OS. Other studies and meta-analyses12,24, however, have shown non-inferiority of 6 months compared to 12 months. These results should be interpreted with caution, as they are based primarily on the Persephone trial12, an older trial that employed chemotherapy-trastuzumab regimens considered nonstandard (taxane-free anthracyclines in 90% and sequential trastuzumab in 53% of cases), in a relatively low-risk population (59% of node-negative patients).
In the case of adjuvant treatment of early breast cancer with trastuzumab, short- and long-term cardiovascular side effects become crucially important in health outcomes25 and this is what we observed also in our real-world study, as patients who did not develop cardiotoxicity, and who do not have hypertension, had better OS than those who did have heart failure or significant decline in LVEF and hypertension. Other authors26 have already established the consensus that patients who do not complete adjuvant trastuzumab because of cardiotoxicity have a significantly increased risk of relapse and death from breast cancer. These findings also support 1 year of adjuvant trastuzumab26.
The use of trastuzumab with chemotherapy in the neoadjuvant setting results in a higher rate of complete disease responses, but the effects on DFS and OS are unclear compared to adjuvant treatment. One study supports early initiation of neoadjuvant trastuzumab, especially in patients with hormone-resistant and HER2-positive tumors27, so we cannot give a plausible explanation for our findings of worse OS and DFS in patients treated in the neoadjuvant setting.
This study was a retrospective observational evaluation of data intended to assess the effectiveness of adjuvant trastuzumab in the real world and is therefore subject to limitations. There may be unmeasured confounders unequally distributed among the groups. There may also be uncaptured patient comorbidities. Another limitation is the need for longer follow-up, as patients with hormone-sensitive breast cancer, even if they are HER2+, may have late recurrences.
There are no similar publications in Spain with detailed data on the effectiveness and cardiac toxicity of adjuvant trastuzumab in the real world and with an extended follow-up of 15 years. For this reason, the results presented here constitute valuable information with which other centers can make comparisons.
In conclusion, our results confirm the significance of the use of trastuzumab in the adjuvant treatment of early-stage breast cancer in the real world, with survival comparable to those of clinical trials. In the real world, factors such as age, hypertension, radiotherapy, neoadjuvant treatment and cardiotoxicity should be taken into consideration to optimize outcomes.
Methods
This is an observational, descriptive, retrospective, single-center, study on a medicinal product for human use. It was carried out at the Medical Oncology Department of a university hospital. All cases treated since the official approval of trastuzumab in 2006 were selected. Patients were identified from the Medical Oncology department’s database, and data related to patients and treatment received were retrospectively collected from Medical Oncology’s medical records. All experimental protocols were approved by the Cádiz Research Ethics Committee.
Patients with breast cancer undergoing surgical treatment with a pathology result showing HER2-positive infiltrating carcinoma were included. Those suitable for inclusion in the study had to have been treated with trastuzumab, either alone or with chemotherapy and/or hormone therapy, between 1 January 2006 and 31 December 2020, and followed up until 1 September 2021.
Female and male patients with HER2-positive infiltrating breast carcinoma (immunohistochemical score 3+ and 2+ with gene amplification), confirmed by histology, at disease stage I, II and III were included. Patients could have received adjuvant or neoadjuvant systemic treatment with hormone therapy, chemotherapy, or neither. Patients who received systemic neoadjuvant therapy were treated with chemotherapy plus trastuzumab, or plus trastuzumab and pertuzumab prior to surgery. These patients did not receive adjuvant chemotherapy but they did receive adjuvant trastuzumab with a goal of 12 months of treatment. Cases of carcinoma in situ, without infiltrating component and patients with metastases were excluded.
Age, sex, functional capacity (measured by the ECOG scale)28, menopausal status, comorbidity (measured by the Charlson scale)29, body mass index and arterial hypertension were the patient-related independent variables.
The independent variables related to the breast tumor were: tumor size at definitive surgery, disease stage (American Joint Committee on Cancer 8th edition), histologic grade, histologic type, extensive peritumoral vascular invasion, estrogen and progesterone receptor status (considered positive if immunohistochemical expression was equal to or greater than 1%), HER2 (positive if immunohistochemical score was 3+ or 2+ with gene amplification by in situ hybridization), Ki67 proliferative index, number of axillary nodes with metastases, number of total axillary nodes analyzed.
The independent variables related to treatment were: type of surgery, radiotherapy, type of adjuvant hormonal treatment, type of adjuvant chemotherapy, route of administration of trastuzumab and form of administration of chemotherapy-trastuzumab (concomitant or sequential). Weekly trastuzumab was administered intravenously at a starting dose of 4 mg/kg of body weight and maintenance dose of 2 mg/kg for 12 months. Trastuzumab was administered three times a week either intravenously at a starting dose of 8 mg/kg and maintenance dose of 6 mg/kg, or subcutaneously at a dose of 600 mg, both also for 12 months.
The independent variables related to safety were cardiotoxicity (subclassified into symptomatic heart failure and asymptomatic LVEF decline), trastuzumab discontinuation (patients were classified into those who had completed the 17–18 three-weekly cycles of trastuzumab treatment and those who have not completed treatment with ≤ 16 cycles), and the reason trastuzumab was discontinued. In case of weekly trastuzumab administration, 3 weeks was considered to be 1 cycle. A baseline LVEF of at least 50% was required to initiate trastuzumab treatment and the test was repeated every 3 months during the year of treatment. Trastuzumab was discontinued if symptomatic heart failure or an asymptomatic decrease in LVEF of 15 points, or 10 points if this put the level below 50%, occurred. In cases of asymptomatic LVEF decline, angiotensin-converting enzyme inhibitors and beta-blockers were prescribed at low doses and repeated on a month-by-month basis. If levels recovered, trastuzumab was reintroduced.
The efficacy dependent variables analyzed were overall survival and disease-free survival30.
A descriptive analysis of the variables (absolute and relative frequency, mean, median and standard deviation) was made. For the comparison of qualitative variables, the chi-square test with Fisher’s correction was applied and, for quantitative variables, the Student’s t-test.
The influence of independent variables on the number of trastuzumab cycles administered was measured using binary logistic regression, using odds ratio (OR) for risk estimation with a corresponding CI of 95%.
Logistic regression analysis with an independent variable was performed to calculate the odds ratio (OR) of each variable in relation to trastuzumab cardiotoxicity.
The Kaplan Meier method and the Log-Rank test for the comparison of survival curves were used to calculate survival. Cox regression with an independent variable was used to calculate the hazard ratio (HR).
Landmark analysis was carried out to monitor immortal time bias in the survival analysis among subgroups with ≤ 16 cycles and 17–18 cycles of trastuzumab. The landmark time is established as the duration of time that it would take to complete 16 cycles. Any patients experiencing event (DFS or OS) prior to this landmark time were dropped from the analytical sample. The groups (≤ 16 cycles versus 17–18 cycles) were defined, using only patients in the group without events prior to the landmark time. The survival analysis was conducted as usual, with t0 set equal to the landmark time.
The SPSS program, version 21, was used for statistical analysis of the data. In the statistical analysis, p < 0.05 was considered to indicate statistical significance.
Ethical considerations
The work described was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments on human subjects. All experimental protocols were approved by the Cádiz Research Ethics Committee (code 152.21), which authorized the waiver of informed consent.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Guy, C. T. et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. U. S. A. 89(22), 10578–10582. https://doi.org/10.1073/pnas.89.22.10578 (1992).
Slamon, D. J. et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182. https://doi.org/10.1126/science.3798106 (1987).
Slamon, D. J. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707–712. https://doi.org/10.1126/science.2470152 (1989).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352. https://doi.org/10.1038/nature10983 (2012).
Yeon, C. H. & Pegram, M. D. Anti-erbB-2 antibody trastuzumab in the treatment of HER2-amplified breast cancer. Invest. New Drugs. 23, 391–409. https://doi.org/10.1038/emboj.2013.19 (2005).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672. https://doi.org/10.1056/NEJMoa052306 (2005).
Romond, E. H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684. https://doi.org/10.1056/NEJMoa052122 (2005).
Slamon, D. et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365, 1273–1283. https://doi.org/10.1056/NEJMoa0910383 (2011).
Conte, P. et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: Final results of the phase III randomized Short-HER study. Ann. Oncol. 29, 2328–2333 (2018).
Joensuu, H. et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs. 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer. JAMA Oncol. 4, 1199–1206 (2018).
Mavroudis, D. et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: A multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann. Oncol. 26, 1333–1340 (2015).
Earl, H. M. et al. 6 vs. 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 393, 2599–2612 (2019).
Pivot, X. et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): Final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 393, 2591–2598 (2019).
Goldvaser, H. et al. Deescalating adjuvant trastuzumab in HER2-Positive early-stage breast cancer: A systemic review and meta-analysis. JNCI Cancer Spectr. 3, pkz033. https://doi.org/10.1093/jncics/pkz033 (2019).
Dempsey, N. et al. Trastuzumab-induced cardiotoxicity: A review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. Breast Cancer Res. Treat. 188(1), 21–36. https://doi.org/10.1007/s10549-021-06280-x (2021).
Curigliano, G. et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 31(2), 171–190. https://doi.org/10.1016/j.annonc.2019.10.023 (2021).
Early Breast Cancer Trialists’ Collaborative group (EBCTCG). Trastuzumab for early-stage, HER2-positive breast cancer: A meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 22, 1139–1150. https://doi.org/10.1016/S1470-2045(21)00288-6 (2021).
Gianni, L. et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375, 377–384. https://doi.org/10.1016/S0140-6736(09)61964-4 (2010).
von Minckwitz, G. et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: Phase III GeparQuattro study. J. Clin. Oncol. 28, 2015–2023. https://doi.org/10.1200/JCO.2009.23.8303 (2010).
Buzdar, A. U. et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): A randomised, controlled, phase 3 trial. Lancet Oncol. 14, 1317–1325. https://doi.org/10.1016/S1470-2045(13)70502-3 (2013).
Telli, M. L., Hunt, S. A., Carlson, R. W. & Guardino, A. E. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J. Clin. Oncol. 25, 3525–3533. https://doi.org/10.1200/JCO.2007.11.0106 (2007).
Halyard, M. Y. et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: Tolerability and adverse event data from the NCCTG Phase III Trial N9831. J. Clin. Oncol. 27(16), 2638–2644. https://doi.org/10.1200/JCO.2008.17.9549 (2009).
Viani, G. A., Afonso, S. L., Stefano, E. J., De Fendi, L. I. & Soares, F. V. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: A meta-analysis of published randomized trials. BMC Cancer 7, 153. https://doi.org/10.1186/1471-2407-7-153 (2007).
Gidwani, R. et al. Survival in the real world: A national analysis of patients treated for early-stage breast cancer. JCO Oncol. Pract. 18(2), e235–e249. https://doi.org/10.1200/OP.21.00274 (2022).
Earl, H. M. et al. Individual patient data meta-analysis of 5 non-inferiority RCTs of reduced duration single agent adjuvant trastuzumab in the treatment of HER2-positive early breast cancer. Ann. Oncol. 32, S1283–S1346. https://doi.org/10.1016/annonc/annonc741 (2021).
Rushton, M. et al. Impact of stopping trastuzumab in early breast cancer: A population-based study in Ontario, Canada. J. Natl. Cancer Inst. 112(12), 1222–1230. https://doi.org/10.1093/jnci/djaa054 (2020).
Palmieri, C. et al. Neoadjuvant chemotherapy and trastuzumab versus neoadjuvant chemotherapy followed by post-operative trastuzumab for patients with HER2-positive breast cancer. Oncotarget 7(11), 13209–13220. https://doi.org/10.18632/oncotarget.4801 (2016).
Oken, M. M. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 5, 649–655 (1982).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chron. Dis. 40, 373–383. https://doi.org/10.1016/0021-9681(87)90171-8 (1987).
Hudis, C. A. et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J. Clin. Oncol. 25, 2127–2132. https://doi.org/10.1200/JCO.2006.10.3523 (2007).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by J.L.G., V.N.A., C.T.H., M.B.G., A.C.B., E.P.Z., L.R.P., J.C.C., M.J.M.B., E.B.R., and J.M.B.C. The first draft of the manuscript was written by J.M.B.C. and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lluch-Gómez, J., Núñez-Álvarez, V., de la Torre-Hita, C. et al. Real world evidence of adjuvant trastuzumab in HER2 positive early breast cancer. Sci Rep 13, 7168 (2023). https://doi.org/10.1038/s41598-023-34429-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34429-9
This article is cited by
-
Enhancing treatment strategies for small bowel cancer: a clinical review of targeted therapy and immunotherapy approaches
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.