Abstract
Camelina (Camelina sativa) is an oil crop with a short growing period, resistance to drought and cold, low fertilizer requirements, and can be transformed using floral dipping. Seeds have a high content of polyunsaturated fatty acids, especially ɑ-linolenic acid (ALA), at 32–38%. ALA is an omega-3 fatty acid that is a substrate for eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the human body. In this study, ALA content was further enhanced by the seed-specific expression of Physaria fendleri FAD3-1 (PfFAD3-1) in camelina. The ALA content increased up to 48% in T2 seeds and 50% in T3 seeds. Additionally, size of the seeds increased. The expression of fatty acid metabolism-related genes in PfFAD3-1 OE transgenic lines was different from that in the wild type, where the expression of CsFAD2 decreased and CsFAD3 increased. In summary, we developed a high omega-3 fatty acid-containing camelina with up to 50% ALA content by introducing PfFAD3-1. This line can be used for genetic engineering to obtain EPA and DHA from seeds.
Similar content being viewed by others
Introduction
Plant oils are composed of triacylglycerols (TAG), which comprise fatty acids (FAs) that form ester bonds with the glycerol backbone. Plant oils can be used in various ways, such as for food and industrial purposes, depending on their FA composition1. Generally, plant oils are mainly composed of five types of FAs: saturated FAs, including palmitic acid (PA; 16:0) and stearic acid (SA; 18:0), and unsaturated FAs, including oleic acid (OA; 18:1), linoleic acid (LA; 18:2), and α-linolenic acid (ALA; 18:3)2.
The aim of this study was to enhance the production of omega-3 FAs. The methyl side of FAs is called omega. If there is a double bond at the third carbon of the methyl group, it is called an omega-3 FA, and if it has a double bond at the sixth carbon, it is called an omega-6 FA3,4. Omega-3 FAs include ALA, eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA)5. ALA is an essential FA because it is not synthesized in humans5. ALA is found in nut and seed oils, while EPA and DHA are mainly found in fish4,6. In the human body, ALA is slightly converted into EPA and DHA. The conversion ratio of ALA to EPA is approximately 8%, but the ratio of ALA to DHA is much lower7,8. An adequate ratio of omega-3 to omega-6 FAs (1:1 to 1:4) should be maintained in the human body, depending on the disease condition, by consuming foods rich in omega-3 FAs9. Fish may be a good source of omega-3 because they contain higher amounts of EPA and DHA10. However, it is concerning that omega-3 FAs, such as EPA and DHA, are abundant in fish, but they contain high levels of mercury. In addition, vegetarians do not eat fish6. Therefore, plant-derived omega-3 FAs are a good alternative to fish-derived omega-3 FAs.
Fatty acid desaturase 3 (FAD3) was identified to synthesize ALA in Arabidopsis (Arabidopsis thaliana). FAD3 synthesizes the ALA by desaturation of 18:2, located at sn-2 of phosphatidylcholine (PC) in the endoplasmic reticulum (ER) membrane11,12. FAD3 contains three histidine boxes with catalytic activities that are conserved in most plants13. In addition, FAD3 contains several transmembrane domains that differ between plant species, and functional studies of the FAD3 gene have been conducted in various plants, including Arabidopsis14,15,16,17,18,19,20,21,22,23,24. Various FAD3s isolated from different plant species were overexpressed in several other plant species to study their effects on FA content and other traits. Overexpression of Plukenetia volubilis FAD3 in tobacco seeds decreased 18:2 and increased 18:3 when compared to that in the wild type (WT)20. Overexpression of Perilla FAD3 in Arabidopsis significantly increased 18:3 content21. Overexpression of Brassica napus FAD3 in tobacco not only increased 18:3 content but also improved drought resistance23. Similarly, the overexpression of Arachis hypogaea L FAD3 in Arabidopsis led to an increase in 18:3 and salt resistance17. However, overexpression of camelina FAD3 in Arabidopsis increased the 18:3 content but also resulted in wrinkled seeds and reduced oil content and seed weight24.
Physaria fendleri FAD3-1 (PfFAD3-1) was used to increase ALA levels in camelina seeds. PfFAD3-1 was chosen because soybean has a low 18:3 content; however, the expression of PfFAD3-1 in soybean significantly increased the 18:3 content by up to 52.4%14,25,26. P. fendleri is a plant species belonging to the Brassicaceae family, with oil containing 60% lesquerolic acid, which is a hydroxy fatty acid27,28. PfFAD3-1 and PfFAD3-2 are two FAD3s found in P. fendleri, both of which are ER membrane-bound proteins with three histidine boxes and four transmembrane domains14,25. Recently, PfFAD3-1 and PfFAD3-2 were expressed in the Arabidopsis fad3-2 mutants. PfFAD3-1 restored the 18:3 content by up to 29.9% when expressed in the fad3-2 mutant, which has a low ALA content, whereas PfFAD3-2 has little desaturase activity14.
Camelina has become an increasingly important research tool for studying biofuels and other biotechnological applications of oil crops29,30,31. It is an allohexaploid (2n = 6x = 40) plant belonging to the Brassicaceae family that is grown primarily in Europe and North America32,33. Camelina has a short life cycle (85–100 days), low fertilizer requirements, and resistance to cold and drought33. It can be easily transformed using the floral dipping method34. Polyunsaturated FAs constitute approximately 50% of camelina seeds; particularly, 32–38% of 18:3 FA. The 18:1 FA and 20:1 FA contents are 12–18% and 12–17%, respectively33.
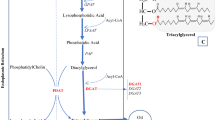
FA biosynthesis is initiated by the conversion of acetyl-coenzyme A (CoA) to malonyl-CoA in the plastids by acetyl-CoA carboxylase (ACCase)35. Malonyl-CoA is then converted into malonyl-ACP by malonyl-CoA: acyl carrier protein (ACP) transacylase36. Malonyl-ACP is converted to 4:0-ACP by FA synthase (FAS) and ketoacyl-acyl carrier protein synthase (KAS) III and is synthesized as 16:0-ACP by KAS I and FAS enzymes37. Thereafter, it is synthesized as 18:0-ACP by FAS and KASII. Stearoyl-ACP desaturase (SAD) generates 18:1-ACP from 18:0-ACP37. Fatty acyl-ACP thioesterase B (FATB) specifically cleaves 16:0-ACP and 18:0-ACP, which are saturated FAs, while 18:1-ACP is specifically cleaved by fatty acyl-ACP thioesterase A (FATA). The three free FAs produced by FATA and FATB are exported from the plastid to the cytosol38. Free FAs (16:0, 18:0, and 18:1) combine with CoA in the cytosol to form an acyl-CoA pool. 18:1-CoA is synthesized into 20:1-CoA and 22:1-CoA in the cytosol by fatty acid elongase 1 (FAE1)39. The desaturation of 18:1 to 18:2 and 18:3 involves a sequential reaction of FAD2 and FAD3, which target 18:1 at the sn-2 position of PC. The 18:1, 18:2, and 18:3 FAs in PC are released into the acyl-CoA pool via the reverse reaction of lysophosphatidylcholine acyltransferase (LPCAT)40. Various FAs in the acyl-CoA pool are transferred to glycerol to form TAG using glycerol-3-phosphate acyltransferase (GPAT), lysophospholipid acyltransferase (LPAT), and diacylglycerol acyltransferase (DGAT)35. In this study, PfFAD3-1 was introduced into camelina to increase ALA content in seeds (Fig. 1).
Schematic diagram of fatty acid synthesis pathway in Camelina sativa seeds. Fatty acid (FA) synthesis in the plastids. Acetyl-CoA is converted to malonyl-CoA by ACCase and then elongated by FAS, KASIII, and KASI. KASII elongates palmitic acid (16:0) to stearic acid (18:0). SAD catalyzes the desaturation of stearic acid into oleic acid (18:1). FAs are transported to plastids by FATA and FATB. Linoleic acid (18:2) and α-linolenic acid (18:3) are synthesized from oleic acid in the PC membrane of the ER by FAD2 and FAD3 enzymes. In addition, oleic acid can be elongated into eicosenoic acid (20:1) and erucic acid (22:1) by FAE1. Black arrows indicate FA flow, and red arrows show the pathway for enhancing linolenic acid by PfFAD3-1 expression. The blue dotted arrow indicates the process by which various FAs transfer to TAG by several enzymes. ACP, acyl carrier protein; CoA, coenzyme A; ER, Endoplasmic reticulum; FAD2, fatty acid desaturase 2; FAD3, fatty acid desaturase 3; FAE1, fatty acid elongase 1; FAS, fatty acid synthase; FATA, fatty acyl-ACP thioesterase A; FATB, fatty acyl-ACP thioesterase B; KASII, β-ketoacyl-acyl carrier protein synthase II; LPCAT, acyl-CoA:lysophosphatidylcholine acyltransferase; SAD, stearoyl-ACP desaturase; TAG, triacylglycerol.
Results
FA composition analysis in seeds across T1 to T3 generations of PfFAD3-1 overexpressed camelina
PfFAD3-1 was overexpressed in camelina to enhance ALA production in seeds. The pBinGlyRed3 vector was used for seed-specific expression41. This vector contains a glycinin promoter, a seed-specific promoter, and DsRed (a red fluorescent protein) as a selection marker. PfFAD3-1 complementary DNA (cDNA) was cloned into pBinGlyRed3 vector (Fig. 2). The FA composition of the T2 seeds of 22 plants was analyzed and compared with that of the WT to observe changes in FA content in PfFAD3-1 OE lines (Fig. 3, Table S1). Transgenic plants are denoted as PfFAD3-1 OE numbers in the following description. In most transgenic plants, 18:3 content was increased compared to that in the WT, leading to a decrease in 18:2 content, which is a substrate of FAD3. The average 18:3 content in the WT was 41.4% and 45.5% in the transformant, indicating an increase of approximately 4%. Furthermore, the average 18:2 content in the WT was 15.8% and 8.7% in the transformant, showing a reduction of approximately 7%. The average 18:1 content was 12% in the WT but increased to an average of 16.9% in the transformant. The contents of other FAs did not significantly differ between the WT and transgenic plants (Fig. 3).
Fatty acid composition of Camelina sativa cv. Suneson and T1 transgenic lines expressing PfFAD3-1 in T2 seeds. FAME was analyzed in the T2 generation seeds of T1 transgenic plants and compared with the WT. Green points indicate the WT. Black points indicate transgenic plants, and PfFAD3-1 OE #6, #19, and #22 lines are marked in blue. PfFAD3-1 OE #6, #19, and #22 lines were selected for T2 generation.
The T1 generation was not a homozygous line; therefore, only PfFAD3-1 OE #6, #19, and #22 lines, which showed the highest 18:3 contents of 48.3%, 48.4%, and 48.5%, respectively, among the 22 transformants, were selected and progressed to the T2 generation. The FA composition was analyzed using gas chromatography (GC) in the T3 seed (Table S2). The average 18:3 content in the WT was 35.7%, whereas that in the PfFAD3-1 OE #6, #19, and #22 lines was 46.1%–49.8%. The average 18:2 content was 16.3% in the WT, whereas that in the PfFAD3-1 OE #6, #19, and #22 lines was 2.9%–4.1%, showing a decrease of more than 12%.
FA analysis was performed five times to determine the FA composition and oil content of the T3 generation lines PfFAD3-1 OE #6-9, #19-5, and #22-1 (Table S3). The average 18:3 content was 48%, 49.4%, and 48.8%, respectively, which was significantly higher than that of the WT (35.7%) (Fig. 4a). The contents of saturated FAs 16:0 and 18:0 in the WT were 7.1% and 3.2%, respectively, and those in the PfFAD3-1 OE #6-9, #19-5, and #22-1 lines were 7.4–7.9% and 3.5–4.0%, respectively, showing no significant differences from those in the WT (Fig. 4a). The 18:2 content in the WT was 16.3%, whereas in PfFAD3-1 OE #6-9, #19-5, and #22-1 it was 2.6–3.4% (Fig. 4a). Furthermore, the 20:1 and 22:1 content in the WT were 12.5% and 2.1%, respectively, and those in PfFAD3-1 OE #6-9, #19-5, and #22-1 were 13.0–13.3% and 1.6–1.7%, showing no significant differences compared to that in the WT. In contrast, the 18:1 content in the WT was 18.9%, but increased slightly to 19.8% in the PfFAD3-1 OE # 6-9 lines and decreased in PfFAD3-1 OE #19-5 (17.4%) and 22-1 (18.2%) lines (Fig. 4a, Table S3).
Fatty acid composition and oil content in T3 seeds of T2 generation transgenic lines. (a) FA composition of three transgenic (PfFAD3-1 OE #6-9, #19-5, #22-1) and WT plants. (b) Total FAME (µg/seed) of transgenic and WT plants. The results were analyzed as five replicates (n = 5). Error bars represent standard error of the mean (SEM). Statistical significance is indicated by different letters using one-way ANOVA and Tukey’s multiple comparison test (*p < 0.05).
The total oil content of the seeds was measured as the total fatty acid methyl ester (FAME). The FAME content of WT was 272.5 μg/seed, and that of PfFAD3-1 OE #6-9, #19-5, and #22-1 lines was 251.8, 263.8, and 268.9 μg/seed, respectively (Fig. 4b). In the PfFAD3-1 OE #19-5 and #22-1 lines, the total FAME (μg/seed) content was similar to that in the WT; however, the total FAME in the PfFAD3-1 OE #6-9 line decreased by 8% compared to that in the WT (Fig. 4b).
In summary, the results of the FA analysis up to the T3 generation revealed that seed-specific overexpression of PfFAD3-1 enhanced 18:3 content in seeds and decreased 18:2 content. Moreover, the effect of PfFAD3-1 was confirmed to be maintained until the T3 generation.
Seed phenotype of PfFAD3-1 OE lines in T3 generation
Seed weight and size were analyzed to investigate the effect of overexpressing PfFAD3-1 on camelina seeds. Seed width, length, and size were measured in triplicate for 50 seeds per line (Fig. 5a). There was no significant difference in seed weight between the WT and PfFAD3-1 OE lines (Fig. 5b). However, the seed width and length of all three transgenic lines were significantly greater than those of the WT (Fig. 5c,d). The average seed width of the WT was 0.83 mm, whereas that of PfFAD3-1 OE #6-9, #19-5, and #22-1 lines was 0.85, 0.88, and 0.87 mm, respectively (Fig. 5c). The average seed length of the WT was 1.62 mm, whereas that of PfFAD3-1 OE #6-9, #19-5, and #22-1 lines was 1.73, 1.74, and 1.66 mm, respectively (Fig. 5d). Seed size was calculated by multiplying the width and length, which showed an increase in the transgenic lines compared with that in the WT (Fig. 5e).
Seed phenotype of transgenic and wild type plants in the T3 generation. (a) Images of seeds of transgenic (PfFAD3-1 OE #6-9, #19-5, #22-1) and WT plants. (b) Seed weight. (c) Seed width. (d) Seed length. (e) Seed size. The results were analyzed in triplicates (n = 30). Scale bar = 2 mm. Error bars represent SEM. Statistical significance is indicated by different letters using one-way ANOVA and Tukey’s multiple comparison test (*p < 0.05).
Analysis of FA composition and FA synthesis-related gene expression in the seed development stage of PfFAD3-1 OE lines
Seven genes (KASIII, SAD, FATA, and FATB, which are involved in FA biosynthesis; FAD2 and FAD3, which are FA desaturase genes; and FAE1, which is an elongase gene) were selected to investigate whether the introduction of PfFAD3-1 affected the expression of endogenous FA synthesis genes in camelina (Fig. 6). Seed development was divided into three stages (S1, S2, and S3), and FA composition and expression of FA biosynthesis genes in the seeds were analyzed for the PfFAD3-1 OE #6-9-3, #19-5-2, and #22-1-3 lines of the T4 generation (Fig. 6a,b).
Fatty acid composition and gene expression in the stage of T4 generation developing seeds. (a) FA composition in transgenic and WT plants. (b) Gene expression patterns in the developing stage of transgenic lines and WT. S1, S2, and S3 were developing seeds harvested 30, 40, and 50 days after flowering, respectively. Error bars represent SD. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001).
In the WT and three PfFAD3-1 OE lines, 16:0 FA and 18:0 FA continued to decrease from S1 to S3, showing no significant differences. The 18:1 content decreased from S1 to S2 and increased in the S3 stage, and the three transgenic plants showed higher content than the WT plants at all stages. The opposite trend was observed between the 18:2 and 18:3 contents; the 18:2 content decreased from S1 to S3, whereas the 18:3 content increased. The decrease and increase in 18:2 and 18:3 content, respectively, in the transformants gradually differed from those in the WT as seed development progressed. The 20:1 and 22:1 contents tended to increase from the S1 to S2 stages, and then slightly decreased at the S3 stage. However, there was no significant difference between the WT and transformants for either 20:1 FA or 22:1 FA (Fig. 6a).
The expression of FA biosynthesis genes in camelina was analyzed in WT and PfFAD3-1 OE plants at each stage of seed development (Fig. 6b). The expression of PfFAD3-1 was not detected in the developing seeds of the WT plants; however, PfFAD3-1 was highly expressed in the developing seeds of all three transgenic lines. CsFAD3, which has the same function as PfFAD3-1, showed different expression patterns between the WT and transgenic plants. At the S2 and S3 stages, the transgenic plants showed a 2.4-fold upregulation of CsFAD3 compared to the that in the WT. The expression patterns of CsSAD, CsFATB, and CsFAD2 showed the same tendencies in the WT and the transformants. However, the expression levels of CsSAD and CsFAD2 were 0.7-fold lower in transgenic plants than in WT plants at S2 stage. During the S2 stage, CsFATB expression was elevated 1.6-fold in the PfFAD3-1 OE #6-9-3 line compared to the WT; however, it showed a 0.4-fold decrease in the PfFAD3-1 OE #19-5-2 and #22-1-3 lines compared to that in the WT. CsKASII, CsFATA, and CsFAE1 showed different expression patterns in the WT and transgenic plants (Fig. 6b). In summary, 18:3 content increased and 18:2 content decreased in the developing seeds of transgenic plants compared to that in WT plants. This was due to the introduction of PfFAD3-1, and the expression of exogenous PfFAD3-1 induced the upregulation of CsFAD3 and downregulation of CsFAD2.
Discussion
In this study, PfFAD3-1, which converts 18:2 FA to 18:3 FA, was transformed into camelina (Fig. 1). Consequently, the ALA (18:3) content increased by up to 48% in the T2 seeds and up to 50% in the T3 seeds of the transgenic camelina plants (Figs. 3, 4). The PfFAD3-1 OE lines showed an average increase of 12.3–13.7% in 18:3 content and a decrease of 13.0–13.7% in 18:2 content (Table S3). This similarity in the increase of 18:3 and decrease of 18:2 suggests that the conversion of 18:2 FA to 18:3 FA in camelina by PfFAD3-1 is both specific and efficient. In a previous study, when PfFAD3-1 was transformed into soybeans under the control of the seed-specific promoter, β-conglycinin, the ALA content increased by up to 52% in T1 seeds and up to 42% in T2 seeds26. In soybean, PfFAD3-1 expression increased ALA content under the control of the phaseolin promoter and 35S promoter; however, the increase in ALA content was the greatest under the control of β-conglycinin26. PfFAD3-1 expression in soybeans did not result in significantly different oil content from that of the WT; however, it increased both seed size and weight26. Similarly, in this study, the seed oil content of PfFAD3-1 OE #6-9 line was slightly lower than that of the WT, whereas that of PfFAD3-1 OE #19-5 and #22-1 lines was similar to that of the WT (Fig. 4b). In the PfFAD3-1 OE #6, #19, and #22 lines, some plants in the next generation exhibited lower ALA content than those in the previous generation, as indicated in Table S2. This may be due to the presence of non-homozygous plants or variation in transgene expression among individuals. Nonetheless, the average ALA content of the progeny of these lines ranged from 46.1 to 49.8%, which was similar or slightly higher than that of the previous generation (Table S2). These findings suggest that the transmission and expression of the PfFAD3-1 transgene is stable and heritable in subsequent generations. Seed weight did not differ between the WT and the three transgenic lines. However, seed width, length, and size increased compared with those of the WT (Fig. 5b–e). This showed a seed phenotype similar to that observed when PfFAD3-1 was expressed in soybean26. Hence, the seed-specific expression of PfFAD3-1 in camelina did not significantly affect seed weight but increased seed size. Since seed size increased without a significant effect on seed oil content, further research is required to determine whether ALA content affects seed size.
Camelina seed oil can be used for various purposes such as baking, frying, and salad dressings42. The camelina developed in this study is beneficial for health because it contains up to 10% or more omega-3 FA compared to that in the WT. Camelina seeds contain eicosenoic acid (20:1) and erucic acid (22:1) at 12–17% and 2–3%, respectively33. The 20:1 and 22:1 FAs were synthesized from 18:1 by FAE143. Camelina was identified as having three copies of FAE1 genes39. When three copies of FAE1 were knocked out in camelina using the CRISPR/Cas9 technology, 20:1 and 22:1 synthesis was blocked, thus increasing the 18:1 content and continuously increasing the ALA content up to 50%44. In this study, PfFAD3-1 expression increased 18:3 content by up to 50% (Fig. 4). Therefore, knocking out CsFAE1 using CRISPR/Cas9 in the PfFAD3-1 overexpression line will further increase the ALA content by over 50%. In addition, under PfFAD3-1 expression, the 18:2 content was 2.6–3.4%, indicating that PfFAD3-1 sufficiently synthesized 18:3 using 18:2 as a substrate. Therefore, if the FAD2 gene is additionally overexpressed in the PfFAD3-1 overexpression line, the flux from 18:1 to 18:2 would increase; thus, PfFAD3-1 could use 18:2 to further enhance ALA content.
Since humans cannot synthesize ALA, it must be obtained through dietary sources, such as rapeseed or walnuts, which have high ALA content45. This study successfully developed a high-ALA camelina that could be used as a source of plant oil for ALA intake. From a clinical standpoint, increasing the ALA intake is important because it has been shown to reduce the risk of incident stroke46. While there are suggestions that ALA may have positive effects on cardiovascular health, the results are conflicting, indicating the need for further research in this area47. In addition to its direct health benefits, ALA can be converted into EPA and DHA in the human body, which also have beneficial effects. However, the efficiency of this conversion process is typically low8. To address this, research has been conducted to develop crops that can produce EPA and DHA directly from ALA through the expression of the desaturase and elongase enzymes involved in the synthesis of these FAs48,49,50. Recently, a transgenic camelina strain was developed using multigene metabolic engineering to produce 12% DHA in seeds49. In addition, this line was crossed with CsFAE1 knockout camelina containing high ALA content in seeds, further increasing omega-3 FA content (ETA, EPA, DPA, and DHA) from 27 to 33%51. Therefore, the high-ALA camelina developed in this study can be used to produce omega-3 FAs such as EPA and DHA.
Materials and methods
Vector construction of PfFAD3-1 gene
cDNA was synthesized using mRNA from developing seeds of P. fendleri using a PrimeScript 1st strand cDNA synthesis kit (Takara, Japan). P. fendleri seed WCL-LY1 (PI596362) was provide by the U.S. National Plant Germplasm System (NPGS). The synthesized cDNA was used as a template, and polymerase chain reaction (PCR) was performed using primers targeting the PfFAD3-1 open reading frame (Table S4). The PfFAD3-1 sequence was obtained from a previous study14. The PCR product was eluted using a PCR purification kit (Cosmogenetech, Korea) and cloned into the pGEMT-Easy vector (Promega, USA). Thereafter, we checked whether there was a PCR error using Sanger sequencing and proceeded to the next step. There is an EcoRI enzyme site on both sides of the pGEMT-Easy vector and a multi-cloning site, including the EcoRI enzyme site, in the pBinGlyRed3 vector equipped with a seed-specific glycine promoter. Therefore, it was cut with EcoRI enzyme, and the PCR product was ligated into the pBinGlyRed3 vector.
Selection of PfFAD3-1 transformed camelina
Camelina sativa cv. Suneson was used for all the experiments in this study. Camelina seeds were provided by Dr. Edgar Cahoon (University of Nebraska-Lincoln, Lincoln, Nebraska, USA). Camelina seeds were germinated on filter paper containing water in a culture chamber at 16 °C under a 16 h light/8 h dark photoperiod. After one week, the seeds from which the cotyledons emerged were transplanted into the soil. Eighteen plants were grown in soil, and transformation was performed using the floral dipping method34. The Agrobacterium strain GV3101 was used. Agrobacterium cells (500 μl) and kanamycin antibiotics (500 μl) were added to 500 ml LB medium and incubated overnight at 28 °C until an optical density value of 1 was obtained. This solution was centrifuged at 3000 × g for 10 min and resuspended in 500 ml solution containing 1% sucrose and 0.05% Silwet L-77. The camelina flowers were then immersed in this solution for 1 min. After floral dipping, plants were placed in a growth chamber for 24 h in the dark. This procedure was performed three times at intervals of 5 d. After harvesting the transformed plants, 22 fluorescent seeds were selected using a green flashlight and a red filter. After germination, these 22 T1 lines and the WT were grown in growth chamber at 20 °C under a 16 h light/8 h dark photoperiod to obtain the next generation. All procedures, including the collection of plant materials and experimental research, were conducted in accordance with institutional, national, and international guidelines.
Analysis of gene expression in PfFAD3-1 OE lines
Reverse transcription real-time PCR (RT-qPCR) was performed on RNA extracted using the method described in the reference52. Developing seeds were taken at the beginning of the desiccation phase (30, 40, and 50 days after flowering) based on reference53. After RNA isolation, 2 μg cDNA was synthesized using a cDNA synthesis kit (Takara, Japan). RT-qPCR analysis was performed using the synthesized cDNA as a template and SYBR Green Master Mix (TOYOBO, Japan) in a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, USA). Camelina ACTIN2 was used as an endogenous gene control, and primers capable of targeting all three copies of the genes were designed and used in this study (Table S4).
Fatty acid analysis
Seven camelina seeds were subjected to GC. Seeds were placed 500 μl of toluene and 1 ml of 5% H2SO4 including pentadecanoic acid (15:0) standard (100 μg/ml) and allowed to react for 2 h in an 85 °C water bath. When the reaction was complete, 1 ml of 0.9% NaCl and 1 ml of hexane were added, mixed thoroughly, and centrifuged at 330 × g for 2 min. The supernatant was transferred to a 6 ml tube. The procedure of adding hexane and centrifuging was repeated three times. A total of 3 ml of the supernatant was purged with nitrogen gas, dissolved in 200 µl hexane, and transferred to a GC vial. A DB-23 column (30 m × 0.25 mm, 0.25 μm film, Agilent, USA) was used, and analysis was performed using a GC-2030 instrument (Shimadzu, Japan). The GC oven temperature was increased from 190 to 230 °C at a rate of 5 °C per min.
Analysis of seed phenotype
Using the WT as a control, seed weight, length, width, and size were measured for the transgenic line. Seeds from each line were analyzed in triplicate to determine the seed phenotype. Seed photographs were captured using an optical microscope (NIKON, Japan). Seed weight was measured using an electronic scale (OHAUS, USA). Seed length and width were measured using ImageJ software, and seed size was calculated by multiplying the seed length and width.
Statistical analysis for data
Statistical analyses were performed using one-way analysis of variance (ANOVA) and Tukey’s multiple comparison test using GraphPad Prism to determine the significance of the differences. Different letters indicate significant changes compared to the WT plants in the FA and seed phenotype analyses. Significant differences in FA composition and gene expression patterns at different seed developmental stages are indicated by *p < 0.05, **p < 0.01, and ***p < 0.001.
Data availability
All data generated or analyzed in this study are included in this published article (and its supplementary information files).
References
Kumar, A., Sharma, A. & Upadhyaya, K. C. Vegetable oil: Nutritional and industrial perspective. Curr. Genom. 17, 230–240. https://doi.org/10.2174/1389202917666160202220107 (2016).
Rustan, A. C. & Drevon, C. A. Fatty acids: Structures and properties. eLS https://doi.org/10.1038/npg.els.0003894 (2005).
Simopoulos, A. P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 54, 438–463. https://doi.org/10.1093/ajcn/54.3.438 (1991).
Simopoulos, A. P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. (Maywood) 233, 674–688. https://doi.org/10.3181/0711-MR-311 (2008).
Saini, R. K. & Keum, Y. S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 203, 255–267. https://doi.org/10.1016/j.lfs.2018.04.049 (2018).
Gebauer, S. K., Psota, T. L., Harris, W. S. & Kris-Etherton, P. M. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 83, 1526S-1535S. https://doi.org/10.1093/ajcn/83.6.1526S (2006).
Burdge, G. C. & Calder, P. C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 45, 581–597. https://doi.org/10.1051/rnd:2005047 (2005).
Swanson, D., Block, R. & Mousa, S. A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 3, 1–7. https://doi.org/10.3945/an.111.000893 (2012).
Simopoulos, A. P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56, 365–379. https://doi.org/10.1016/s0753-3322(02)00253-6 (2002).
Amjad Khan, W. et al. Bioengineered plants can be a useful source of omega-3 fatty acids. Biomed. Res. Int. 2017, 7348919. https://doi.org/10.1155/2017/7348919 (2017).
Lemieux, B., Miquel, M., Somerville, C. & Browse, J. Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. Theor. Appl. Genet. 80, 234–240. https://doi.org/10.1007/BF00224392 (1990).
Browse, J., McConn, M., James, D. Jr. & Miquel, M. Mutants of Arabidopsis deficient in the synthesis of alpha-linolenate. Biochemical and genetic characterization of the endoplasmic reticulum linoleoyl desaturase. J. Biol. Chem. 268, 16345–16351. https://doi.org/10.1016/S0021-9258(19)85427-3 (1993).
Los, D. A. & Murata, N. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta 1394, 3–15. https://doi.org/10.1016/s0005-2760(98)00091-5 (1998).
Lee, K.-R. et al. Lesquerella FAD3-1 gene is responsible for the biosynthesis of trienoic acid and dienoic hydroxy fatty acids in seed oil. Ind. Crops Prod. 134, 257–264. https://doi.org/10.1016/j.indcrop.2019.04.008 (2019).
Liu, K., Zhao, S., Wang, S., Wang, H. & Zhang, Z. Identification and analysis of the FAD gene family in walnuts (Juglans regia L.) based on transcriptome data. BMC Genom. 21, 299. https://doi.org/10.1186/s12864-020-6692-z (2020).
Xue, Y. et al. Omega-3 fatty acid desaturase gene family from two omega-3 sources, Salvia hispanica and Perilla frutescens: Cloning, characterization and expression. PLoS One 13, e0191432. https://doi.org/10.1371/journal.pone.0191432 (2018).
Peng, Z. et al. The family of peanut fatty acid desaturase genes and a functional analysis of four ω-3 AhFAD3 members. Plant Mol. Biol. Rep. 38, 209–221. https://doi.org/10.1007/s11105-019-01191-0 (2020).
Hernandez, M. L., Sicardo, M. D. & Martinez-Rivas, J. M. Differential contribution of endoplasmic reticulum and chloroplast omega-3 fatty acid desaturase genes to the linolenic acid content of olive (Olea europaea) Fruit. Plant Cell Physiol. 57, 138–151. https://doi.org/10.1093/pcp/pcv159 (2016).
Yurchenko, O. P. et al. Genome-wide analysis of the omega-3 fatty acid desaturase gene family in Gossypium. BMC Plant Biol. 14, 312. https://doi.org/10.1186/s12870-014-0312-5 (2014).
Liu, G., Wu, Z., Shang, X., Peng, Y. & Gao, L. Overexpression of PvFAD3 gene from Plukenetia volubilis promotes the biosynthesis of alpha-linolenic acid in transgenic tobacco seeds. Genes (Basel) https://doi.org/10.3390/genes13030450 (2022).
Duan, W. et al. Genome-wide analysis of the fatty acid desaturase gene family reveals the key role of PfFAD3 in alpha-linolenic acid biosynthesis in Perilla seeds. Front. Genet. 12, 735862. https://doi.org/10.3389/fgene.2021.735862 (2021).
Torres-Franklin, M. L. et al. Omega-3 fatty acid desaturase (FAD3, FAD7, FAD8) gene expression and linolenic acid content in cowpea leaves submitted to drought and after rehydration. Environ. Exp. Bot. 65, 162–169. https://doi.org/10.1016/j.envexpbot.2008.12.010 (2009).
Zhang, M. et al. Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 44, 361–371. https://doi.org/10.1111/j.1365-313X.2005.02536.x (2005).
Yin, Y. et al. Ultra-high alpha-linolenic acid accumulating developmental defective embryo was rescued by lysophosphatidic acid acyltransferase 2. Plant J. 103, 2151–2167. https://doi.org/10.1111/tpj.14889 (2020).
Kim, H. U. & Chen, G. Q. Identification of hydroxy fatty acid and triacylglycerol metabolism-related genes in lesquerella through seed transcriptome analysis. BMC Genom. 16, 230. https://doi.org/10.1186/s12864-015-1413-8 (2015).
Yeom, W. W. et al. Increased production of alpha-linolenic acid in soybean seeds by overexpression of lesquerella FAD3-1. Front. Plant Sci. 10, 1812. https://doi.org/10.3389/fpls.2019.01812 (2019).
Dierig, D. A. et al. Lesquerella: New crop development and commercialization in the U.S.. Ind. Crops Prod. 34, 1381–1385. https://doi.org/10.1016/j.indcrop.2010.12.023 (2011).
Cocuron, J. C., Anderson, B., Boyd, A. & Alonso, A. P. Targeted metabolomics of Physaria fendleri, an industrial crop producing hydroxy fatty acids. Plant Cell Physiol. 55, 620–633. https://doi.org/10.1093/pcp/pcu011 (2014).
Iskandarov, U., Kim, H. J. & Cahoon, E. B. In Plants and BioEnergy (eds McCann, M. C. et al.) 131–140 (Springer, 2014).
Bansal, S. & Durrett, T. P. Camelina sativa: An ideal platform for the metabolic engineering and field production of industrial lipids. Biochimie 120, 9–16. https://doi.org/10.1016/j.biochi.2015.06.009 (2016).
Vollmann, J. & Eynck, C. Camelina as a sustainable oilseed crop: Contributions of plant breeding and genetic engineering. Biotechnol. J. 10, 525–535. https://doi.org/10.1002/biot.201400200 (2015).
Kagale, S. et al. The emerging biofuel crop Camelina sativa retains a highly undifferentiated hexaploid genome structure. Nat. Commun. 5, 3706. https://doi.org/10.1038/ncomms4706 (2014).
Moser, B. R. Camelina (Camelina sativa L.) oil as a biofuels feedstock: Golden opportunity or false hope?. Lipid Technol. 22, 270–273. https://doi.org/10.1002/lite.201000068 (2010).
Lu, C. & Kang, J. Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep. 27, 273–278. https://doi.org/10.1007/s00299-007-0454-0 (2008).
Abdullah, H. M. et al. Transcriptome profiling of Camelina sativa to identify genes involved in triacylglycerol biosynthesis and accumulation in the developing seeds. Biotechnol. Biofuels 9, 136. https://doi.org/10.1186/s13068-016-0555-5 (2016).
Lessire, R. & Stumpe, P. K. Nature of the fatty acid synthetase systems in parenchymal and epidermal cells of Allium porrum L. Leaves. Plant Physiol. 73, 614–618. https://doi.org/10.1104/pp.73.3.614 (1983).
Li-Beisson, Y. et al. Acyl-lipid metabolism. Arabidopsis Book 11, e0161. https://doi.org/10.1199/tab.0161 (2013).
Rodriguez-Rodriguez, M. F., Salas, J. J., Garces, R. & Martinez-Force, E. Acyl-ACP thioesterases from Camelina sativa: Cloning, enzymatic characterization and implication in seed oil fatty acid composition. Phytochemistry 107, 7–15. https://doi.org/10.1016/j.phytochem.2014.08.014 (2014).
Hutcheon, C. et al. Polyploid genome of Camelina sativa revealed by isolation of fatty acid synthesis genes. BMC Plant Biol. 10, 233. https://doi.org/10.1186/1471-2229-10-233 (2010).
Klinska, S., Jasieniecka-Gazarkiewicz, K. & Banas, A. Acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) of Camelina sativa seeds: Biochemical properties and function. Planta 250, 1655–1670. https://doi.org/10.1007/s00425-019-03248-6 (2019).
Zhang, C. et al. Genetic and biochemical basis for alternative routes of tocotrienol biosynthesis for enhanced vitamin E antioxidant production. Plant J. 73, 628–639. https://doi.org/10.1111/tpj.12067 (2013).
Zubr, J. Oil-seed crop: Camelina sativa. Ind. Crops Prod. 6, 113–119. https://doi.org/10.1016/S0926-6690(96)00203-8 (1997).
Millar, A. A. & Kunst, L. Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. Plant J. 12, 121–131. https://doi.org/10.1046/j.1365-313x.1997.12010121.x (1997).
Ozseyhan, M. E., Kang, J., Mu, X. & Lu, C. Mutagenesis of the FAE1 genes significantly changes fatty acid composition in seeds of Camelina sativa. Plant Physiol. Biochem. 123, 1–7. https://doi.org/10.1016/j.plaphy.2017.11.021 (2018).
Blondeau, N. et al. Alpha-linolenic acid: An omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic?. Biomed. Res. Int. 2015, 519830. https://doi.org/10.1155/2015/519830 (2015).
de Goede, J., Verschuren, W. M., Boer, J. M., Kromhout, D. & Geleijnse, J. M. Alpha-linolenic acid intake and 10-year incidence of coronary heart disease and stroke in 20,000 middle-aged men and women in the Netherlands. PLoS ONE 6, e17967. https://doi.org/10.1371/journal.pone.0017967 (2011).
Mozaffarian, D. & Wu, J. H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 58, 2047–2067. https://doi.org/10.1016/j.jacc.2011.06.063 (2011).
Usher, S., Haslam, R. P., Ruiz-Lopez, N., Sayanova, O. & Napier, J. A. Field trial evaluation of the accumulation of omega-3 long chain polyunsaturated fatty acids in transgenic Camelina sativa: Making fish oil substitutes in plants. Metab. Eng. Commun. 2, 93–98. https://doi.org/10.1016/j.meteno.2015.04.002 (2015).
Petrie, J. R. et al. Metabolic engineering Camelina sativa with fish oil-like levels of DHA. PLoS ONE 9, e85061. https://doi.org/10.1371/journal.pone.0085061 (2014).
Ruiz-Lopez, N., Haslam, R. P., Napier, J. A. & Sayanova, O. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J. 77, 198–208. https://doi.org/10.1111/tpj.12378 (2014).
Han, L., Haslam, R. P., Silvestre, S., Lu, C. & Napier, J. A. Enhancing the accumulation of eicosapentaenoic acid and docosahexaenoic acid in transgenic Camelina through the CRISPR-Cas9 inactivation of the competing FAE1 pathway. Plant Biotechnol. J. 20, 1444–1446. https://doi.org/10.1111/pbi.13876 (2022).
Onate-Sanchez, L. & Vicente-Carbajosa, J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res. Notes 1, 93. https://doi.org/10.1186/1756-0500-1-93 (2008).
Rodríguez-Rodríguez, M. F., Sánchez-García, A., Salas, J. J., Garcés, R. & Martínez-Force, E. Characterization of the morphological changes and fatty acid profile of developing Camelina sativa seeds. Ind. Crops Prod. 50, 673–679. https://doi.org/10.1016/j.indcrop.2013.07.042 (2013).
Acknowledgements
This work was supported by grants from the Mid-Career Researcher Program of the National Research Foundation of Korea (NRF-2020R1A2C2008175) and New Breeding Technologies Development Program (Project No. PJ01653301), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
M.-E.P. and H.U.K. performed the experiments and analyzed the data. M.-E.P., H.U.K., and H.-A.C. wrote the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, ME., Choi, HA. & Kim, H.U. Physaria fendleri FAD3-1 overexpression increases ɑ-linolenic acid content in Camelina sativa seeds. Sci Rep 13, 7143 (2023). https://doi.org/10.1038/s41598-023-34364-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34364-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.