Abstract
Gastroschisis has increased globally over recent decades, and this increase has not been explained by identified risk factors. We conducted a population-based study of infants born in Canada, 2004–2020. We used “winter” months (i.e., September through June) and northern areas of residence as indicators of less sunlight/less active lifestyle, while “summer” (i.e., July and August) and southern areas were considered as reference. Rate of gastroschisis for infants conceived in winter (3.4 per 10,000) was higher than for infants conceived in summer (2.2 per 10,000; p < 0.001). Exposure to winter, and northern area, hypothyroidism, substance or tobacco uses and depressive disorder were initially identified as risk factors for gastroschisis. There was a significant interaction between women < 24 years of age and 2-month conception intervals (rate ratio (RR): 1.42 (95% confidence interval [CI] 1.19–1.70). The association of maternal depression (mean ratio 2.19, 95% CI 0.87–3.50, p = 0.001) with infant gastroschisis was mediated by hypothyroidism (mean ratio 1.04, 95% CI 1.01–1.07, p < 0.001), whereas substance use, hypothyroidism, tobacco smoking and gestational diabetes showed 5.5-, 3.1-, 2.7-, and 1.2-fold associations, respectively, with maternal depression. In contrast to the summer conception interval of low gastroschisis risk, an elevated risk of gastroschisis spans the other ten months in association with higher levels of stress adaptation, thermoregulation and metabolism, reproduction, and growth effector hormones. Our findings suggest that periconception depression with mediation by hypothyroidism, may play a causal role in offspring gastroschisis.
Similar content being viewed by others
Introduction
Gastroschisis is a serious defect with midgut prolapse into the amniotic cavity1. Its prevalence at birth varies greatly by geographic region2,3. Gastroschisis has increased globally over recent decades, and this increase is not explained by demographic changes in maternal age or other identified risk factors. The cause is believed to be multifactorial and primarily non-genetic/epigenetic, but its etiology remains unknown3,4,5,6. Lifestyle behaviours, environmental exposures, substance or alcohol use, tobacco smoking, poor nutrition, lower dietary intake of vitamin D and sociodemographic risk factors have been frequently or occasionally associated with an increase in gastroschisis6,7,8, young maternal age is the only confirmed risk factor, while the etiology remains obscure. For example, a 7 times higher risk of gastroschisis is typically observed in women < 20 years of age than women aged 25–29 years. Our previous study reported a significantly increasing temporal trend in the maternal age-adjusted rate, and identified maternal hypothyroidism, substance use and northern residence as significant risk factors for gastroschisis in Canada9. A recent study of global gastroschisis patterns among 24 countries showed an extremely wide range in the prevalence for teen women (< 20 years): from 2.1 in Slovak Republic or 3.7 per 10,000 births in Spain to 21.5 in Utah (U.S) and 26.2 per 10,000 in Canada3. However, researchers have paid little attention to why gastroschisis varies with location (e.g., northern area) of residence and the complex relationship and interplay between maternal characteristics, conditions and identified risk factors, as well as the mechanistic pathway.
We have assumed that circannual endocrine function, photoperiod, residential latitude and climate may influence the relationship between anterior pituitary hormones plus their respective effector hormones and risk of offspring gastroschisis10,11,12,13,14. We have used “winter” months (i.e., September through June) and northern geographic latitudes as proxy indicators of shorter photoperiod/less outdoor lifestyle exposure, while “summer” (July and August) and southern latitudes were considered as the reference exposures that may influence fetal gastroschisis development. As gastroschisis develops between the 5th and 10th week of gestation, a negative relationship with pre- or perinatal thyroid function (circulating hormones) and vitamin D status in winter versus summer season is plausible8,11,15,16,17. Tendler et al. analyzed an Israeli medical record of 46 million person-years, and they found that within the normal ranges of each respective hormone, levels of most anterior pituitary hormones were relatively higher in calendar summer months, while their target-tissue effector hormones were relatively higher across the calendar fall, winter and spring months. Specifically, the study reported that thyrotropins (TSH) increased after May, and peaked in August, and the seasonality increased with latitude12. Fu et al. conducted a study among women of reproductive age in Shenyang, northern China and found that central sensitivity to thyroid hormones was influenced by seasonal factors and age18. Studies on residents in the Antarctica suggested that prolonged cold exposure was associated with increased TSH and T3 levels during winter compared to summer, and increased sunlight and vitamin D3 exposure in summer was found to be positively associated with steroid hormone production of sex hormones19.
We examined whether fetuses conceived in winter season or in residential areas with high latitudes (shorter photoperiod along with cold climate) have an increased risk of gastroschisis compared to summer or southern areas in Canada. Specifically, we intended to assess how extrinsic (e.g., substance use, tobacco smoking) and intrinsic factors (e.g., maternal age, depression, hypothyroidism, gestational diabetes and chronic illness) interacted with conception seasonal variation in infant gastroschisis prevalence, in an attempt to explore the etiological role and possible underlying pathway in fetal gastroschisis development.
Methods
Study design and population
We completed a retrospective population-based cohort study. We included all live births (N = 4 435,732) in Canada between April 1, 2004 and March 31, 2020. The data were drawn from the Canadian Institute for Health Information’s Discharge Abstract Database (DAD), which contains records of live-born infants linked to their mothers20. The records were collated by trained medical record personnel using standardised definitions and included information on gestational age, plurality, birthweight, maternal and newborn diagnoses (up to 25 diagnosis fields available), and interventions (up to 20 procedure fields) that were noted during the delivery admission21. Diagnoses among infants and mothers were coded using the enhanced Canadian version of the 10th revision of the International Classification of Diseases (ICD‐10 CA). The hospital discharge database has been routinely checked for accuracy, and validation studies have shown the information to be complete and accurate20,22. Information on pregnancy conditions and birth outcomes was well recorded in the database, and the database has been previously used for perinatal research9,23,24,25.
Stillbirths and terminated pregnancies were not included as variables such as gestational age and birth date for fetal deaths necessary to determine conception date were unavailable in the DAD for privacy concern. Information on gestational age at birth in the DAD allows for accurate estimation of conception date for live-born infants26. After exclusions for gestational age < 22 weeks, birth weight < 500 g, missing information on birth date, gestational age, birth weight, non-Canadian residence or maternal age at conception ≥ 45 years (n = 26,016); a total of 4,409,716 (99.4%) mother-liveborn dyads remained eligible for the study [s-Fig. 1].
Conception season (exposure)
Month of conception and maternal residential latitude estimate were used as proxies for exposure to photoperiod and climate variation around the time of conception and in early fetal development. The month of conception, a primary exposure measure, was determined using the birth month and the number of completed weeks of gestation at birth, using the following formula:
where (1) 7 is used to convert gestational weeks to days; (2) 14 + x indicates two weeks (i.e., 14 days) of the last menstrual period (LMP) and x represents the missing incomplete gestational week ranging from 1 to 6 days, and (3) 30.4 (i.e., 365 days divided by 12 months) is used to convert gestational days to define conception months such as January, February …… and December.
Pre-defined sub-regions based on postal codes of maternal residence were used to classify pregnant women into four geographic regions with differential latitudes reflecting degree of daylight exposure:27,28 (i) Far North consisting of the three Territories: Yukon, Northwest Territories and Nunavut; (ii) North including northern British Columbia, Alberta, Saskatchewan and Manitoba, northern Ontario, northern Quebec (iii) North-to-South consisting of other parts of British Columbia, Alberta, Saskatchewan and Manitoba, and Labrador; and (iv) South including western, central, and eastern Ontario, metropolitan Toronto, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and southern Quebec.
In this study we used winter months (i.e., September through June) and northern areas of residence as indicators of less sunlight/less active lifestyle, while summer (i.e., July and August) and southern areas were considered as the reference.
Outcome and covariates
Gastroschisis was collected from the birth record using ICD-10 code (s-Table 1). Gastroschisis is usually detected prenatally and confirmed at birth. In this study, we used hypothyroidism to be a surrogate variable for maternal thyroid dysfunction.
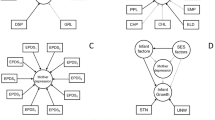
Maternal characteristics and covariates studied included maternal age at conception derived using gestational age and date of delivery, depressive disorder, hypothyroidism, use of substances (i.e., opioids, cannabinoids, cocaine, alcohol and other specified/unspecified drugs or medications), tobacco use, gestational diabetes, obesity, and maternal chronic conditions or illnesses (e.g., pre-gestational type 1 or type 2 diabetes, anxiety and other specific mental disorders, migraine, lupus, epilepsy, hyperthyroidism or thyroiditis) (s-Table 1). Other characteristics and covariates included parity (i.e., 1st child, 2nd child, ≥ 3rd child and missing), multifetal pregnancy and infant sex. Rural or urban maternal residence was identified using the forward sortation area of residential postal code. We included this variable (i.e., rural vs. urban) to potentially account for variations in accessibility to prenatal screening/diagnosis and subsequent termination, and because this variable is a partial surrogate for socioeconomic status (SES)28. A directed acyclic graph (DAG) was used to illustrate our data analysis and hypothetical relationships between risk factor, covariates and outcome in this study (Fig. 1).
Statistical analysis
We examined gastroschisis prevalence in each month of conception. We used July and August (summer), months with the longest daytime non-exposure, as the reference. We compared individual 2-month winter seasons of exposure period, such as January–February, March–April, May–June, September–October and November–December, and then compared exposure season combined (i.e., year-around) to the reference. Maternal age at conception was grouped as < 19, 19–23, 24–28, 29–33, 34–38 and 39–44 years.
A log-binomial regression model with a Poisson distribution was used to model the association of infant gastroschisis between exposure to conception season and covariates. Univariable and multivariable rate ratios with 95% confidence intervals were estimated. Specifically, interactions between season of exposure (i.e., 2-month conception season) and maternal age at conception (< 24 vs. 24–44 years) were assessed, adjusting for residential latitude, hypothyroidism, substance use, gestational diabetes and other covariates. We compared five two-month winter (exposure) conception seasons with summer (non-exposure) season, respectively, to assess if there were associations and interactions between maternal age, characteristics, covariates and conception season in infant gastroschisis.
Next, in order to understand the relationships of various risk or “protective” factors/covariates that may be involved in the causal pathway between the hypothesized exposure and risk of offspring gastroschisis, we conducted mediation analysis29,30. Specifically, we focused on the assessments of hypothyroidism as a primary mediator for substance use, tobacco smoking, depressive disorder, gestational diabetes and obesity in univariate and multivariate models. We also tested proxy latitudes of maternal residence, gestational diabetes, hypothyroidism and depressive disorder for potential mediating role in offspring gastroschisis development in winter versus summer. We used the CAUSALMED procedure for this purpose31. This method decomposes total effects into several two-, three-, and four-way decompositions. We used causal mediation analysis to decompose the total direct effect of a given risk factor into controlled direct effect, reference interaction, mediated interaction and pure indirect effect32. Each potential risk factor was alternatively included in a mediation analysis model as a covariate wherein all other characteristics and factors were controlled. Based on Poisson regression modelling, the value of total effect is equal to the product of mean ratio of the direct and indirect effect.
Secondary analysis
Gestational diabetes is most often manifest in later pregnancy (after 24 weeks) in response to naturally hormonal changes32. It was identified as a confounding in several previous studies of risk factors for gastroschisis9,33,34. In our post hoc analysis, we evaluated whether women with gestational diabetes manifested with conception seasonal trend similar to gastroschisis35. In addition, we assessed possible mediating role for proxy latitude, gestational diabetes, hypothyroidism and depressive disorder associated with lower gastroschisis prevalence in summer months using mediation analysis.
We used omphalocele and chromosomal anomalies (s-Table 1) as negative controls because both are known to be causally distinct from gastroschisis and primarily genetic. We included these two anomalies as negative controls36 and applied the same participant inclusion criteria and repeated assessments of the conception seasonal variations, and mediation analysis.
This study was carried out under the surveillance and applied research mandate of the Public Health Agency of Canada, and ethics approval was not required.
Results
Characteristics of study cohort
A total of 3,689,855 and 719,861 mother-infant dyads were identified to be conceived in exposure and non-exposure groups, respectively, yielding gastroschisis prevalence rates of 3.40 (95% confidence interval (CI) 3.22–3.60) and 2.21 (95% CI 1.88–2.58) per 10,000 infants. There were moderate temporal variations while no significant differences between the five 2-month periods were identified in the exposure season (χ2 = 4.7, p = 0.32).
Rates of gastroschisis appeared to vary by month of conception across the 10-month span of the designated winter season with a low of 2.94 (95% CI 2.40–3.57) per 10,000 in April to the high values of 3.62 (95% CI 3.02–4.30) in May and 3.66 (95% CI 3.07–4.33) per 10,000 in October. Gastroschisis rates for infants conceived in November (3.53, 95% CI 2.96–4.17) per 10,000 and December (3.60, 95% CI 3.03–4.25) were significantly higher than those conceived in July (2.27, 95% CI 1.81–2.82) and August (2.14, 95% CI 1.67–2.68) per 10,000 infants, respectively (Fig. 2). There were statistically significant differences in geographic region, maternal age at conception, parity, rural or urban residence and substance use disorders. However, the differences in maternal hypothyroidism, depressive disorder, tobacco smoking, obesity, chronic illness and infant sex between the two birth cohorts were statistically non-significant (Table 1).
Risk or “protective” factors
In the adjusted analysis, compared with the 2-month summer, the rate of gastroschisis in infants conceived in winter seasons remained higher with an adjusted rate ratio (aRR) 1.55 (95% CI 1.31–1.82). Gastroschisis prevalence increased from 2.4 per 10,000 livebirths in South to 8.8 in far North, with an approximately twofold South-North increase (aRR 1.83, 95% CI 1.34–2.51). Women with hypothyroidism, substance use, depressive disorder, or tobacco smoking showed a significantly higher prevalence rate ratio, while women with gestational diabetes or obesity showed an inverse association with risk of gastroschisis. Specifically, maternal hypothyroidism showed a significant association with gastroschisis (aRR 2.86, 95% CI 1.68–4.85). Substance use disorders remained significantly associated with infant gastroschisis; however, the strength of association was attenuated substantially, from a crude RR: 4.20 (95% CI 3.23–5.46) to an adjusted RR: 2.22 (95% CI 1.70–2.91). The adjusted rate ratio for depressive disorder changed only slightly. In addition, both gestational diabetes and obesity remained negatively associated with infant gastroschisis, although the strength of association reduced considerably after adjustments (Table 2).
Interaction, two-month comparison and varying rate ratio for the influential factors
All two-month exposure intervals within the winter season showed statistically lower adjusted rate ratios of infant gastroschisis compared with summer, while there appeared little variation for the overall comparison (i.e., aRR 1.55 vs. cRR 1.54). Interaction terms indicated statistically significant associations (i.e., 6- or sevenfold higher rate ratio) for maternal age (< 24 years vs. 24–44) with specific 2-month winter conception seasons and year-around comparisons. Adjusted for covariates, the interaction term showed a significant association with conception season in March–April (aRR 1.96, 95% CI 1.34–2.85), November–December (aRR 1.39, 95% CI 1.01–1.91), and the entire winter months (aRR 1.42 (95% CI, 1.19–1.70) compared to summer (Table 3).
Based on the two-month conception seasonal comparisons, rate ratios for hypothyroidism, depressive disorder, substance or tobacco use and gestational diabetes showed significant or substantial changes between the respective crude and fully adjusted models, and between two-month winter conception intervals and summer. For example, in the comparisons of March–April versus July–August, rate ratios for hypothyroidism and gestational diabetes doubled after adjustments, while adjusted rate ratio for the outcome risk in March–April was attenuated to be non-significant (aRR 0.88, 95% CI 0.63–1.22). Similar changes to rate ratios were also observed for the comparisons between November–December and summer. There appeared to be substantial changes in rate ratios for hypothyroidism and gestational diabetes in the 2-month comparisons, indicating that the comparison of entire winter versus summer underlie the seasonal variations in the outcome measure for exposure winter versus non-exposure summer, while the variations in rate ratios for hypothyroidism and gestational diabetes appeared to primarily vary with the two-month conception intervals (Table 3). It is also notable that tobacco smoking was no longer identified as a risk factor, although substance use remained significantly associated with risk of infant gastroschisis. Taken together, unlike the entire winter versus summer comparisons, these two-month conception seasonal comparisons seemed to better reflect the risk or protective (instead of confounding) roles for the influential factors of interest in offspring gastroschisis.
Mediational effects and causal pathway
Maternal depressive disorder, substance use and tobacco smoking showed significant positive total effects of 2.19, 2.18, and 1.69, respectively, on infant gastroschisis, while hypothyroidism was identified to have statistically significant mediational and interactive effects with the first, as well as the third individual risk factors. In particular, depressive disorder showed statistically significant total effects (mean ratio: 2.19 (95% CI 0.87–3.50) on infant gastroschisis while hypothyroidism mediated the relationship (mean ratio: 1.04 (95% CI 1.01–1.07, p < 0.001) with 5.3% of total effects (95% CI 1.6–8.9%, p < 0.01) and 1.8% as interaction (95% CI 0.8%-2.9%) for risk of infant gastroschisis, suggesting that hypothyroidism impacts offspring gastroschisis development through a mediational path in depressed women. Further, the four-way decomposition of total effect of periconception depression strengthens that pure mediation effect (mean ratio: 0.029; 95% CI 0.015 to 0.044; p < 0.0001) may underlie the causal relationship: periconception depression → hypothyroidism (mediator) → grastroschisis (Table 4).
In addition, we also have showed that hypothyroidism may impact offspring gastroschisis through a negative mediational path in pregnant women with substance use disorder (0.998; 95% CI 0.996 to 0.999; p < 0.01) or through both mediational and interactive paths in women with tobacco smoking (p < 0.05). Gestational diabetes and obesity showed statistically significant negative overall effect (p < 0.001) on the risk of infant gastroschisis, respectively, through mediational and/or interactive pathways with maternal hypothyroidism (Table 4).
Higher infant gastroschisis rate in winter and periconception depression-associated factors
Exposure to winter showed significantly higher direct effect (mean ratio: 1.55 (95% CI 1.29–1.80) on risk of offspring gastroschisis that was mediated by latitude (p = 0.001). Gestational diabetes in winter showed less mediational or “protective” effects (mean ratio: -0.0015 (95% CI − 0.0026 to − 0.0004, p < 0.001), while latitude also impacted (i.e., less protective) significantly (mean ratio: − 0.0009 (95% CI − 0.0015 to − 0.0004, p = 0.001) on the risk of infant gastroschisis. Specifically, depressive disorder appeared to have additional mediational effect through an interaction of two-month conception seasonal variation with young (< 24 years) pregnant women with a reduced risk of gastroschisis among infants conceived in summer (Table 5).
The rate of maternal depression increased by 50%, from 26.1 per 10,000 in 2004–2007 to 39.0 per 10,000 in 2016–2020, while the fully adjusted rate increased by 19%. In particular, a 1.6-fold increase in depressive disorder was observed in teen women (i.e., age 13–18 years at conception), compared with women aged 24–28 years. More importantly, we observed a 5.5-fold (95% CI 5.2–5.6) association with substance use, 3.1-fold (95% CI 3.8–4.3) risk of hypothyroidism and 2.7-fold increased risk of tobacco smoking in women with depressive disorder. The fully adjusted rate ratio for depressive disorder was significantly lower in summer (aRR 0.956, 95% CI 0.915–0.999; p < 0.05), highlighting a significantly reduced risk of gastroschisis in infants conceived in summer (Table 6).
Secondary analysis
Gestational diabetes rates according to conception month varied greatly throughout the year, with a significantly lower rate starting in August and returning to an average level in December (s-Fig. 2). Nevertheless, winter (i.e., September through June) showed a significantly higher risk for gestational diabetes (95% CI 1.019, 1.009–1.029, p = 0.0002) compared to summer (July and August) regardless of the covariates (s-Table 2).
We did not observe any statistically significant conception seasonal patterns, decreased or increased prevalence in summer compared to other seasons for prevalence of chromosomal anomalies or omphalocele in livebirths (s-Fig. 3, s-Table 3). Mediation analyses for these two negative controls showed that neither residential latitudes nor hypothyroidism had indirect effects on risk of infant omphalocele or chromosomal anomalies (p > 0.05). No mechanistic path was identified through the mediation analysis (s-Table 4, s-Table 5).
Discussion
In this Canada-wide population-based study, we found that infants conceived in July and August were at a significantly lower rate of gastroschisis than those conceived in any other 2-month intervals of the year. This lower rate in summer months appeared to be associated with decreased maternal depression at least in part mediated by thyroid function, presumably attributable to the circannual endocrine changes driven by increased exposure to sunlight. Substance use and tobacco smoking are also identified to have direct effects on offspring gastroschisis in the associations of maternal factors with infant gastroschisis. Diagnosed maternal depression around conception showed significant independent effects on offspring gastroschisis development, and our mediation analysis indicates that such effects appear to work through the mechanistic pathway of maternal hypothyroidism. Periconception depression appeared to influence the conception seasonal variations in risk, along with several other associated factors (e.g., residential latitude, young maternal age, and gestational diabetes). Our data suggest that these factors play an interactive role in the development of offspring gastroschisis.
Our findings on these associations and their respective strength and seasonal variation of gastroschisis prevalence with identified risk factors suggest that young women with depressive disorder are at significantly elevated risk of offspring gastroschisis. Exposure to summer season appeared to play as an exclusive modifiable factor for the relationship between periconception depression and infant gastroschisis. Our findings provide data supporting the hypothesis that increased periconceptional exposure to summer (i.e., longer daytime, lower latitude and more active lifestyle) could protect against gastroschisis during fetal development, in particular, for young women.
There is a substantial body of evidence that shows maternal perinatal mental disorders are associated with an increase in a range of adverse developmental outcomes in offspring. Prenatal stress and associated epigenetic changes in pregnant women with mental disorders may increase the risk of adverse child outcomes37. Another example, a recent study from Quebec, Canada reported that maternal depression and stress and anxiety was significantly associated with an increased risk of hypertrophic pyloric stenosis in the newborns38.
Two meta-analyses assessed the association between antenatal depression and fetal and neonatal outcomes10,39. One reported that studies controlling for women taking antidepressant drugs or smoking generated small (non-significant) odds ratios39, whereas the other concluded that the summary relative risk was comparable for depressed women treated and not treated with antidepressants. Antidepressants or smoking can be markers for more severe depression, showing stronger association with antidepressants and mental health disorders10. Selective serotonin reuptake inhibitors are widely used for depression and anxiety and have been associated with birth defects in several studies40,41. Previous studies reported that use of antidepressants, depression, and stressful pregnancy events were associated with up to 4 times the odds of having a child with gastroschisis34,42.
Hypothyroidism is considered a cause of or a strong risk factor for depression or depressive disorders43. Associated with clinical depression, maternal hypothyroidism is relatively common during pregnancy, with an overall prevalence of 0.61% for overt hypothyroidism and 5.1% for subclinical hypothyroidism44. We observed 0.72% of hypothyroidism in this data (Table 1). Some past studies have identified maternal hypothyroidism as a possible risk factor for birth defects45,46. Light exposure has been associated with seasonal fluctuation of thyroid function related to circulating hormones, and external stimuli such as light and warm temperature can influence thyroid function11. Normal maternal thyroid function is essential for optimal pregnancy outcomes, especially during the early gestational period. Firstly, temperature variation is a critical stimulus to the central regulation of hypothalamus-pituitary-thyroid (HPT) axis via changes in secretion of thyroid-stimulating hormone (TSH)47. One systematic review and meta-analysis found that individuals with hypothyroidism had significantly lower vitamin D levels compared to healthy people48. Secondly, light is associated with seasonal fluctuations in thyroid function. The alteration in thyroid hormone regulation could also be a part of metabolic adaptation to seasonal climate changes11. Thirdly, hypothyroidism has been causally associated with decreased sex hormone concentrations such as sex hormone binding globulin (SHBG) and free androgen index (FAI) in women13. In addition, increased sunlight and vitamin D3 exposure in summer was found to be positively associated with steroid hormone production of sex hormones18. Exposure to winter sunlight in Northern Canada does not promote previtamin D3 synthesis in human skin49.
Based on the findings of our study, we propose the following hypothetical mechanistic pathway by which maternal hypothyroidism could be a key causal factor in the occurrence of many cases of gastroschisis. Thyroid hormone is essential for normal embryonic and fetal development. Maternal thyroid hormone is important for the well-being of the embryo/fetus throughout pregnancy but is the sole source prior to onset of thyroid hormone synthesis and secretion by the fetal thyroid tissue beginning at approximately 16 weeks of pregnancy50. Prior to that time, multiple major embryonic events unfold and are solely dependent upon that maternal source of thyroid hormone. While it is well-known that normal neurocognitive development depends upon normal thyroid exposure of developing neural tissues51, it is also well understood that the process of angiogenesis including developmental neovascularization is also thyroid hormone dependent52. Specifically, the alphaVbeta3 integrin is a key mediator of angiogenesis in adult tissues, tumors and during development, and is directly regulated by a non-genomic thyroid hormone receptor within alphaVbeta3 integrin53,54. Accordingly, absent or reduced thyroid hormone signaling compromises angiogenesis. Since vascular insufficiency or compromise of the relevant abdominal wall vessels is one of the favored hypothetical mechanisms by which gastroschisis occurs, reduced or absent angiogenesis could be a key factor in the occurrence of gastroschisis. In brief, maternal thyroid hormone is the sole source for the embryo, and that maternal thyroid hormone acts locally on the plasma membrane receptor located within alphaVbeta3 integrin to stimulate angiogenesis in the embryo. If maternal thyroid hormone supplied to the embryo is modestly, moderately or severely reduced, the angiogenesis that is essential for normal development of the abdominal wall of the embryo/fetus would be compromised.
To our knowledge, this is the first study to report the role of light and presumptive active lifestyle exposure associated with maternal depression, hypothyroidism, and potential mood/mental changes in the causality of fetal gastroschisis development. The strengths of our study include use of data from a vast geography with differential latitude/climate (e.g., far North) along with distinctive seasonal variation. Information on postal codes for maternal residence is complete and accurate and has been used in the past to account for differences in pregnancy-related endpoints such as prenatal screening or pregnancy termination. Our secondary analysis of gestational diabetes by conception season may also elucidate the inverse associations of gastroschisis with gestational diabetes observed in previous studies9,34,35.
However, several limitations must be noted. Firstly, some risk factors that are associated with gastroschisis may be tested after the occurrence of a congenital anomaly or other adverse pregnancy outcome. As antenatal data could not be available for our analysis, subclinical hypothyroidism and depression cases could have been missed in the childbirth hospitalization data. The clinical manifestations may represent a maternal persistent condition. Secondly, misclassification or lack of diagnosis may occur, though probably not in a differential manner, and thus it is not plausible that the coding of hypothyroidism, clinical depression or other conditions would be different for women who conceived in different months. While this potential misclassification may have underestimated the outcome rates, the rate ratio would still be unchanged. Thirdly, while fetal death or stillbirths due to gastroschisis have become more uncommon in Canada, absence of the data may impact the seasonal prevalence and regional variation. In a previous study including stillbirths or more severe or fatal gastroschisis, we reported an increased gastroschisis risk for maternal hypothyroidism but not for depression in Canadian births9. We speculate that maternal depression may be more likely to be associated with a somewhat less severe spectrum of gastroschisis in liveborn neonates. Future studies are needed to confirm our observation.
Our study uses data from a vast geography with differential latitudes (i.e., sunlight or active lifestyle exposure), and demonstrates a distinctive seasonal variation. Information on postal codes for maternal residence is complete and accurate and has been successfully used to define residential location (i.e., rural or urban areas) in the past28,55. Nevertheless, remaining variations in socioeconomic status, education, diet, ethnicity, vitamin D supplementation, etc., could have confounded our analysis when comparing geographic regions of maternal residence.
In conclusion, given the seasonal and geographic variations in gastroschisis occurrence in Canada and many other parts of the world, particularly in the Northern hemisphere, we observed that northern regions with less daylight length and presumably less active lifestyle might be associated with an increased risk of maternal depression and associated hypothyroidism that is linked to offspring gastroschisis. Our study has demonstrated that conceptions occurring in shorter photoperiod months or higher latitudes are at increased risk of infant gastroschisis compared to longer light months of summer and/or lower latitudes in which maternal hypothyroidism may play a significant mediating role in depressed women. Our findings that infant gastroschisis is associated with seasonal and regional factors indicative of low light exposure suggest that seasonal changes in other hormones, such as sex hormones, might also play a role in the etiology of fetal gastroschisis development. Further studies are warranted to confirm this suggestion in other areas of the world, and to identify any biological mechanisms that may link variations in sex hormones with maternal mood (e.g., depression) and hypothyroidism, and in turn with offspring gastroschisis development.
Data availability
All data files are available through the Canadian Institute for Health Information (https://www.cihi.ca/en/data-andstandards/access-data).
References
Opitz, J. M., Feldkamp, M. L. & Botto, L. D. An evolutionary and developmental biology approach to gastroschisis. Birth Defects Res. 111, 294–311. https://doi.org/10.1002/bdr2.1481 (2019).
Castilla, E. E., Mastroiacovo, P. & Orioli, I. M. Gastroschisis: international epidemiology and public health perspectives. Am. J. Med. Genet. C Semin. Med. Genet. 148C, 162–179. https://doi.org/10.1002/ajmg.c.30181 (2008).
Feldkamp, M. L., et al. Global gastroschisis prevalence patterns among 27 surveillance programs (representing 24 countries), International Clearinghouse for Birth Defects Surveillance and Research, 1980–2017. Birth Defects Res. in press (2023).
Feldkamp, M., Carey, J. C. & Sadler, T. Development of gastroschisis: Review of hypotheses, a novel hypothesis, and implications for research. Am. J. Med. Genet. Part A 143, 639–652. https://doi.org/10.1002/ajmg.a.31578 (2007).
Rasmussen, S. A. & Frias, J. L. Non-genetic risk factors for gastroschisis. Am. J. Med. Genet. Part C Semin. Med. Genet. 148C, 199–212 (2008).
Hughes, C. & Adibe, O. G. The increasing prevalence of gastroschisis: associated factors, possible mechanisms, and potential mitigative interventions. Glob. Clin. Transl. Res. 1(1), 4–20 (2019).
Baldacci, S. et al. Lifestyle and sociodemographic risk factors for gastroschisis: a systematic review and meta-analysis. Arch. Dis. Child. 105, 756–764. https://doi.org/10.1136/archdischild-2019-318412 (2020).
Adrien N, Orta OR, Nestoridi E, Carmichael SL, Yazdy M for the National Birth Defects Prevention Study. Early pregnancy vitamin D status and risk of select congenital anomalies in the National Birth Defects Prevention Study. Birth Defects Res. https://doi.org/10.1002/bdr2.2101 (2022).
Liu, S. et al. Time trends, geographic variation and risk factors for gastroschisis in Canada. A population-based cohort study 2006–2017. Paediatr. Perinat. Epidemiol. 35, 664–673. https://doi.org/10.1111/ppe.12800 (2021).
Stein, A. et al. Effects of perinatal mental disorders on the fetus and child. Lancet 384, 1800–1819 (2014).
Watts, H. E. Seasonal regulation of behaviour: What role do hormone reception play?. Proc. Biol. Sci. 287(1930). https://doi.org/10.1098/rspb.2020.0722 (2020).
Trendler, A. et al. Hormone seasonality in medical records suggests circannual endocrine circuits. Proc. Natl. Acad. Sci. USA 118(7), e2003926118. https://doi.org/10.1073/pnas.2003926118 (2021).
Kjaergaard, A. D. et al. Thyroid function, sex hormones and sexual function: a Mendelian randomization study. Eur. J. Epidemiol. 36, 335–344 (2021).
Yoshimura, T. Thyroid hormone and seasonal regulation of reproduction. Front. Neuroendocrinol. 34, 157–166 (2013).
Meyer, N., Harvey, A. G., Lockley, S. W. & Dijk, D. Circadian rhythms and disorders of the timing of sleep. Lancet https://doi.org/10.1016/S0140-6736(22)00877-7 (2022).
Miliku, K. et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am. J. Clin. Nutr. 103(6), 1514–1522. https://doi.org/10.3945/ajcn.115.123752 (2016).
Koster, M. P. H. et al. A compromised maternal vitamin D status is associated with congenital heart defects in offspring. Early Hum. Dev. 117, 50–56 (2018).
Fu, J., et al. Seasonal changes of thyroid function parameters in women of reproductive age between 2012 and 2018: A retrospective, observational, single-center study. Front. Endocrinol. 12, 719225. https://doi.org/10.3389/fendo.2021.719225.
Hofer, D. et al. Testicular synthesis and vitamin D action. J. Clin. Endocrinol. Metabal. 10, 3766–3773. https://doi.org/10.1210/jc.2014-1690 (2014).
Canadian Institute for Health Information (CIHI). Data Quality Documentation, Discharge Abstract Database—Multi-Year Information Standards and Data Submission. Ottawa: Canadian Institute for Health Information. Available: https://www.cihi.ca/en/dad_multi-year_en.pdf. Accessed 10 Apr 2023.
Public Health Agency of Canada. Congenital Anomalies in Canada 2013: A Perinatal Health Surveillance Report. http://www.phacaspc.gc.ca/ccasn-rcsac/cac-acc-2013-eng.php.
Joseph, K.S, Fahey, J., for the Canadian Perinatal Surveillance System. Validation of perinatal data in the Discharge Abstract Database of the Canadian Institute for Health Information. Chronic Dis. Can. 29, 96–100 (2009).
Liu, S. et al. Association of maternal risk factors with the recent rise of neural tube defects in Canada. Paediatr. Perinat. Epidemiol. 33, 145–153 (2019).
Liu, S. et al. Association between maternal chronic conditions and congenital heart defects: a population-based cohort study. Circulation 128, 583–599 (2013).
Liu, S., Joseph, K. S., Luo, W., et al. For the Canadian Perinatal Surveillance System. Effect of folic acid food fortification in Canada on congenital heart disease subtypes. Circulation 2016;134:647‐655.
Mei-Dan, E., Ray, J. G. & Simone, N. V. Perinatal outcomes among women with bipolar disorder: a population-based cohort study. Am. J. Obstet. Gynecol. 212, 367.e1–e8.
Martens, P. J. et al. North-South gradients in adverse birth outcomes for First Nations and others in Manitoba, Canada. Open Women’s Health J https://doi.org/10.2174/1874291201004020046 (2010).
McNive, C. & Puderer, H. Delineation of Canada's North: An examination of the North-South relationship in Canada. Statistics Canada Catalogue no.92F0138MIE, no.2000-3. ISSN: 1481-174X.
VanderWeele, T. J. et al. Genetic variants on 15q25.1, smoking, and lung cancer: An assessment of mediation and interaction. Am. J. Epidemiol. 175(10), 1013–1020 (2012).
Yung, Y., Lamm, M. & Zhang, W. Causal Mediation Analysis with the CAUSALMED Procedure Paper SAS1991-2018 (SAS Institute Inc., Cary, NC, 2018).
VanderWeele, T. J. A unification of mediation and interaction: A 4-way decomposition. Epidemiology 25, 749–761 (2014).
McIntyre, H. D. et al. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 5, 47. https://doi.org/10.1038/s41572-019-0098-8 (2019).
Tinker, S. C. et al. Specific birth defects in pregnancies of women with diabetes: National Birth Defects Prevention Study, 1997–2011. Am. J. Obstet. Gynecol. 222(176), e171–e176. https://doi.org/10.1016/j.ajog.2019.08.028 (2020).
Skarsgard, E. D. et al. Maternal risk factors for gastroschisis in Canada. Birth Defects Res. A Clin. Mol. Teratol. 103, 111–118 (2015).
Faal, S. et al. Sex hormone binding globulin for prediction of gestational diabetes mellitus in pre-conception and pregnancy: A systematic review. Diabetes Res. Clin. Pract. 152, 39–52 (2019).
Lipsitch, M., Tchetgen, E. T. & Cohen, T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 21, 383–388 (2010).
DeSocio, J. E. Epigenetics, maternal prenatal psychosocial stress, and infant mental health. Arch. Psychiatr. Nurs. 32, 901–906. https://doi.org/10.1016/j.apnu.2018.09.001 (2018).
Le-Nguyen, A., Piché, N., Lee, G. E. & Auger, N. Maternal mental disorders and risk of pathological abdominal conditions in children. Arch. Womens Ment. Health 24, 925–932. https://doi.org/10.1007/s00737-021-01126-3 (2021).
Grigoriadis, S. et al. The impact of maternal depression during pregnancy on perinatal outcomes: A systematic review and meta-analysis. J. Clin. Psychiatry 74, e321–e341 (2013).
Wogelius, P. et al. Maternal use of selective serotonin reuptake inhibitors and risk of congenital malformations. Epidemiology 17, 701–704. https://doi.org/10.1097/01.ede.0000239581.76793.ae (2006).
Alwan, S. et al. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N. Engl. J. Med. 356, 2684–2692. https://doi.org/10.1056/NEJMoa066584 (2007).
Given, J. E. et al. Gastroschisis in Europe—a case-malformed-control study of medication and maternal illness during pregnancy as risk factors. Paediatr. Perinat. Epidemiol. 31, 549–559. https://doi.org/10.1111/ppe.12401 (2017).
Bode, H. et al. Association of hypothyroidism and clinical depression. A systematic review and meta-analysis. JAMA Psychiat. 78(12), 1375–1383. https://doi.org/10.10001/jamapsychiatry.2021.2506 (2021).
Teng, W., Shan, Z., Patil-Sisodia, K. & Cooper, D. S. Hypothyroidism in pregnancy. Lancet Diabetes Endocrinol. 1(3), 228–237. https://doi.org/10.1016/S2213-8587(13)70109-8 (2013).
Khoury, M. J., Becerra, J. E. & D’Almada, P. J. Maternal thyroid disease and risk of birth defects in offspring: a population-based case-control study. Paediatr. Perinat. Epidemiol. 4, 347–474. https://doi.org/10.1111/j.1365-3016.1989.tb00528.x (1989).
Grattan, M. J. et al. Maternal hypothyroidism may be associated with CHD in offspring. Cardiol. Young 7, 1247–1253. https://doi.org/10.1017/S1047951114001887 (2015).
Neumann, A. M., Schmidt, C. X., Brockmann, R. M. & Oster, H. Circadian regulation of endocrine systems. Auton. Neurosci. Basic Clin. 216, 1–8. https://doi.org/10.1016/j.autneu.2018.10.001 (2019).
Taheriniya, S. et al. Vitamin D and thyroid disorders: a systematic review and meta-analysis of observational studies. BMC Endocr. Disord. 21, 171. https://doi.org/10.1186/s12902-021-00831-5 (2021).
Webb, A. R., Kline, L. & Holick, M. F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endo Metab. (JCE & M) 67(2), 373–379 (1988).
Hartoft-Nielsen, M. L., Boas, M., Bliddal, S., Rasmussen, A. K., Main, K. & Feldt-Rasmussen, U. Do thyroid disrupting chemicals influence foetal development during pregnancy? J. Thyroid Res.Article ID 342189. https://doi.org/10.4061/2011/342189 (2011).
Kooistra, L., Crawford, S., van Baar, A. L., Brouwers, E. P. & Pop, V. J. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics 117, 161–167. https://doi.org/10.1542/peds.2005-0227 (2006).
Eliceiri, B. P. & Cheresh, D. A. The role of alphaV integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J. Clin. Invest. 103, 1227–1230 (1999).
Bergh, J. et al. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146, 2864–2871. https://doi.org/10.1210/en.2005-0102 (2005).
Davis, P. J., Leonard, J. L. & Davis, F. B. Mechanisms of nongenomic actions of thyroid hormone. Front. Neuroendocrinol. 29, 211–218. https://doi.org/10.1016/j.yfrne.2007.09.003 (2008).
Guay, B., Johnson-Obaseki, S., McDonald, J. T., Connell, G. & Corsten,. Incidence of differentiated thyroid cancer by socioeconomic status and urban residence: Canada 1991–2006. Thyroid https://doi.org/10.1089/thy.2013.0308 (2014).
Acknowledgements
The authors are grateful to Nathalie Auger (University of Montreal), Amélie Boutin (Québec-Université Laval), Michael S. Kramer (McGill University), Julian Little (University of Ottawa), Chantal Nelson and Sebastian Srugo (Public Health Agency of Canada) for comment on earlier analysis or manuscript. We thank the Canadian Institute for Health Information for providing access to the Discharge Abstract Database at the Public Health Agency of Canada.
Author information
Authors and Affiliations
Contributions
S.L.: Study conception, data analysis, manuscript drafting and revision. C.H.: Manuscript review, comment and editing. S.J.Y.: Manuscript review and comment. D.C.: Manuscript review and comment. All authors approve the submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Claude, H., Yong, S.J. et al. Association of maternal depression and hypothyroidism with infant gastroschisis: a population-based cohort study in Canada. Sci Rep 13, 7540 (2023). https://doi.org/10.1038/s41598-023-34090-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34090-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.