Abstract
Recently, several probiotic products have been developed; however, most probiotic applications focused on prokaryotic bacteria whereas eukaryotic probiotics have received little attention. Saccharomyces cerevisiae yeast strains are eukaryotes notable for their fermentation and functional food applications. The present study investigated the novel yeast strains isolated from Korean fermented beverages and examined their potential probiotic characteristics. We investigated seven strains among 100 isolates with probiotic characteristics further. The strains have capabilities such as auto-aggregation tendency, co-aggregation with a pathogen, hydrophobicity with n-hexadecane,1,1-diphenyl-2-picrylhydrazyl scavenging effect, survival in simulated gastrointestinal tract conditions and the adhesion ability of the strains to the Caco-2 cells. Furthermore, all the strains contained high cell wall glucan content, a polysaccharide with immunological effects. Internal transcribed spacer sequencing identified the Saccharomyces strains selected in the present study as probiotics. To examine the effects of alleviating inflammation in cells, nitric oxide generation in raw 264.7 cells with S. cerevisiae showed that S. cerevisiae GILA could be a potential probiotic strain able to alleviate inflammation. Three probiotics of S. cerevisiae GILA strains were chosen by in vivo screening with a dextran sulfate sodium-induced colitis murine model. In particular, GILA 118 down-regulates neutrophil–lymphocyte ratio and myeloperoxidase in mice treated with DSS. The expression levels of genes encoding tight junction proteins in the colon were upregulated, cytokine interleukin-10 was significantly increased, and tumor necrosis factor-α was reduced in the serum.
Similar content being viewed by others
Introduction
Intestinal inflammation, such as inflammatory bowel disease (IBD), is a chronic relapsing inflammatory disorder of the gastrointestinal tract (GIT)1. The most common drugs used for the treatment of inflammatory bowel disease include sulfasalazine, mesalamine (Asacol, Pentasa, Colazal, and Salofalk), azathioprine, 6-mercaptopurine, cyclosporine, infliximab (Remicade), adalimumab (Humira), and corticosteroids (prednisone); however, long-term drug therapy should be avoided to evade their side effects2. A common alternative treatment is probiotics. Probiotic research including product development has received increasing attention3,4,5. Probiotics are active microorganisms that improve health and prevent illness6. Lactobacillus and Bifidobacterium are present in most probiotics, especially lactic acid bacteria (LAB) in the pharmaceutical industry7,8. It has been suggested that Saccharomyces boulardii, a well-known probiotic, can be effective in IBD by inhibiting the Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κb) signaling pathway9. In particular, probiotic research on S. boulardii has indicated positive outcomes in treating environment-related guts and anti-inflammatory effects10,11.
Prokaryotic probiotics have been studied more extensively than eukaryotic probiotics, and the differences between the prokaryotic (bacteria) and eukaryotic (yeast) probiotics lie in their sizes, cell wall composition, and optimal growth conditions12. Yeasts are ten times larger than bacteria, and their cell wall comprises chitin, glucan, mannose, phosphopeptidomannan, and phospholipomannan. Glucan, a cell wall component, modulates the immune system to improve immune functions13, presenting a benefit for probiotic applications. Eukaryotic (yeast) probiotics can tolerate stress, survive, and adhere to the gastrointestinal tract. However, yeast application as a probiotic is limited while bacteria use a wide range of animals12. Therefore, we need to provide insights into the yeast strain that will benefit the host.
Similar to other microorganisms, the principal function of yeast is fermentation. For instance, S. cerevisiae converts glucose to ethanol and carbon dioxide in traditional Korean rice wines such as Makgeolli and Dongdongju, contributing to their characteristic properties14. Yeast also protects rice wine from contamination by other bacteria14. Some yeast strains also have high nutrient value15, and the ability of yeast to change cereals to fermented and functional foods is closely related to health issue16. Because of their safety and technological applications, S. cerevisiae species have been extensively studied and used in various sectors, including bakeries and breweries. Several reports have demonstrated and documented the benefits of S. cerevisiae strains on human health17,18,19. Functions of S. cerevisiae’s including its anti-infective properties17, antioxidant activities18, and other probiotic properties, have also been studied. Recently, yeast S. cerevisiae UFMG A-905 showed probiotic properties when treated in mice infected by Salmonella Typhimurium20. These findings suggest that the probiotic S. cerevisiae varieties should be carefully selected for the optimum host health. Therefore, screening of probiotic S. cerevisiae is of utmost importance, and our findings highlight the probiotic potential of S. cerevisiae.
The objective of the present study, we demonstrated the probiotic potential of S. cerevisiae strains derived from Korean rice wine. The Immunomodulatory activity of S. cerevisiae was compared with S. boulardii to determine nitric oxide production by RAW 264.7 cells. Furthermore, in vivo studies were done to choose the S. cerevisiae GILA stain that alleviated intestinal inflammation functionality in a DSS-induced colitis model.
Results
Tolerance to in vitro GI tract conditions
Yeast cell growth was less resistant at pH 2.0 than S. boulardii CNCM I-745. We only chose similar or higher growth yeast than S. boulardii CNCM I-745. Table 1 shows the results for identified yeast strains. According to resistance to pH 2.0 and bile condition, test S. cerevisiae GILA strain showed a similar survival rate. S. cerevisiae were chosen to compare the growth in the bile condition with S. boulardii CNCM I-745. S. boulardii control strains had almost 100% survivability in 3.0% Oxgall. The strains with over 90% survivability were selected, where most strains showed > 90% survivability.
The survival rates of all S. cerevisiae GILA strains were similar when compared with control strain S. boulardii, CNCM I-745, which showed over 95% survival in GIT model at mouth, stomach and intestine (Fig. 1). S. cerevisiae GILA106 showed a significantly lower survival rate (p < 0.01 and p < 0.001) than other S. cerevisiae GILA strain in the stomach and intestine, whereas all strains showed about a 90.0% survival rate.
Aggregation and adhesion properties to caco-2 cell
Coaggregation with pathogen was high in S. boulardii CNCM I-745. We selected S. cerevisiae GILA stains with more significant or similar to coaggregation ability than S. boulardii CNCM I-745 (Table 2). Most of the isolated yeasts showed high autoaggregation properties. Aggregation characteristics of yeast are related to sporulation21. Notably, 80% autoaggregation after 24 h of incubation was based on colony formation22, which affects host colonization after entry. Those strains with autoaggregation ability could also have coaggregation ability with the pathogen. S. boulardii CNCM I-745 had between 60 and 85% coaggregation with the three pathogens, Staphylococcus aureus ATCC 25922, Enterococcus faecalis ATCC 29212 and Escherichia coli K88.
Adhesion assay to Caco-2 cell show all S. cerevisiae GILA strain’s adhesion ability (4% to 15%). When compared with the control yeast strain, S. boulardii CNCM I-745, S. cerevisiae GILA 100, 118, 137, 197 were not significantly (p > 0.05) different. S. cerevisiae GILA 106 was significantly (p < 0.05) lower than GILA 100 (Fig. 2).
Antioxidant ability by DPPH assay
The DPPH scavenging effect of S. cerevisiae GILA strains was evaluated to examine their antioxidant ability. The scavenging results for S. boulardii CNCM I-745 were 91.35 ± 0.13%; results for other yeasts were less than 90.00%. For this reason, we chose those strains with more than 85.00% activity (Fig. 3). In the final stage of probiotic research, we compared these results with cell wall β-glucan.
Phylogenetic analysis of the ITS region
High homology of ITS sequences was observed closest location of S. cerevisiae GILA 137 to S. cerevisiae GILA 106 and closer to S. cerevisiae GILA 118 in the 15 yeasts (Fig. 4). S. cerevisiae GILA 100 is closely related to S. cerevisiae GILA 106, 118, and 137. Due to the high homology of S. cerevisiae GILA, phenotypic differences such as the probiotics potential of S. cerevisiae GILA were investigated.
Alleviating inflammation in 264.7 cells and splenocyte
NO is related to various immunological procedures such as host defense, immunoregulation and signal transduction and are importance mediators triggering gastrointestinal disease23. NO is produced from L-arginine by an enzyme of nitric oxide synthase (NOS) and the inducible isoform of NO (iNOS) during inflammation where iNOS is activated by pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6). For examining the effects of selected S. cerevisiae GILA, 10 ng/ml of LPS was treated in 264.7 cells for 48 h to induce inflammation and NO production. Selected S. cerevisiae GILA significantly (p < 0.001) suppressed NO production induced by LPS compared with the positive control treated LPS only. S. boulardii CNCM I-745 has shown anti-inflammatory effects compared with the LPS treatment group (Fig. 5).
Nitric oxide production of heat-killed S.boulardii and S.cerevisiae strains in LPS (1 µg/ml) induced RAW 264.7 murine macrophage cells. The concentration of Nitric oxide production was determined by calculating standard curve. Values are mean ± S.D of triplicates for each group. ***p < 0.001, compared with treatment of only LPS.
Alleviating the intestinal inflammation in a dextran sulfate sodium-induced colitis mouse model
During DSS treatment with yeast period, the S. cerevisiae GILA115 group showed body weight loss more than the normal group (p < 0.01) on day11. In contrast the other S. cerevisiae GILA groups were not significantly (p > 0.05) different (Fig. 6a). Stool consistency and bleeding score were significantly lower in the S. cerevisiae GILA 59, 100, 118, and 137 groups than in the DSS group, although S. cerevisiae GILA 59 and 100 groups lose more weight (8–10%) than S. cerevisiae GILA 118 and 137 groups (4–8%). Consequently, S. cerevisiae GILA 118 and 137 groups were significantly (p < 0.05) lower than the DSS treatment group when compared with the disease activity index (DAI) score (Fig. 6b). The relative colon length rate was not significantly different (Fig. 6c,d) whereas the relative spleen weight rate was significantly different between the normal and DSS treatment groups (Fig. 6e,f). S. cerevisiae GILA100 and 118 groups were similar spleen weight rates to the normal group (Fig. 6e). Standard scores were calculated using normal and DSS group scores. The total score showed GILA 100, 118, and 137 groups were similar to the normal group than the other groups (Fig. 6g).
In vivo screening of S.cerevisiae GILA strain. Pathological and physiological status through the indicators of inflammation. (a) Body weight (%) compared to the normal group. (b) DAI score of disease from C57BL/6 J mouse group. Relative colon length rate compared with that of Normal (c) and DSS (d) group. Relative spleen weight rate compared with that of Normal (e) and DSS (f) group. (g) S.c GILA strain’s in vivo screening total score. Statical significance is indicated as follows: *p < 0.05, **p < 0.01 and ***p < 0.001.
To investigate the therapeutic properties of S. cerevisiae GILA strains in vivo, the DSS group showed IBD-colitis symptoms, including increased neutrophil count, neutrophil–lymphocyte ratio (NLR) in blood, myeloperoxidase (MPO) in feces (Fig. 7a), and proinflammatory cytokine (TNF-α) in serum (Fig. 7b). Stool consistency and bleeding score results were related to NLR from complete blood cell count (Fig. 7a)24,25. This finding suggested that the DSS-induced increase in neutrophils may affect other biomarkers and cytokines. Neutrophil expression in blood is one of the main features of colitis26,27,28. Further analysis was conducted to investigate the amelioration of intestinal inflammation. The gene expression levels of mucin-2 (Muc-2), zonula occludens-1 (ZO-1), occludin and epithelial cadherin (E-cadherin) significantly increased compared with those in the DSS group. (Fig. 7c).
Alleviation of Intestinal Inflammation in Mice between Treatments of S. cerevisiae GILA. (a) Neutrophil, Neutrophil–lymphocyte ratio from complete blood cell count and Amount of MPO in feces. (b) Analysis of pro-inflammatory cytokine TNF-α, IL-6 and anti-inflammatory cytokine IL-10 in serum. (c) Relaive gene expression of Muc-2, ZO-1, Occludin, E-cadherin in colon tissue. Statical significance is indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
We found that the S. cerevisiae GILA group had significantly decreased NLR in the blood (p < 0.01), MPO in feces (p < 0.001), and TNF-α in serum (p < 0.05, p < 0.01 and p < 0.001) (Fig. 7a,b). No significant changes were observed for IL-6 in serum. Meanwhile, IL-10 in serum significantly increased in the S. cerevisiae GILA 118 group compared with that in the other groups. The S. cerevisiae GILA 118 group also showed significantly increased serum IL-10 levels compared with the DSS group (Fig. 7b). These results suggested that S. cerevisiae GILA 118 effectively inhibits the biomarker of IBD and the expression of IL-10, thereby ameliorating colitis in mice.
Discussion
In our investigation, we selected a probiotic candidate when we applied S. cerevisiae GILA, which had a high survival rate in low pH and bile conditions. These results indicate that yeast which had high resistance to harsh condition also had high survival in GIT simulation model. S. cerevisiae GILA has almost over 90.0% survival rate in GIT model. Tolerance to GI tract could be considered in vivo conditions. Comparing the viable counts in the murine gastrointestinal tract could be shown survival rate in vivo29. Survival was an important factor of probiotics, but safety was also studied for selected probiotic potential yeast. None of the selected S. cerevisiae GILA strain had hemolytic activity and biogenic amine production. Yeast is also known to have antibiotic resistance. Yeasts aggregate via their cell wall mannose30; a prominent aggregation ability indicates ample mannose. S. cerevisiae GILA stain showed 90% hydrophobicity (data not shown). The adhesion ability for Caco-2 cells, such as microbial adhesion to mucosa model31 was 4–15%. These results show that S. cerevisiae GILA stain possesses cell adhesion ability to be used in probiotic preparation.
The DPPH scavenging effect measures the antioxidant capacity related to the cell wall β-glucan content. β-Glucan is a β-d-glucose polysaccharide group and a component of the yeast cell wall. Its structure is a long, β-(1,6)-branched, β-(1,3)-glucan32. β-Glucan has an excellent antioxidant capacity33; however, this could not explain the results for cell wall β-glucan (Fig. S1). Yeast itself may then possess an intrinsic antioxidant ability34. Therefore, there should be another experiment to quantify cell wall β-glucan. β-Glucan is recognized by the receptors on the host’s immune cells35, enhancing immune function resulting in anticancer and anti-inflammatory effects35,36. Furthermore, yeast β-Glucan benefits host health by protecting it against pathogens37. The content of β-Glucan was calculated from total glucan by subtracting α-glucan. A large amount of β-glucan in the yeast cell walls was comparable to a previous study38. The S. cerevisiae GILA strain had more than 36% β-glucan; this result could imply a probiotic potential (Fig. S1). Commercial probiotics, such as Lactobacillus rhamnosus GG have 5% β-glucan (data not shown), respectively. S. cerevisiae has more than triple the amount of β-glucan than these Lactobacillus strains. Moreover, the structure of β-glucan should also be considered in future research. Since β-glucan modulates cytokines in human blood, it should be confirmed whether all structures of β-glucan are beneficial to host health39. This result indicated that β-glucan benefits the host’s health and immune system. A future probiotic approach could consider yeast’s β-glucan characteristics. Our results revealed the quantity of β-glucan in the yeast cell walls. This method could be applied during probiotics-related yeast and β-glucan screening. The result was unexpected from the DPPH scavenging effect, but all experimental strains had more than 36% β-glucan, which is a sufficient level38.
The cell wall had more than 36% β-glucan, and nitric oxide production was significantly lower than the control. The β-glucan quantity could be edequate, but we need more evidence of related probiotic functionality. Previous studies have reported the prevention of inflammation by yeast fermentate40; there are also reports on β-glucan-mediated induction of proinflammatory cytokines41. Given that the quantity of β-glucan did not influence this outcome, another factor, such as the probiotic properties of the S. cerevisiae GILA strain, may have been responsible for reducing in proinflammatory cytokines. The physiological impact of yeast probiotics against the host was determined by their ability to relieve oxidative stress measured by fecal MPO level42. Activated neutrophils release MPO, a marker of oxidative stress, and destroy epithelial cells26. As demonstrated by DPPH scavenging capacity of Saccharomyces cerevisiae GILA in vitro (Fig. 3), in vivo results also prove this ability to relieve oxidative stress (Fig. 7a). The S. cerevisiae strain was resistant to ETEC infection17, and S. cerevisiae cell wall glucan had an immune-modulatory effect, which could affect colitis reduction13. Spleen weight could be due to alleviating intestinal immune response43. Moreover, the inflammation biomarkers were similar to those in the CBC test-neutrophil lymphocyte rate results28 (Fig. 7a,b). There was no significant difference in the relative expression rate of the Muc-2 gene between the normal and DSS groups (Fig. 7c). Recovery through a 6-days period (Fig. S2) is considered maintaining in the DSS group. In this study, an increase in Muc-2 gene expression was regarded as an improved capacity of epithelial protection44. As a result, similar to this experiment, colitis was alleviated by increasing Muc-2 expression in intestinal goblet cells45. An increase in Muc-2 gene expression is assumed to reduce colitis (Fig. 7c). Furthermore, we elucidated that anti-inflammatory cytokine IL-10 in serum was more upregulated by S. cerevisiae GILA 118 than other S. cerevisiae GILA strains. These findings warrant further experiments, especially S. cerevisiae GILA 118 structure study with the DSS-colitis model. For this reason, S. cerevisiae could be developed as a useful probiotic in the future. Nevertheless, more evidence as a potential gut microbiota modulator46 is required for the S. cerevisiae GILA strain-related probiotics. Thus, further additional research and development are required to characterize these probiotic candidates' functionality.
Conclusion
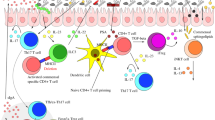
We screened and selected seven S. cerevisiae strains as probiotic properties were similar to or higher than S. boulardii CNCM I-745. The strain S. cerevisiae GILA100, GILA118, and GILA137 met the criteria for a probiotic which had to alleviate inflammatory effect in a DSS-induced colitis mouse, especially S. cerevisiae GILA 118 administration increased IL-10 in serum and also alleviated intestinal inflammation in mice compared with S. cerevisiae GILA 100 and GILA137 (Fig. 8).
Materials and methods
Isolation and culture conditions
Eight samples of rice wine were obtained from Gangwon-do and three from Chungcheong-do, both in Korea. Ninety-two strains of yeast were from makgeolli, and eight strains were from dongdongju. All isolates were confirmed by gram staining and cell morphology47.
Yeasts were cultured in yeast extract–peptone–dextrose (YPD) broth, consisting of 1% (w/v) yeast extract, 2% (w/v) peptone, and 2% dextrose. To screening yeasts that aggregate with a pathogen, Staphylococcus aureus ATCC 25922, Enterococcus faecalis ATCC 29212, and Escherichia coli K88 were cultured in brain heart infusion broth.
Saccharomyces boulardii CNCM I-745, supplied by Jarrow Formulas (Los Angeles, USA), was used as a control for comparison with the isolated yeast strain. The yeast strains were incubated aerobically at 37℃ for 24 h before use simulating the conditions in a human host48. All broth and agar materials were obtained from Difco (USA).
Resistance to low pH and bile conditions
To determine the ability of yeast strains to survive in GI conditions, the isolates were incubated in YPD broth at 37 °C for 24 h. Then, the cultured yeasts were centrifuged at 5500×g for 10 min at 4 °C. The pellets were incubated for 2 h in YPD broth, and adjusted to pH 2.0 with 1 N HCl. The sample (100 μL), diluted in phosphate-buffered saline (PBS), was spread on YPD agar according to the drop-plating method49. After incubation in YPD broth (pH 2.0) at 37 °C, the resistance of yeast to bile was estimated similarly. The pellets were incubated for 12 h in YPD broth with 3.0% bovine bile (Oxgall, Difco, USA). The survival rate at pH 2.0 and in 3.0% Oxgall was calculated using the following formula: acid and bile tolerance (%) = [yeast after 24 h incubation (log cfu/mL)/yeast after 2 h incubation at pH 2.0 (log cfu/mL) and in 3.0% Oxgall (log cfu/mL)] × 10050.
Autoaggregation and coaggregation with pathogen
Yeasts were grown for 24 h at 37 °C in YPD broth, then harvested by centrifugation at 5500×g for 15 min. The pellets were washed twice with PBS, and then resuspended in PBS. Cell suspensions (4 mL) were mixed by vortexing for 10 s, and autoaggregation was determined after 24 h of incubation at 37 °C. The upper suspension layer (0.1 mL) was transferred to another tube with 3.9 mL PBS, and the absorbance (A) was measured at 600 nm. The autoaggregation percentage was calculated as follows: [1 − (A24/A0)] × 10051.
For the coaggregation test, 2 mL each of yeast and pathogen, Staphylococcus aureus ATCC 25922, Enterococcus faecalis ATCC 29212, and Escherichia coli K88 were vortexed together. The level of coaggregation with pathogen was calculated according to the equation of Handley et al.52 as follows: coaggregation (%) = [((Ax + Ay)/2) − A(x + y)/((Ax + Ay)/2)] × 100, where x and y represent the yeast and pathogen in the control tube, respectively, and (x + y) is the mixture.
Hydrophobicity
The hydrophobicity of the yeast cell surface [H(%)] was estimated using adhesion to n-hexadecane (Sigma, USA) according to the method by Rosenberg et al53 with a slight modification as follows: H(%) = [(1 − OD4)/OD0] × 100, where OD is the optical density at 0 and 4 h.
1-Diphenyl-2-picrylhydrazyl (DPPH) scavenging effect
The DPPH scavenging assay was performed to compare the antioxidant abilities of the yeast strains. The yeast pellets were harvested, washed twice, and resuspended in 1 mL PBS. The resulting suspensions (800 μL) were added to 1 mL DPPH solution (0.2 mM in 80% methanol) and mixed by vortexing, followed by incubation for 30 min in the dark. After the incubation, the solutions were centrifuged at 12,400×g for 5 min, and 300 μL of each sample was transferred to a 96-well plate to measure the absorbance at 517 nm. The reconstitution of the standard was performed by adding ascorbic acid to 80% (v/v) methanol at a concentration of 1 mg/mL to 400 μL/mL. Five twofold serial dilutions were performed, and 80% methanol served as the zero standard54.
In vitro gastrointestinal tract (GIT) models
The constituents and concentrations of the various synthetic juices of the in vitro GIT model are shown in Table 3. All materials were obtained from Sigma-Aldrich (USA) or Difco (USA). The inorganic and organic solutions are mixed with distilled water. The pH of the juices and incubation time are adjusted to human physiological traits with a minor modification55,56. 7 ml of the S. boulardii CNCM I-745 and S. cerevisiae GILA strain were centrifuged, then resuspended in 1 ml PBS. Saliva was added and incubated for 5 min. After the incubation, gastric juice was mixed and incubated for 2 h. 12 ml of duodenum juice with 6 ml of bile juice were mixed and incubated for 2 and 5 h with agitation (60×g) at 37 °C. Yeast samples were harvested three times and serially diluted and plated onto YPD agar.
Adhesion assay
The Caco-2 cell line, obtained from the Korea cell line bank (KCLB, Seoul, Korea), was used between passages 40–60 for all experiments. The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; corning, USA) supplemented with 10% heat inactivated FBS, 2 mM l-glutamine ml−1, 100 U penicillin ml−1, and 100 µg, streptomycin ml−1 at 37 °C in a 5% CO2 atmosphere.
After harvesting yeast, overnight cultures of yeast strains were suspended with preheated fresh DMEM media and adjusted to O.D 600 nm at 2.0 density (approximately 1 × 107 cfu ml−1). One millimeter of each yeast was inoculated to 12-well plates and incubated for 2 h at 37 in a 5% CO2 atmosphere. Then, non-adherent yeasts were removed by washing with PBS twice, and the Caco-2 cells and attached yeast were lysed with 1 ml of 0.05% Trypsin–EDTA (Gibco, USA). The adherent yeast was enumerated by diluting the solution serially (1:10) with PBS from the initial and using the drop-plating method on YPD agar4.
Hemolytic activity and biogenic amine production
Safety assessments were conducted by measuring the hemolytic activity and biogenic amine production. The hemolytic activity was evaluated using blood agar plates supplemented with 5% (v/v) defibrinated sheep blood (KisanBio, Korea). The appearance of clear zones around the colonies confirmed by β-hemolysis. After the colony of each strain was streaked on the blood agar, the plates were incubated aerobically at 37 °C for 48 h57.
Biogenic amine production was analyzed according to the method of Bover-Cid and Hozapfel58. The isolates were streaked on decarboxylase media and incubated aerobically at 37 °C for 4 days. Decarboxylase activity was detected by the color change from yellow to blue.
ITS region sequencing and phylogenetic analysis
Single colonies were submitted to SolGent Corporation (South Korea) for ITS sequencing. DNA extraction was performed using a boiling method by Chelex bead. The screened and selected strains were identified using amplified internal transcribed spacer ITS 1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS 4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) sequencing59. The polymerase-chain-reaction (PCR) reaction was performed in a BigDye® Terminatorv3.1 cycle sequencing kits. Sequencing was analyzed by ABI 3730XL DNA Analyzer (50 cm capillary). The primary measurement (identity, %) was compared to the yeast strain. Sequences were aligned by the NCBI GenBank database using the BLASTn. Phylogenetic analysis proceeded with MEGA software version 11 with neighbor-joining analysis of the ITS region identified S.cerevisiae with their type strains. Bootstrap analysis included 1,000 replicates. In addition, sequences were compared with others in the NCBI GenBank database using the BLASTn technique for identification.
Determination of nitric oxide production
RAW 264.7 cell lines of murine macrophages were obtained from the Korea cell line bank (KCLB, Seoul, Korea). The cells were cultivated in Dulbecco’s modified eagles medium (DMEM, Gibco, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS, Gibco, USA) and 1% antibiotic–antimycotic (Gibco, USA) at 37 ℃ in a 5% CO2 atmosphere. RAW 264.7 cells were seeded at 1 × 105 cells ml−1 in 24-well plates and stabilized for 2 h. To stabilize overnight cultures of S. bouladii CNCM I-745 and S. cerevisiae were centrifuged. The cell pellet were washed with PBS twice and adjusted with OD 600 nm at 1.0 density. S. bouladii CNCM I-745 and S. cerevisiae were heated for 15 min at 110 °C to remove activity of S. bouladii CNCM I-745 and S. cerevisiae, and then the cells were stimulated with 450 μL of lipopolysaccharides (LPS, 1 μg/mL; Sigma-Aldrich, USA) and 50 μL of heat-killed S. bouladii CNCM I-745 and S. cerevisiae for 48 h. The incubated cells were centrifuged at 600×g, 4 °C for 10 min, and the cell supernatant was transferred to new tubes. The measurement of nitric oxide (NO) concentration was estimated using the Griess reagent (Promega Inc., USA) as the manufacture’s instruction. After mixing the cell supernatant and Griess reagent with the same volumes, the mixture was incubated for 10 min at room temperature, and absorbance at 540 nm was determined by a microplate reader (SpectraMax M4 Microplate/Cuvette Reader, Molecular Devices, USA). The concentration of NO was calculated by comparing it a standard curve60.
In vivo experimental design
Six-week-old female C57BL/6J mice were purchased from Daehan Bio Link Co., Ltd. (Korea). Mice were randomly distributed into 11 groups. After one week of stabilization, the mice were treated with 1.5% dextran sulfate sodium (DSS, MW 36,000–50,000; MP Biomedicals, USA) in distilled water with S. boulardii CNCM I-745 or S. cerevisiae strain (107 CFU/day) once a day for 14 days, followed by 6 days of recovery (Fig. S2). All mice were subsequently euthanized by carbon dioxide (CO2) asphyxiation.
Colitis evaluation
Mice were examined daily for weight, stool consistency, and total blood in feces for colitis evaluation. DAI was evaluated using the method of Cooper et al.61 with minor modification. Scores for weight loss, stool consistency, and bleeding were monitored after 7 days of DSS treatment. After sacrifice, the mouse, colon length and spleen weight per body weight were compared in each group (n = 8).
RNA isolation, RT-PCR and ELISA
Colon tissue in Buffer RLT was well homogenized. After disruption, the RNA was isolated by RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) protocol. cDNA was synthesized by the PrimeScipt RT reagent Kit (Takara Korea Biomedical Inc.,Seoul, Korea) protocol. Gene amplification was done by the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) protocol. Data were normalized with the housekeeping β-actin expression level. The primers used are listed in Table 4. Serum, feces were quantified by ELISA kits (R&D Systems, Minneapolis, MN, USA) for inflammatory cytokine and myeloperoxidase (MPO), according to the manufacturer’s instructions.
Cell wall β-glucan
β-Glucan (%, w/w) was measured using the yeast glucan assay kit (Megazyme, Ireland). Before the calculation of β-glucan, the yeast cell wall was autolyzed and hydrolyzed following the procedures of Pengkumsri et al.38. Briefly, yeast cells were incubated in pH 5.0 water at 50 °C for 48 h with shaking at 160 × g, then at 80 °C for 15 min in a water bath. After incubation, yeast cells were harvested by centrifugation for 10 min at 4 °C at 6900×g. The autolyzed yeast cells were mixed with 1.0 M NaOH/HCl and incubated at 80 °C with a stirrer for 2 h. Finally, the hydrolyzed cells’ β-glucan content (%, w/w) was calculated following the assay kit protocol.
Statistical analyses
The results are the means ± standard deviations of triplicate analyses. Pearson’s correlation and Duncan’s test were performed with SPSS (version 18.0), and results were analyzed to ANOVA using the GraphPad Prism software (GraphPad Software, San Diego, CA, USA).
Research involving animal participants
For the in vivo experiment, this study was carried out in accordance with the guidelines by the Korean Association for Laboratory Animals, and the protocol was approved by the Institutional Animal Care and Use Committee of Seoul National University (Approval No. SNU-200706-6-2). All studies were performed in compliance with the ARRIVE guidelines.
Data availability
The datasets generated during the current study are available in the GenBank (Web link: https://www.ncbi.nlm.nih.gov/genbank/) repository, accession number: OQ247983, OQ247986, OQ248002, OQ248003, OQ248004, OQ248507, and OQ253417. Cell wall β-glucan content of selected S. cerevisiae strains and an overview of in vivo screening are available in Supplementary Figs. 1 and 2.
References
De Souza, H. S. & Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13, 13–27 (2016).
Kornbluth, A. & Sachar, D. B. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am. J. Gastroenterol. 105, 501–523 (2010).
Day, R. L., Harper, A. J., Woods, R. M., Davies, O. G. & Heaney, L. M. Probiotics: Current landscape and future horizons. Future Sci. OA 5, 391 (2019).
Menezes, A. G. T. et al. Probiotic potential, antioxidant activity, and phytase production of indigenous yeasts isolated from indigenous fermented foods. Probiotics Antimicrob. Proteins 12, 280–288 (2020).
Wen, J. et al. Effects of probiotic litchi juice on immunomodulatory function and gut microbiota in mice. Food Res. Int. 137, 109433 (2020).
Group, J. F. W. W. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food (World Health Organization Food Agriculture organization of the United Nations, Rome, Italy, 2002).
Tachedjian, G., Aldunate, M., Bradshaw, C. S. & Cone, R. A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 168, 782–792 (2017).
Evivie, S. E., Huo, G.-C., Igene, J. O. & Bian, X. Some current applications, limitations and future perspectives of lactic acid bacteria as probiotics. Food Nutr. Res. 61, 1318034 (2017).
Pothoulakis, C. anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment Pharmacol. Ther. 30, 826–833 (2009).
Everard, A., Matamoros, S., Geurts, L., Delzenne, N. M. & Cani, P. D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. mBio. 5, e01011–01014 (2014).
Rodríguez-Nogales, A. et al. Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on microRNAs expression and gut microbiota composition. J. Nutr. Biochem. 61, 129–139 (2018).
Nayak, S. K. In Biology of Eukaryotic Probiotics, Probiotics 29–55 (2011).
Stier, H., Ebbeskotte, V. & Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1, 3/1, 6-D-glucan. Nutr J. 13, 1–9 (2014).
Park, H.-J., Lee, S. M., Song, S. H. & Kim, Y.-S. Characterization of volatile components in Makgeolli, a traditional Korean rice wine, with or without pasteurization, during storage. Molecules 18, 5317–5325 (2013).
Gallone, B. et al. Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell. 166, 1397–1410. e1316 (2016).
Melini, F., Melini, V., Luziatelli, F., Ficca, A. G. & Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 11, 1189 (2019).
Roussel, C. et al. Anti-infectious properties of the probiotic Saccharomyces cerevisiae CNCM I-3856 on enterotoxigenic E. coli (ETEC) strain H10407. Appl. Microbiol. Biotechnol. 102, 6175–6189 (2018).
Fakruddin, M., Hossain, M. & Ahmed, M. M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement Altern Med. 17, 1–11 (2017).
Puppala, K. R., Ravi Kumar, V., Khire, J. & Dharne, M. Dephytinizing and probiotic potentials of Saccharomyces cerevisiae (NCIM 3662) strain for amelioration of nutritional quality of functional foods. Probiotics Antimicrob. Proteins 11, 604–617 (2019).
Oliveira, S. R. et al. Evaluation of a functional craft wheat beer fermented with Saccharomyces cerevisiae UFMG A-905 to treat Salmonella Typhimurium infection in mice. Probiotics Antimicrob. Proteins 14, 1–13 (2022).
Cullen, P. J. & Sprague, G. F. Jr. The regulation of filamentous growth in yeast. Genetics 190, 23–49 (2012).
Gil-Rodríguez, A. M., Carrascosa, A. V. & Requena, T. Yeasts in foods and beverages: In vitro characterisation of probiotic traits. Food Sci. Biotechnol. 64, 1156–1162 (2015).
Schoedon, G. et al. Nitric oxide and infection: Another view. Clin. Infect. Dis. 21, S152–S157 (1995).
Dinler Ay, C. Neutrophil to lymphocyte ratio as a prognostic biomarker in puppies with acute diarrhea. J. Vet. Emerg. Crit Care 32, 83–89 (2022).
Langley, B. O. et al. Inflammatory bowel disease and neutrophil-lymphocyte ratio: A systematic scoping review. J. Clin. Med. 10, 4219 (2021).
Fournier, B. & Parkos, C. The role of neutrophils during intestinal inflammation. Mucosal. Immunol. 5, 354–366 (2012).
Kucharzik, T., Walsh, S. V., Chen, J., Parkos, C. A. & Nusrat, A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 159(6) (2001).
Torun, S. et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: A promising marker in predicting disease severity. Clin. Res. Hepatol. Gastroenterol. 36, 491–497 (2012).
Hedin, K. A. et al. Effects of broad-spectrum antibiotics on the colonisation of probiotic yeast Saccharomyces boulardii in the murine gastrointestinal tract. Sci. Rep. 12, 8862 (2022).
Tiago, Fd. C. P. et al. Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. J. Med. Microbiol. 61, 1194–1207 (2012).
Saikia, D. et al. Hypocholesterolemic activity of indigenous probiotic isolate Saccharomyces cerevisiae ARDMC1 in a rat model. J. Food Drug Anal. 26, 154–162 (2018).
Volman, J. J., Ramakers, J. D. & Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 94, 276–284 (2008).
Jaehrig, S. C., Rohn, S., Kroh, L. W., Fleischer, L.-G. & Kurz, T. In vitro potential antioxidant activity of (1→ 3), (1→ 6)-β-d-glucan and protein fractions from Saccharomyces cerevisiae cell walls. J. Agric. Food Chem. 55, 4710–4716 (2007).
Horiguchi, H., Yurimoto, H., Kato, N. & Sakai, Y. Antioxidant system within yeast peroxisome: Biochemical and physiological characterization of CbPmp20 in the methylotrophic yeast Candida boidinii. J. Appl. Biol. Chem. 276, 14279–14288 (2001).
Chan, G.C.-F., Chan, W. K. & Sze, D.M.-Y. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2, 1–11 (2009).
Lee, K.-H. et al. Bacterial β-(1, 3)-glucan prevents DSS-induced IBD by restoring the reduced population of regulatory T cells. Immunobiology 219, 802–812 (2014).
Auinger, A., Riede, L., Bothe, G., Busch, R. & Gruenwald, J. Yeast (1, 3)-(1, 6)-beta-glucan helps to maintain the body’s defence against pathogens: A double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur. J. Nutr. 52, 1913–1918 (2013).
Pengkumsri, N. et al. Extraction of β-glucan from Saccharomyces cerevisiae: Comparison of different extraction methods and in vivo assessment of immunomodulatory effect in mice. Food Sci. Technol. 37, 124–130 (2016).
Noss, I., Doekes, G., Thorne, P. S., Heederik, D. J. & Wouters, I. M. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate Immun. 19, 10–19 (2013).
Evans, M., Reeves, S. & Robinson, L. E. A dried yeast fermentate prevents and reduces inflammation in two separate experimental immune models. Evid. Based Complement Alternat. Med. 2012, 973041 (2012).
Javmen, A. et al. β-Glucan from Saccharomyces cerevisiae induces IFN-γ production in vivo in BALB/c mice. In Vivo 29, 359–363 (2015).
Hansberry, D. R., Shah, K., Agarwal, P. & Agarwal, N. Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus 9, e1004 (2017).
Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 104, 15.25.11-15.25.14 (2014).
Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006).
Ma, S., Yeom, J. & Lim, Y.-H. Dairy Propionibacterium freudenreichii ameliorates acute colitis by stimulating MUC2 expression in intestinal goblet cell in a DSS-induced colitis rat model. Sci. Rep. 10, 5523. https://doi.org/10.1038/s41598-020-62497-8 (2020).
Li, B. et al. Saccharomyces boulardii alleviates DSS-induced intestinal barrier dysfunction and inflammation in humanized mice. Food Funct. 13, 102–112 (2022).
Harrington, A., McCourtney, K., Nowowiejski, D. & Limaye, A. Differentiation of Candida albicans from non-albicans yeast directly from blood cultures by Gram stain morphology. Eur. J. Clin. Microbiol. Infect. Dis. 26, 325–329 (2007).
Leite, A. M. et al. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J. Dairy Sci. 98, 3622–3632 (2015).
Herigstad, B., Hamilton, M. & Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 44, 121–129 (2001).
Ogunremi, O., Sanni, A. & Agrawal, R. Probiotic potentials of yeasts isolated from some cereal-based N igerian traditional fermented food products. J Appl Microbiol. 119, 797–808 (2015).
Kos, B. et al. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 94, 981–987 (2003).
Handley, P. S. et al. A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriate strains of Streptococcus salivarius. J. Gen. Microbiol. 133, 3207–3217 (1987).
Rosenberg, M., Gutnick, D. & Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9, 29–33 (1980).
Patel Rajesh, M. & Patel Natvar, J. In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. J. Adv. Pharm. Educ. Res. 1, 52–68 (2011).
Oomen, A. et al. Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants. Arch. Environ. Contam. Toxicol. 44, 0281–0287 (2003).
Yeo, S. et al. Development of putative probiotics as feed additives: Validation in a porcine-specific gastrointestinal tract model. Appl. Microbiol. Biotechnol. 100, 10043–10054 (2016).
Saravanan, V. & Vijayakumar, S. Isolation and screening of biosurfactant producing microorganisms from oil contaminated soil. J. Acad. Indus. Res. 1, 264–268 (2012).
Bover-Cid, S. & Holzapfel, W. H. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol. 53, 33–41 (1999).
Cavalieri, D., McGovern, P. E., Hartl, D. L., Mortimer, R. & Polsinelli, M. Evidence for S. cerevisiae fermentation in ancient wine. J. Mol. Evol. 57, S226–S232 (2003).
Lee, H.-S. et al. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. Int. Immunopharmacol. 8, 574–580 (2008).
Cooper, H. S., Murthy, S., Shah, R. & Sedergran, D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69, 238–249 (1993).
Yin, R. et al. Selection and evaluation of stable housekeeping genes for gene expression normalization in carbon nanoparticle-induced acute pulmonary inflammation in mice. Biochem. Biophys. Res. Commun. 399, 531–536 (2010).
Floyd, A. M. et al. Mucin deficiency causes functional and structural changes of the ocular surface. PLoS ONE 7, e50704 (2012).
Liu, T. et al. Vitamin D treatment attenuates 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis but not oxazolone-induced colitis. Sci. Rep. 6, 32889 (2016).
Shiohira, S. et al. Sphingosine-1-phosphate acts as a key molecule in the direct mediation of renal fibrosis. Physiol. Rep. 1, e00172. https://doi.org/10.1002/phy2.172 (2013).
Funding
This work is supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. 316005-5).
Author information
Authors and Affiliations
Contributions
B.J.K., Y.J.P., C.H.Y., and C.S.H. conceived and designed the experiments; B.J.K., Y.J.P., H.J.P., C.H.Y., and C.S.H. performed the experiments; B.J.K., and C.S.H. analyzed the data; Y.J.P., HJP, JWK, and C.H.Y. contributed data or analysis tools; and C.S.H. wrote the paper; B.J.K., JWK, C.H.Y., and C.S.H. reviewed and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kil, B.J., Pyung, Y.J., Park, H. et al. Probiotic potential of Saccharomyces cerevisiae GILA with alleviating intestinal inflammation in a dextran sulfate sodium induced colitis mouse model. Sci Rep 13, 6687 (2023). https://doi.org/10.1038/s41598-023-33958-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33958-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.