Abstract
Previous studies indicate differences in short-term postoperative outcomes depending on the surgical starting time of the day, but long-term data are lacking. The aim of this study was to clarify if surgical starting time of the day influences long-term survival in gastric cancer patients. This cohort study consecutively included 2728 patients who underwent curatively intended gastrectomy for gastric cancer in 2011–2015 at a high-volume hospital in China, with follow-up until June 2019. Cox regression provided hazard ratios (HRs) with 95% confidence intervals (CIs) for 3-year all-cause mortality, adjusted for age, sex, health insurance, pathological tumor stage, surgical approach, neoadjuvant therapy, and weekday of surgery. Compared with patients with early starting time of gastrectomy (08:00–09:29), the point estimates for 3-year all-cause mortality were modestly increased in patients with a starting time in the middle of day (09:30–13:29; HR 1.15, 95% CI 0.97 to 1.37) and later (13:30–21:25; HR 1.10, 0.91 to 1.32). The corresponding HRs were increased particularly in patients who underwent laparoscopic gastrectomy (HR 1.54, 1.10 to 2.14 and HR 1.59, 1.12 to 2.25, respectively) and in those with stage II tumors (HR 1.74, 1.11 to 2.73 and HR 1.60, 1.00 to 2.58, respectively). Our study indicated that in patients who underwent laparoscopic gastrectomy and in those who with stage II tumors, starting surgery in the early morning might be associated with better long-term survival.
Similar content being viewed by others
Introduction
Gastric cancer is the fifth most common cancer and the fourth leading cause of cancer-related death worldwide, with an annual incidence of over one million new cases and nearly 800,000 deaths1. The rates are highest in East Asia (42% of all cases worldwide occurring in China), East Europe, and South America2. The 5-year overall survival in gastric cancer is below 40%3. Surgical resection with total or subtotal gastrectomy is the main curatively intended treatment. Optimization of this surgery would improve the survival.
‘Time of day’ variations in various interventions may influence short-term outcomes in several diseases4,5,6, including cancer7,8. Some surgeons in high-volume centers perform two or three major surgeries each day9,10, although the procedures are demanding and time-consuming, requiring high surgical skills and concentration11. Whether ‘time of day’ variation in gastrectomy influences long-term survival in gastric cancer is unknown and evidence of how this influences short-term outcomes is scarce12. Interestingly, some studies have found increased postoperative mortality in patients who undergo various types of surgery later as opposed to earlier in the week13,14,15. Some research specifically suggests that later weekday of gastrectomy independent of other prognostic factors increases the long-term disease-specific mortality in gastric cancer16. A possible explanation is a negative influence of the cumulative workload during the working week. We hypothesized that similar mechanisms exist for the timing of gastrectomy during the day, resulting in worse long-term survival and short-term outcomes in gastric cancer patients if the procedure is conducted later in the day. This hypothesis was examined in a cohort study from a high-volume center of gastrectomy in China.
Methods
Design
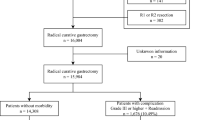
This cohort study consecutively included all patients who underwent total or subtotal gastrectomy for gastric cancer in a cancer center in Beijing, China between January 1, 2011 and December 31, 2015, with follow-up until June 30, 2019. The start date was chosen because minimally invasive surgery was broadly used in this hospital from 2011 onwards. An earlier version of this cohort has been described elsewhere17. The following patients were eligible for the cohort: (1) age ≥ 18 years, (2) histologically confirmed primary gastric adenocarcinoma, (3) pathological tumor stage I-III according to the American Joint Committee on Cancer TNM staging system, 7th edition18, (4) curatively intended gastrectomy, and (5) planned gastrectomy performed during the working week (Monday to Friday). Patients were excluded if they had (1) emergency gastrectomy, (2) palliative gastrectomy, (3) gastric stump cancer, or (4) other histological gastric tumor types than adenocarcinoma, (5) gastrectomies performed in weekends (Fig. 1).
This study was performed in accordance with the Declaration of Helsinki. The institutional review board of Chinese PLA General Hospital approved this retrospective study (reference number, S2019-040-01), in which patient informed consent from individual participant was waived due to retrospective nature and anonymous process of this study.
Data collection
Data on the baseline characteristics of patients, including sex, age, hospital stays, comorbidity, and health insurance, were extracted directly from the electronic medical records (EMR) system held by the Department of Medical Big Data in the cancer center. The surgical and post-operative details were independently reviewed by two researchers (WL and HX), who were blinded to the study hypothesis. Comorbidity was assessed using the well-validated Charlson comorbidity index19, and the insurance coverage was used as an indicator for socioeconomic status. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines20.
Exposure
The study exposure was the starting time of first incision for gastrectomy, which was extracted as a structural characteristic from the anesthesiology charts in the EMR system. Patients received curatively-intended gastrectomies by a total of 16 senior surgeon teams who were trained and qualified for both open and minimally invasive gastrectomy. The distribution of surgical starting time is displayed in supplemental Fig. S1. The starting time range was 08:00 to 21:25. The patients were categorized into three approximately equal-sized groups (tertiles) according to surgical starting time: an early morning group with starting time between 08:00 and 09:29 (N = 929), an intermediate group with starting time between 09:30 and 13:29 (N = 955), and a late group with starting time between 13:30 and 21:25 (N = 844). To further minimize selection bias, analysis by four approximately equal time interval groups (08:00–11:00, 11:00–14:00, 14:00–17:00, 17:00-after) was also performed (Table S1).
Outcomes
The primary outcome was 3-year all-cause mortality. The 3-year cut-off was chosen instead of 5 years for three reasons: (1) The 3-year mortality mirrors longer term survival, (2) the clinical follow-up practice in the center, and (3) it was possible to follow all patients in the cohort for 3 years within the study period. Data on 3-year all-cause mortality were collected from the patients’ medical records or via telephone follow-up (every 3 months for the first 2 years and every 6 months the third year). Secondary outcomes were total number of retrieved lymph nodes and length of postoperative hospital stay. These data were extracted directly from the medical records and pathology reports.
Statistical analysis
The patients were followed up from the date of gastrectomy until the end of study or death, whichever occurred first. The association between surgical starting time of the day and 3-year all-cause mortality was assessed using multivariable Cox proportional hazards regression, providing hazard ratios (HRs) with 95% confidence intervals (CIs). Seven predefined covariates were included in a multivariable model because of their known prognostic influence in combination with possible influence on the surgical starting time of the day: (1) age at surgery (continuous), (2) sex (male or female), (3) health insurance coverage (yes or no), (4) neoadjuvant therapy (yes or no), (5) pathological tumor stage (0-I, II, or III), and (6) surgical approach (open, laparoscopic or robotic), and (7) weekday of surgery (Monday-Wednesday or Thursday-Friday). The proportional hazards assumption was tested by Schoenfeld residuals and was met in all analyses. To explore associations within specific subgroups, analyses were stratified by the aforementioned 7 covariates using the same categorization, as well as by Charlson comorbidity (0, 1, or ≥ 2) and tumor location (cardia or non-cardia). Survival curves for different surgical starting time groups were generated using Kaplan–Meier estimates and compared by the log-rank test.
The secondary outcomes, i.e. postoperative stay and number of retrieved lymph nodes, were both treated as binary variables with the cut-off set at the median values, i.e. 11 days of postoperative stay and 23 lymph nodes. The association between surgical starting time of the day and these outcomes was assessed using multivariable logistic regression, providing odds ratios (ORs) with 95% CIs, adjusted for the seven covariates (with the same categorization) presented above.
All statistical analyses followed a detailed pre-defined study protocol and were performed by first author (YG) and checked by an experienced statistician (FM) using the SPSS software, version 25 (SPSS Inc, Chicago, IL). All tests were 2-sided and statistical significance was set at P < 0.05.
Results
Patients
A total of 2728 patients who underwent planned and curatively intended gastrectomy for gastric cancer were included in the cohort. Most patients were men (2065, 75.7%) and the median age was 60 years (interquartile range 52–67). There were no major differences in the distribution of age, sex, tumor location, pathological tumor stage, comorbidity, or neoadjuvant therapy among the three surgical starting time groups, but there was a trend of more self-paid patients in the two later surgical starting time groups compared to the early group (Table 1). The distribution of surgical approach, reconstruction method, and extent of the lymph node dissection was similar in the three surgical starting time groups (Table 2). The patients were followed up for a median of 50 months (interquartile range 29–73). Among all participants, 54 (2%) were lost to follow-up and were censored.
3-year all-cause mortality
The 3-year all-cause mortality rate was 26.0% in the entire cohort and no major differences were observed among the three surgical starting time groups in crude analysis (Fig. 2a, log-rank test, P = 0.269). Compared to the early starting group, the adjusted point estimates for the intermediate starting time group (HR 1.15, 95% CI 0.97 to 1.37) and the late starting group (HR 1.10, 0.91 to 1.32) were increased, but not statistically significant (Table 3). In patients who underwent laparoscopic gastrectomy, the adjusted HRs were increased in the intermediate starting time group (HR 1.54, 1.10 to 2.14) and in the late group (HR 1.59, 1.12 to 2.25), compared to the early starting group (Table 3 and Fig. 2b). A similar trend was suggested in patients who had undergone robotic surgery in late starting group (HR 1.36, 0.58 to 3.17), although the estimates were not statistically significant. No such association was indicated for open surgery. In patients diagnosed with pathological tumor stage II, the 3-year all-cause mortality was increased in the intermediate starting time group (HR 1.74, 1.11 to 2.73) and in the late group (HR 1.60, 1.00 to 2.58), compared to the early group (Table 3 and Fig. 2c). No association between gastrectomy starting time and 3-year all-cause mortality was found in patients diagnosed with early (I) or advanced pathological tumor stage (III). No clear differences in associations were found for subgroups of sex, age, comorbidity, tumor location, health insurance coverage, neoadjuvant therapy, or weekday of surgery, although almost all point estimates were above 1.0 in the two later starting time groups compared to the first (Table 3).
In analysis by quartile of time groups (Table S1), subgroup analysis also demonstrated that the 3-year all-cause mortality was increased in intermediate time group (14:00–17:00, HR 1.55, 95%CI 1.10–2.18) compared to early starting time group (08:00–11:00) in laparoscopic approach patients. Similarly, in patients with stage II gastric cancer, elevated mortality was observed in intermediate (11:00–14:00, HR 1.93, 1.25–2.97) and late starting time (17:00-after, HR 2.15, 1.00–4.64) groups.
Lymph node retrieval and length of postoperative stay
Gastrectomy performed later during the day was not associated with obvious increased odds of lymph node retrieval (Table 4, OR 0.88, 95% CI 0.73 to 1.06 for 09:30–13:29 and OR 1.06, 0.88 to 1.28 for 13:30–21:25) or length of postoperative stay (OR 0.98, 0.81 to 1.17 for 09:30–13:29 and OR 0.83, 0.69 to 1.01 for 13:30–21:25) compared to early starting time (08:00–09:29).
Discussion
This study indicated an increased risk of 3-year all-cause mortality in gastric cancer patients if the surgical starting time was later in the day than in the early morning, particularly in those who underwent laparoscopic gastrectomy and with pathological stage II tumor. The surgical starting time during the day did not influence the lymph node yield or length of postoperative hospital stay.
Some methodological issues need to be discussed in order to interpret the findings. First, it was not feasible to randomly assign the surgical starting time of the day, which left us with an observational design. Second, this study was based on one of the largest cancer centers in China and focused on gastric cancer, thus providing a large sample size and counteracting disease heterogeneity. The single-center high-volume approach also allowed complete and detailed clinical data and at least partly counteracted bias resulting from different surgeon volumes. On the other hand, the results from this single center might be less generalizable. Third, the assessment of the gastrectomy starting time (exposure), 3-year all-cause mortality (main outcome) and covariates was objective and accurate. The surgery day rotation system at the center enabled each consultant surgeon to have similar opportunities to arrange their operation schedule, meaning that factors like age or experience of the surgeon would not influence the surgical starting time. However, due to lack of data on cause-specific death, we could not assess disease-specific mortality. Fourth, potential confounding by the main prognostic factors was carefully adjusted for in the analyses, but residual confounding cannot be ruled out.
To the best of our knowledge, this is the first study examining the role of ‘time of the day’ variations in surgery in relation to long-term survival in gastric cancer. The finding that gastric cancer patients who underwent laparoscopic surgery and those diagnosed with pathological tumor stage II tended to have worse survival if the gastrectomy was started later in the day is interesting. Chance cannot be excluded as an explanation, but the findings may also be true. Speculatively, the workload accumulation during the day might influence the performance of the surgeons and the surgical team21,22. This could be more of an issue for laparoscopic surgery than for open surgery, because laparoscopic procedures tend to be more time-consuming and technically demanding23,24. However, statistically differences were not observed in robotic assisted gastrectomy. We speculated that limited sample size of robotic surgery might mainly account for this finding, which calls for further studies with larger cohort of robotic surgeries to address. A possible explanation for the tumor stage II-specific finding is a that the fine-tuning of the surgical accuracy may be less critical in patients with earlier tumor stage who usually have a very high survival rate anyway, while those with more advanced tumors more often have invisible tumor spread beyond surgical cure25,26. Patients with stage II tumors, on the other hand, may benefit most from the best possible surgical treatment. The absence of better survival in the middle starting group than that the last starting group may speculatively be due to the fact that surgeons did not get a break or any food before starting surgery around lunch time.
Because lymphadenectomy27,28 and postoperative complications and re-operations might influence the long-term survival29,30, we explored the surgical starting time during the day in relation to lymph node retrieval and length of hospital stay. The lack of associations with these outcomes indicate that these factors were not mediators of the worse survival in gastric cancer patients who underwent surgery later in the day.
The findings from this first study examining the topic need confirmation in future research before any clinical implications can be considered. Large population-based studies examining a more detailed grouping of the surgical starting time may be particularly useful in this respect. If proven true, these results indicate a need to tailor the starting time of gastrectomy. Although this study focused on surgery for gastric cancer, it is possible that similar mechanism and results might be generalizable to other challenging surgical cancer procedures, for example, surgery for colorectal, hepatobiliary and pancreatic cancers.
Conclusions
In conclusion, this current study indicated that in patients with gastric cancer, especially those who undergo laparoscopic gastrectomy and those diagnosed with stage II tumors, initiating surgery in the early morning was possibly associated with better prognosis, which still needs further prospective clinical trials to verify.
Data availability
The anonymous data during the current study are available from the corresponding author upon reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Chen, W. et al. Cancer statistics in China, 2015. CA Cancer J. Clin. 66, 115–132. https://doi.org/10.3322/caac.21338 (2016).
Allemani, C. et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385, 977–1010. https://doi.org/10.1016/S0140-6736(14)62038-9 (2015).
Kelz, R. R. et al. Time of day is associated with postoperative morbidity: An analysis of the national surgical quality improvement program data. Ann. Surg. 247, 544–552. https://doi.org/10.1097/SLA.0b013e31815d7434 (2008).
Magid, D. J. et al. Relationship between time of day, day of week, timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment elevation myocardial infarction. JAMA 294, 803–812. https://doi.org/10.1001/jama.294.7.803 (2005).
Uusaro, A., Kari, A. & Ruokonen, E. The effects of ICU admission and discharge times on mortality in Finland. Intensive Care Med. 29, 2144–2148. https://doi.org/10.1007/s00134-003-2035-1 (2003).
Ishiyama, Y. et al. Surgical starting time in the morning versus the afternoon: Propensity score matched analysis of operative outcomes following laparoscopic colectomy for colorectal cancer. Surg. Endosc. 33, 1769–1776. https://doi.org/10.1007/s00464-018-6449-9 (2019).
Slaughter, K. N. et al. Minimally invasive surgery for endometrial cancer: Does operative start time impact surgical and oncologic outcomes. Gynecol. Oncol. 134, 248–252. https://doi.org/10.1016/j.ygyno.2014.06.007 (2014).
Choi, Y. Y. et al. Ten thousand consecutive gastrectomies for gastric cancer: Perspectives of a master surgeon. Yonsei Med. J. 60, 235–242. https://doi.org/10.3349/ymj.2019.60.3.235 (2019).
Kim, E. Y., Song, K. Y. & Lee, J. Does hospital volume really affect the surgical and oncological outcomes of gastric cancer in Korea. J. Gastric Cancer 17, 246–254. https://doi.org/10.5230/jgc.2017.17.e31 (2017).
Brenkman, H. J., Haverkamp, L., Ruurda, J. P. & van Hillegersberg, R. Worldwide practice in gastric cancer surgery. World J. Gastroenterol. 22, 4041–4048. https://doi.org/10.3748/wjg.v22.i15.4041 (2016).
Sessler, D. I., Kurz, A., Saager, L. & Dalton, J. E. Operation timing and 30-day mortality after elective general surgery. Anesth. Analg. 113, 1423–1428. https://doi.org/10.1213/ANE.0b013e3182315a6d (2011).
Aylin, P., Alexandrescu, R., Jen, M. H., Mayer, E. K. & Bottle, A. Day of week of procedure and 30 day mortality for elective surgery: Retrospective analysis of hospital episode statistics. BMJ 346, f2424. https://doi.org/10.1136/bmj.f2424 (2013).
Lagergren, J., Mattsson, F. & Lagergren, P. Weekday of esophageal cancer surgery and its relation to prognosis. Ann. Surg. 263, 1133–1137. https://doi.org/10.1097/SLA.0000000000001324 (2016).
Zare, M. M., Itani, K. M., Schifftner, T. L., Henderson, W. G. & Khuri, S. F. Mortality after nonemergent major surgery performed on Friday versus Monday through Wednesday. Ann. Surg. 246, 866–874. https://doi.org/10.1097/SLA.0b013e3180cc2e60 (2007).
Lagergren, J., Mattsson, F. & Lagergren, P. Weekday of cancer surgery in relation to prognosis. Br. J. Surg. 104, 1735–1743. https://doi.org/10.1002/bjs.10612 (2017).
Gao, Y. et al. Comparison of robotic- and laparoscopic-assisted gastrectomy in advanced gastric cancer: Updated short- and long-term results. Surg. Endosc. 33, 528–534. https://doi.org/10.1007/s00464-018-6327-5 (2019).
Washington, K. 7th edition of the AJCC cancer staging manual: stomach. Ann. Surg. Oncol. 17, 3077–3079. https://doi.org/10.1245/s10434-010-1362-z (2010).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383. https://doi.org/10.1016/0021-9681(87)90171-8 (1987).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 4, e296. https://doi.org/10.1371/journal.pmed.0040296 (2007).
Eastridge, B. J. et al. Effect of sleep deprivation on the performance of simulated laparoscopic surgical skill. Am. J. Surg. 186, 169–174. https://doi.org/10.1016/s0002-9610(03)00183-1 (2003).
Gawande, A. A., Zinner, M. J., Studdert, D. M. & Brennan, T. A. Analysis of errors reported by surgeons at three teaching hospitals. Surgery 133, 614–621. https://doi.org/10.1067/msy.2003.169 (2003).
Berguer, R., Smith, W. D. & Chung, Y. H. Performing laparoscopic surgery is significantly more stressful for the surgeon than open surgery. Surg. Endosc. 15, 1204–1207. https://doi.org/10.1007/s004640080030 (2001).
Lee, H. J. et al. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann. Surg. https://doi.org/10.1097/SLA.0000000000003217 (2019).
Nashimoto, A. et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer 16, 1–27. https://doi.org/10.1007/s10120-012-0163-4 (2013).
Sano, T. et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 20, 217–225. https://doi.org/10.1007/s10120-016-0601-9 (2017).
Ajani, J. A. et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 14, 1286–1312 (2016).
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14, 101–112. https://doi.org/10.1007/s10120-011-0041-5 (2011).
Gertsen, E. C. et al. Identification of the clinically most relevant postoperative complications after gastrectomy: a population-based cohort study. Gastric Cancer https://doi.org/10.1007/s10120-019-00997-x (2019).
Raziee, H. R. et al. Systematic review of the predictors of positive margins in gastric cancer surgery and the effect on survival. Gastric Cancer 15(Suppl 1), S116-124. https://doi.org/10.1007/s10120-011-0112-7 (2012).
Acknowledgements
The authors wish to thank the clinical follow-up team, consisting of Drs. Baohuang Wang, Shaoqin Li, Chuang Wang, Ziwei Zhuang, Bo Cao, Yi Liu, Pengpeng Wang, Wang Zhang, Yixun Lu and Tianyu Xie, for their contribution to their routine follow-up of the patients and the collection of survival data.
Funding
Open access funding provided by Karolinska Institute. This study was supported by the Swedish Research Council (340-2013-5478), National Natural Science Foundation of China (81972790) and Beijing Nova Program (Z181100006218011).
Author information
Authors and Affiliations
Contributions
S.-H.X. and Y.G. conceived and designed the Study. Y.G, H.X. and W.L. acquired and cleaned the Data. Y.G. and F.M. performed the statistical analysis. Y.G. L.C. and S.-H.X. drafted the manuscript preparation. J.L., Y.G. and S.-H.X. revised and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, Y., Xi, H., Mattsson, F. et al. Surgical starting time of the day and survival in gastric cancer. Sci Rep 13, 6955 (2023). https://doi.org/10.1038/s41598-023-33692-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33692-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.