Abstract
Patients with early gastric cancer (EGC) who undergo non-curative endoscopic resection (ER) require additional surgery. The aim of the study was to validate surgical and oncological outcomes according to the timing of additional surgery after non-curative endoscopic resection. We retrospectively analyzed long-term follow-up data on the 302 patients enrolled between January 2007 and December 2014. We validated our earlier suggestion that the optimal time interval from non-curative ER to additional surgery was 29 days. All patients were divided into two groups by reference to time intervals from ER to additional surgery of ≤29days (n = 133; group A) and >29 days (n = 169; group B). The median follow-up duration was 41.98 ± 21.23 months. As in our previous study, group B exhibited better surgical outcomes. A total of 10 patients developed locoregional or distant recurrences during the follow-up period, but no significant difference was evident between the two groups. Interestingly, the survival rate was better in group B. Group B (>29 days) exhibited better surgical and oncological outcomes. Thus, additional gastrectomy after non-curative ER should be delayed for 1 month to ensure optimal surgical and oncological outcomes.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is a major health problem worldwide, with an estimated 1 million new cases per year1. Early gastric cancer (EGC) is defined as a lesion confined to the mucosa or the submucosa, regardless of the presence of regional lymph node metastasis (LNM), and some of them can be cured via endoscopic resection (ER)2,3,4. ER for EGC plays a central role in the treatment of EGC, and due to the development of the endoscopic technology and instrument, extended criteria are being applied beyond the absolute indication for ER, nowadays5,6,7. Although ER has the advantage of preserving the stomach, is minimally invasive, and affords a better quality of life than open surgery, ER sometimes fails to completely remove a lesion8. The rates of non-curative ER range from 15.3 to 16.7%9,10,11. Patients with non-curative ER typically require additional treatment such as re-ER, ablation therapy, and/or gastrectomy12,13,14. However, additional surgical gastrectomy with lymph node dissection is generally recommended after non-curative ER because of the possibility of LNM and the unfavorable prognosis7,13,15,16. However, the optimal timing of additional surgery after non-curative ER remains unclear. In our previous study, we evaluated the time interval between ER and additional surgery in terms of surgical and oncological outcomes17. We found that the interval affected the surgical outcomes17.
ER-associated electrocoagulation creates a large iatrogenic ulcer requiring 4–8 weeks for complete healing. Also, ER may cause edema, fibrosis, and even adhesions of both the stomach and surrounding tissues, which may be increasing the surgical difficulties during subsequent gastrectomy18,19. However, waiting for the healing of edema or ulceration after ER in cancer patients may allow for tumor to grow and increase the risk of recurrence. Another essential aspect that must also be taken to account is the potential impact of treatment delay on patient anxiety. Although patients know that it is necessary to wait for a treatment, it is experienced as a suffering time of anxiety and fear. Many surgeons plan the timing of operation after non-curative ER by reference to their surgical schedules or other subjective factors. It is important to objectively evaluate the optimal effect of the time interval of additional gastrectomy after ER on surgical outcome.
Our previous study suggested an optimal time interval between non-curative ER and additional surgery; however, the work had certain limitations, including a relatively small number of patients and only short-term follow-up. We thus could not confirm the oncological outcomes. Here, we analyze long-term follow-up data to validate the surgical outcomes and evaluate the oncological outcomes associated with our previously proposed optimal time interval from non-curative ER to additional surgery.
Results
Clinicopathological characteristics and surgical outcomes
Baseline clinicopathological characteristics and surgical outcomes are shown in Table 1. The mean patient age was 60.53 (±12.23) years. ER was non-curative in a total of 85 patients (28.1%) for at least two reasons, including lateral or vertical margin involvement combined with LVI. Most patients underwent laparoscopic gastrectomy and the mean operative time was 188.97 ± 73.96 min. After gastrectomy, 44 (14.6%) patients had residual cancer and 26 (9.5%) LNM. In our previous short-term follow up study, there are 16.2% residual cancer and 9.7% LNM17. A total of 145 (48%) patients developed postoperative complications and the incidence of major complications was 5.3%. The mean follow-up period after surgery was 41.98 ± 21.23 months, which is longer than 26.7 ± 16.4 months of the previous study.
The relationship between time interval and major complications
To validate the perioperative safety of patients who underwent additional surgery, we analyzed the relationship between the time from ER to additional surgery and the major complications by drawing a receiver-operating characteristic (ROC) curve. The area under the curve (AUC) was 0.579 (95% CI, 0.521–0.635), associated with a sensitivity of 50% and a specificity of 56.7% (Supplemental Fig. 1). These results suggest that the time interval of additional surgery did not affect the incidence of major complications.
The optimal time interval between ER and additional surgery
We sought correlations between the time to additional surgery and the difficulty of that surgery. The raw data on the time from ER to surgery, and the operative time and EBL, are plotted in Supplemental Fig. 2. The operative time and the EBL decreased significantly as the time interval increased (r = −0292, P < 0.001, and r = −0.135, P = 0.019, respectively).
We next performed one-way MANOVA to validate the optimal time interval (29 days) between non-curative ER and surgery identified in our previous study. In our previous study, we analyzed the correlation between the time interval of additive surgery and the difficulty of surgery by using one- way MANOVA. The time interval point, at which the operative time and the EBL of the earlier operation group and the later operation group showed the greatest disparities. This difference was most pronounced at day 2917. In this study, differences between the early and later surgical group were evident over the time interval (25–35 days). The differences were greatest on day 29, at which time the slopes of the graphs changed direction. We thus confirmed that 29 days was the appropriate cut-off point. No significant between-group difference was apparent from after day 36 (Fig. 1, Supplemental Fig. 3).
The time intervals since endoscopic resection (days) associated with the greatest differences between the amounts of blood loss and the operative times in the early and later groups were evaluated with the aid of the MANOVA test. The relationship between the time elapsed since endoscopic resection and a combination of operative time and the amount of intraoperative blood loss. The greatest difference and the change of the slope of the graph was around day 29 (the cut-off point), and no significant difference was evident from after day 36.
Short-term surgical outcomes of both groups
Table 2 compares the two groups by time to surgery after ER. Of the 302 patients, 133 (44.0%) were in the earlier operation group (≤29 days; group A), and 169 (56%) in the later operation group (>29 days; group B). In our previous study, there are 78 (50.6%) were categorized as group A, and 76 (49.4%) as group B17. The percentage of patients in group B was more than in our previous study. The two groups did not differ significantly in terms of any of age, sex, body mass index, comorbidities except for tumor size, or ER specimen size. In the later operation group, the operative time, the EBL, the number of perioperative transfusions, the time to drain removal, the drainage volume on postoperative day (POD) 1, the maximal postoperative C-reactive protein (CRP) level, and the duration of postoperative hospital stay were all better than that of the early operation group. The groups did not differ in terms of the numbers of overall or major postoperative complications, or locoregional or distant recurrences.
Supplemental Fig. 4 shows the data on perioperative surgical outcomes, including the CRP levels, the white blood cell (WBC) counts, and drainage volumes. There was no significant between-group difference except in terms of the POD 1 drainage volume, as mentioned above.
Long-term oncological outcomes of both groups
The median follow-up times after surgery were 37.02 ± 20.54 and 44.18 ± 19.49 months in the early operation and later operation groups, respectively (P = 0.002). During the follow-up period, six patients of the early operation group and four of the later operation group experienced recurrences. In the early operation group, distant metastasis occurred in four patients and locoregional recurrence in two. The former four patients died; their characteristics are described in Supplementary Table 1. The median survival time was estimated to be 58.6 months in the Group A (≤29days) and 62.7 months in the Group B (>29days). In the all patients, the median survival time was 61.8 months. The 5-year survival rates were as follows: Group A (≤29days): 92%, Group B (>29days): 99%. There was no significant difference in the 5-year survival rate of two groups.
The recurrence rates did not differ significantly between the groups. Interestingly, the survival rate was better in the later operation group than the early group (Fig. 2). Of factors possibly affecting recurrence and survival, LNM was significant in both univariate and multivariate analyses (Tables 3 and 4; Supplementary Fig. 5).
Kaplan–Meier plots of overall recurrence and survival. (A) The recurrence-free rate curve of those for whom the interval between endoscopic resection (ER) and additional surgery was 29 days. (B) The survival rate curve of such patients. The recurrence-free rate did not differ after the later operation, but the survival rate did.
Discussion
In this long-term follow-up study, we found that additional surgery performed about 1 month after ER afforded better short-term surgical outcomes and long-term oncological safety. Minimally invasive surgery for the treatment of EGC has increased in popularity, and many reports have addressed learning curve effects in terms of laparoscopy-assisted gastrectomy; operative time, extent of intraoperative bleeding, and postoperative complication rate fell with increasing surgical experience20,21,22. Another study found that a longer operation time, more bleeding, and more frequent transfusion were all associated with more challenging and difficult operations23. We evaluated perioperative patient safety using the Clavien–Dindo system; such safety is the highest priority when planning surgery24,25,26. We found no significant relationship between the major complication rate and the time to surgery after ER. This means that there is no relationship between the additional surgery time interval after ER and the important complications that may occur in the patient.
Second to surgical complications, the operative time, and EBL would be applied as next endpoints to estimate operative feasibility. In these respects, we evaluated the association between time interval of additioanl surgery after non-curative ER and the two factors, operative time and EBL. As shown in the results, these parameters tended to decrease as the time interval increased. In our previous study, we used the MANOVA test to define the time from ER at which the later operative time and the EBL differed maximally between an early and later operation group; we employed the same method here. The greatest differences (reflected by changes in the slopes of the graphs) were evident 29 days after ER. Many studies have reported that ER-induced ulcers are usually in the healing or scarring stage 4–8 weeks after ER27,28,29,30. ER-induced inflammation and the lack of ulcer healing may render early operation (within 4 weeks) more difficult than later operation. Also, the postoperative hospital stay was significantly shorter in the later operative group. In recent years, the length of stay has been emphasized not only as an indicator of healthcare costs, but also because it is closely related to complications associated with surgery, and surgical outcomes31,32,33. Between-group differences were evident in terms of the WBC counts, CRP levels, and drainage volumes; these are all markers of surgical trauma34,35. Therefore, our long-term follow-up study validated our earlier suggestion that the optimal time interval from ER to additional surgery was about 1 month.
We performed subgroup analysis by surgical experience (Supplementary Table 2). We defined a group of experienced surgeons in previous studies as surgeons with more than five years of experience in gastric surgery. Group A was 46.6% (62/133) and Group B was 65.7% (111/169). Because the experienced surgeon rate of the earlier operation group (≤29days; group A) is lower than the later operation group (>29 days; group B), we performed subgroup analysis for patients in the experienced surgeon group in order to correct the surgeon specific variable factor. On subgroup analysis in the experienced surgeon group, the operative time and postoperative hospital stay of patients in the early operation group were significantly longer than in the later group. In the late group, the EBL was significantly lower than in the early operation group. But there is no difference recurrence between two groups.
In this long-term follow-up study, we also evaluated oncological outcomes in terms of the optimal timing of surgery. As mentioned above, during follow-up, 10 patients experienced locoregional or distant recurrences, of whom 6 were in the early and 4 in the later operation group. The recurrence incidence did not differ between the two groups, but the survival rate did; more patients operated upon early rather than late developed LNM, suggesting that the biological behavior of the cancer was prognostically more important than the time between ER and surgery. Recently, the requirement for perioperative blood transfusion and the extent of intraoperative blood loss have been suggested to be potentially (negatively) prognostic in terms of long-term outcomes36,37,38. This may be why the survival rate of our early operation group was poorer than that of the later group.
The limitations of our study include the retrospective design of the work and the inclusion of patients treated in only two tertiary centers. Additional prospective multicenter studies are needed to validate our findings. However, we validated our earlier study on the optimal timing of additional gastrectomy after non-curative ER, and our work may be of assistance to other surgeons.
In conclusion, we suggest that the interval between non-curative ER and additional gastrectomy should be about 1 month. This was associated with better surgical outcomes and oncological safety than earlier surgery.
Methods
Study population
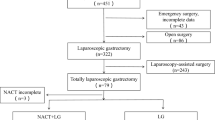
We retrospectively collected data on patients diagnosed with EGC who underwent ER at the Severance and Gangnam Severance Hospitals, Yonsei University College of Medicine, Seoul, Korea, between January 2007 and December 2014. A total of 2,743 such patients were enrolled. Of these, 330 (12.0%) underwent non-curative ER as revealed histologically, and therefore required additional surgery. We excluded patients with any other malignancies, those who underwent combined operations, those who underwent emergency operations to treat ER complications (such as perforation or bleeding), and those who died because of other malignancies. We performed long-term follow-up on 154 patients enrolled in our previous study and a further 148 patients. Thus, in total, we analyzed 302 patients who underwent additional gastrectomy after non-curative ER.
The indications for ER included the expanded criteria: (1) a differentiated intramucosal adenocarcinoma ≤3 cm in diameter, without lymphovascular invasion (LVI), irrespective of ulceration status; (2) a differentiated intramucosal adenocarcinoma without LVI or ulceration, irrespective of tumor diameter; (3) an undifferentiated intramucosal cancer ≤2 cm in diameter, without LVI or ulceration; and, (4) a differentiated adenocarcinoma ≤ 3 cm in diameter exhibiting minimal submucosal invasion, without LVI7,39. EGC patients who underwent non-curative ER included those with incomplete margin resections, or LVI, or who otherwise did not fall within (exceeded) the expanded ER criteria39.
We analyzed clinicopathological characteristics, comorbidities, American Society of Anesthesiologists (ASA) scores, surgical outcomes (operative time and estimated intraoperative blood loss [EBL]), postoperative complications, and oncological outcomes (locoregional and distant recurrences). Postoperative complications were graded using the Clavien–Dindo classification; complications of grade ≥III were defined as ‘major,’ being potentially life-threatening25,40.
The patients were divided into two groups depending on the time interval (a cutoff between 1 and 60 days; please see below) between ER and surgery. Then, the surgical outcomes of the earlier operation group (group A) and the later operation group (group B) were compared to identify the optimal time interval from non-curative ER to surgery.
Informed consent was obtained from all patients before the procedures. This study was approved by the Institutional Review Board of Yonsei University College of Medicine and was conducted in accordance with the ethical principles of the Declaration of Helsinki (IRB No. 3-2018-0022).
Statistical analysis
Categorical variables are presented as numbers with percentages and were compared using the chi- squared or Fisher’s exact test. Continuous variables are presented as means ± standard deviations and were compared with the aid of Student’s t-test. A P value <0.05 was considered to reflect statistical significance. The relationship between the time interval from ER to surgery, and major complications, was evaluated by calculating the area under the receiver operating characteristic (AUROC) curve. Multivariate analyses of variance (MANOVAs) were used to explore the effects of the time interval between non-curative ER and additional surgery on the later operative time and the EBL. When data points lay >1.5-fold of the interquartile range (IQR) above the third or below the first quartile (outliers), we treated them as missing when calculating operative times and EBLs. The recurrence-free and overall survival rates of the two groups were calculated using the Kaplan–Meier method. To identify risk factors for recurrence and survival after additional surgery, we performed both univariate and multivariate logistic regression analyses. Cox’s regression hazard model was used for the multivariable analysis. We included only those variables that exhibited P values <0.1 on univariate analysis in the multivariable analysis. All statistical calculations were performed with the aid of SPSS version 23.0 (SPSS Inc., Chicago, IL, USA) and SAS MANOVA version 9.3 (SAS Inc., Cary, NC, USA).
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136, E359–386, https://doi.org/10.1002/ijc.29210 (2015).
Saragoni, L. et al. Early gastric cancer: diagnosis, staging, and clinical impact. Evaluation of 530 patients. New elements for an updated definition and classification. Gastric Cancer 16, 549–554, https://doi.org/10.1007/s10120-013-0233-2 (2013).
Seto, Y. et al. Lymph node metastasis and preoperative diagnosis of depth of invasion in early gastric cancer. Gastric Cancer 4, 34–38, https://doi.org/10.1007/s101200100014 (2001).
Ono, H. et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48, 225–229 (2001).
Kim, Y. I. et al. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy 47, 293–301, https://doi.org/10.1055/s-0034-1391284 (2015).
Soetikno, R., Kaltenbach, T., Yeh, R. & Gotoda, T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol 23, 4490–4498, https://doi.org/10.1200/JCO.2005.19.935 (2005).
Gotoda, T. et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3, 219–225 (2000).
Korenaga, D. et al. Pathological appearance of the stomach after endoscopic mucosal resection for early gastric cancer. Br J Surg 84, 1563–1566 (1997).
Choi, M. K. et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc 27, 4250–4258, https://doi.org/10.1007/s00464-013-3030-4 (2013).
Nagano, H. et al. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer 8, 149–154, https://doi.org/10.1007/s10120-005-0328-5 (2005).
Jung, H. et al. Surgical outcome after incomplete endoscopic submucosal dissection of gastric cancer. Br J Surg 98, 73–78, https://doi.org/10.1002/bjs.7274 (2011).
Yokoi, C., Gotoda, T., Hamanaka, H. & Oda, I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc 64, 212–218, https://doi.org/10.1016/j.gie.2005.10.038 (2006).
Oda, I. et al. Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg 95, 1495–1500, https://doi.org/10.1002/bjs.6305 (2008).
Kitamura, T., Tanabe, S., Koizumi, W., Mitomi, H. & Saigenji, K. Argon plasma coagulation for early gastric cancer: technique and outcome. Gastrointest Endosc 63, 48–54, https://doi.org/10.1016/j.gie.2005.08.009 (2006).
Ryu, K. W. et al. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol 14, 3428–3434, https://doi.org/10.1245/s10434-007-9536-z (2007).
Song, K. Y. et al. Is gastrectomy mandatory for all residual or recurrent gastric cancer following endoscopic resection? A large-scale Korean multi-center study. J Surg Oncol 98, 6–10, https://doi.org/10.1002/jso.21074 (2008).
Kim, M. J. et al. Is there an optimal surgery time after endoscopic resection in early gastric cancer? Ann Surg Oncol 21, 232–239, https://doi.org/10.1245/s10434-013-3299-5 (2014).
Jiang, X. et al. Laparoscopy-assisted gastrectomy in patients with previous endoscopic resection for early gastric cancer. Br J Surg 98, 385–390, https://doi.org/10.1002/bjs.7358 (2011).
Tanaka, M., Ono, H., Hasuike, N. & Takizawa, K. Endoscopic submucosal dissection of early gastric cancer. Digestion 77(Suppl 1), 23–28, https://doi.org/10.1159/000111484 (2008).
Jin, S. H. et al. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc 21, 28–33, https://doi.org/10.1007/s00464-005-0634-3 (2007).
Kunisaki, C. et al. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg Laparosc Endosc Percutan Tech 18, 236–241, https://doi.org/10.1097/SLE.0b013e31816aa13f (2008).
Lee, S. I. et al. Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg 202, 874–880, https://doi.org/10.1016/j.jamcollsurg.2006.02.028 (2006).
An, J. Y. et al. Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: their risk factors and clinical relevance. Ann Surg Oncol 19, 2452–2458, https://doi.org/10.1245/s10434-012-2267-9 (2012).
Mazeh, H. et al. Application of a novel severity grading system for surgical complications after colorectal resection. J Am Coll Surg 208, 355–361, https://doi.org/10.1016/j.jamcollsurg.2008.12.008 (2009).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240, 205–213 (2004).
Clavien, P. A. et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250, 187–196, https://doi.org/10.1097/SLA.0b013e3181b13ca2 (2009).
Lee, S. Y. et al. Healing rate of EMR-induced ulcer in relation to the duration of treatment with omeprazole. Gastrointest Endosc 60, 213–217 (2004).
Tomita, T. et al. Prospective randomized controlled trial to compare the effects of omeprazole and famotidine in preventing delayed bleeding and promoting ulcer healing after endoscopic submucosal dissection. J Gastroenterol Hepatol 27, 1441–1446, https://doi.org/10.1111/j.1440-1746.2012.07144.x (2012).
Ohya, T. R. et al. A prospective randomized trial of lafutidine vs rabeprazole on post-ESD gastric ulcers. World J Gastrointest Endosc 2, 36–40, https://doi.org/10.4253/wjge.v2.i1.36 (2010).
Goto, O. et al. Short-term healing process of artificial ulcers after gastric endoscopic submucosal dissection. Gut Liver 5, 293–297, https://doi.org/10.5009/gnl.2011.5.3.293 (2011).
Correia, M. I. & Waitzberg, D. L. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 22, 235–239 (2003).
Taheri, P. A., Butz, D. A. & Greenfield, L. J. Length of stay has minimal impact on the cost of hospital admission. J Am Coll Surg 191, 123–130 (2000).
Macario, A., Vitez, T. S., Dunn, B., McDonald, T. & Brown, B. Hospital costs and severity of illness in three types of elective surgery. Anesthesiology 86, 92–100 (1997).
Christensen, M. B., Eriksen, T. & Kjelgaard-Hansen, M. C-reactive protein: quantitative marker of surgical trauma and post-surgical complications in dogs: a systematic review. Acta Vet Scand 57, 71, https://doi.org/10.1186/s13028-015-0164-5 (2015).
Warschkow, R. et al. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg 256, 245–250, https://doi.org/10.1097/SLA.0b013e31825b60f0 (2012).
Hyung, W. J. et al. Adverse effects of perioperative transfusion on patients with stage III and IV gastric cancer. Ann Surg Oncol 9, 5–12 (2002).
Kamei, T., Kitayama, J., Yamashita, H. & Nagawa, H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg 33, 1240–1246, https://doi.org/10.1007/s00268-009-9979-4 (2009).
Liang, Y. X. et al. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J Gastroenterol 19, 5542–5550, https://doi.org/10.3748/wjg.v19.i33.5542 (2013).
Japanese Gastric Cancer, A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20, 1–19, https://doi.org/10.1007/s10120-016-0622-4 (2017).
Lee, J. H., Park, D. J., Kim, H. H., Lee, H. J. & Yang, H. K. Comparison of complications after laparoscopy-assisted distal gastrectomy and open distal gastrectomy for gastric cancer using the Clavien-Dindo classification. Surg Endosc 26, 1287–1295, https://doi.org/10.1007/s00464-011-2027-0 (2012).
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant no. 2015R1C1A2A01053924).
Author information
Authors and Affiliations
Contributions
C.J.H. substantial contributions to conception and design, or analysis and interpretation of data and drafting the article or revising it critically for important content; J.K. and H.K. substantial contribution to conception and design, final approval of the version to be published and agreement to be accountable for all aspects of the work; D.H.J., J.J.P., Y.H.Y., H. P., S.H.C., J.C., W.J.H. and S.H.N. revising the article critically for important intellectual content; All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41598_2019_54778_MOESM1_ESM.docx
Supplementary Table 1. The characteristics of patients with distant recurrence in Group A (≤ 29days) and Group B (>29days)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cha, J.H., Kim, JH., Kim, HI. et al. The optimal timing of additional surgery after non-curative endoscopic resection to treat early gastric cancer: long-term follow-up study. Sci Rep 9, 18331 (2019). https://doi.org/10.1038/s41598-019-54778-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54778-8
This article is cited by
-
Short-term efficacy of additional laparoscopic-assisted radical gastrectomy after non-curative endoscopic submucosal dissection for early gastric cancer
Langenbeck's Archives of Surgery (2023)
-
Effect of endoscopic resection on short-term surgical outcomes of subsequent laparoscopic gastrectomy: a meta-analysis
World Journal of Surgical Oncology (2021)
-
Gastrointestinal Malignancies and the COVID-19 Pandemic: Evidence-Based Triage to Surgery
Journal of Gastrointestinal Surgery (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.