Abstract
Mono-cropping of maize–wheat, mechanical disintegration of soils, and continuous chemical fertilization have deteriorated soil health in the Indo-Gangetic Plains. We studied the long-term impact of pulse-based cropping systems with integrated nutrient management on soil physical and chemical properties and yield sustainability. We evaluated four different cropping systems: (1) maize–wheat (M–W), (2) maize–wheat–mungbean (M–W–Mb), (3) maize–wheat–maize–chickpea (M–W–M–C), (4) pigeonpea–wheat (P–W) each with three degrees of soil fertilization techniques: (1) unfertilized control (CT), (2) inorganic fertilization (RDF), and (3) integrated nutrient management (INM). The field experiment was undertaken in a split-plot design with three replications each year with a fixed layout. P–W and M–W–Mb systems enhanced soil properties such as volume expansion by 9–25% and porosity by 7–9% (p < 0.05) more than M–W, respectively. P–W and M–W–Mb increased soil organic carbon by 25–42% and 12–50% over M–W (RDF). P–W system enhanced water holding capacity and gravimetric moisture content by 10 and 11% (p < 0.05) than M–W. Pulse-based systems (P–W and M–W–Mb) had higher available nitrogen (8–11%), phosphorus (42–73%), and potassium (8–12%) over M–W (p < 0.05). M–W–Mb increased 26% maize yield and 21% wheat yield over M–W (p < 0.05) at the thirteenth crop cycle. P–W system had a higher sustainable yield index (p < 0.05) of wheat over the M–W. Thus, pulse inclusion in the cropping system in combination with INM can enhance physical and chemical properties vis-à-vis sustainable yield index over the cereal-cereal system.
Similar content being viewed by others
Introduction
Soil physical properties are a significant part of the soil system working and empower to assess the ecosystem sustainability1, 2. The physical properties of soil as bulk density, soil structure, and water-holding capacity are signs of good soil well-being in the long-run3. The water-holding capacity of soil is regulated by: (1) pore size distribution, (2) surface area of soil, and (3) aggregate stability. Fundamentally, soil aggregates (macro–and micro–aggregates) minimize organic matter mineralization, sequestering soil organic carbon (SOC) and nutrients4. Theoretically, increased water-holding capacity, soil moisture content, and aggregate stability are indicators of the positive impact of crop diversification and balanced fertilization on soil health1, 5. Soil aggregated nutrients strongly influenced by crop management practices that include tillage/mechanical disruption, crop rotation, and fertilization techniques6. Rice–wheat (9.64 m ha area) and maize–wheat (1.83 m ha area) are the two dominant cropping systems in the Indo-Gangetic plains (IGP)6, 7. These cropping systems reduced crop productivity and soil ecology because of SOC depletion, macro- and micro-nutrient deficiency, the decline in soil microbial/biological properties, and groundwater table depletion8, 9. Pulse crops in the cropping system might reduce soil health deterioration and increase yield sustainability9. A long-term study indicated that the chickpea yield was more sustainable than the maize10. Therefore, it is necessary to evaluate the long-term impact of pulses in cropping systems on soil physical and chemical properties and crop yields in the IGP.
Soil water holding capacity has a significant role in the soil–plant–atmospheric water balance. Water holding capacity is regulated by various soil physico-chemical and biological indices11. However, the effects of soil factors on water-holding capacity cannot be generalized across regions. For example, Khaleel et al. saw that 80% of fluctuation in water-holding capacity was because of texture and carbon12. While a meta-analysis study reported a mean increase of only 1.2% volumetric available water capacity with a 1% mass increase in soil organic carbon11. However, these studies stated a definite impact of soil carbon on water-holding capacity. Hence, the management-induced changes in carbon content eventually change the water-holding capacity and crop productivity over time. Evaluation of crop rotation with diverse crop phenology as rooting behavior of cereals, pulses, and biomass accumulation is of great value to designing a sustainable cropping intensification13. Previous studies reported the beneficial effects of pulse crops on biological nitrogen fixation and carbon dynamics4, 14, 15. The added residues of pulse crops provide macro- and micro-nutrients to the soil that enhance soil fertility, improve soil aggregates, and encourage adaptive capacity of plants to adverse environments16. For instance, an eight-year study from Alberta in Canada stated that pulse-based rotation (pea-wheat) increased SOC from a baseline level of 10.3–11.2 g kg−1 which was higher than wheat monoculture17. Also, a pulse-based intensive cropping system increases biomass return into the soil and carbon sequestration18. Despite increments in SOC under pulse-based cropping systems, but long-term impacts of pulses in cropping systems on soil physical and chemical functions are less reported in the subtropical climate.
Nutrient management practices influence soil health by altering physical and chemical properties of soil in the long-run. Hence, inappropriate nutrient management results in decline in crop productivity and deterioration in soil health19. Organic amendments retain soil moisture, increasing soil infiltration and favoring crop growth and yield20. Besides, the addition of organic amendments (farmyard manure + crop residues) in soil not only proliferates the microbial density in rhizospheric soil but also enhances the microbial diversity in rhizospheric soil. Thus, it can support soil structure-building processes through favorable interaction between soil aggregate and organic matter21. Results from Sanborn University of Missouri (Columbia) after 100 years of corn-wheat-red clover rotation depicted that manure addition decreased bulk density (by 0.12 g cm−3) and increased saturated hydraulic conductivity (by 9 times) than unfertilized control22. A long-term study of 5 years from vertisol of India indicated that integrated nutrient management (INM) comprising chemical fertilizers and farm-yard manure (5 Mg ha−1) increased 20.9% and 13.1% mean grain yield of maize and chickpea, respectively over chemical fertilization10. An 18 years study with a rice–wheat cropping system in a Typic Haplustept of Indian IGP deciphered that INM (chemical fertilizers + farm-yard manure + crop residues) enhanced soil aggregation, aggregate-associated carbon, and carbon stock23. Mean weight diameter, commonly used to express aggregate stability, was higher in manure application than in control treatments24. An improved understanding of soil physical and chemical properties and yields of crops under different cereal-pulse rotations with fertilization techniques will help to intensify cropping systems with pulse crops in a sustainable manner10. However, the impact of pulses and organic amendments in systems on bulk density and associated soil physical properties and crop productivity is less studied in the subtropical IGP1.
In this regard, long–term experiments provide valuable information on management-induced alteration of physical and chemical properties of soil25. Maize–wheat–red clover significantly increased soil strength, aggregate stability, and yields than wheat–maize rotation in Missouri (Columbia)22. Effects of cropping system/fertilizer management on soil physical properties are affected by soil type, antecedent soil properties, climate, land use, and soil structure formation process20. Pulse crops provide annual input of biological N fixation at ~ 3 Tg enhancing the SOC concentration and overall soil health26. It is necessary to assess the regional-specific soil properties under variable crop management for yield sustainability. The degraded cultivated soils must be urgently restored in IGP using sustainable practices and adapted soil management strategies. Therefore, an integrated assessment of soil quality and crop yield is needed for crop management practices for agricultural sustainability in the region.
Therefore, the present study focused on two objectives: (1) to evaluate the long-term impact of pulses in the cropping system and fertilization techniques on soil physical and chemical properties and yields of component crop/system, (2) to determine the effects of pulse-based cropping systems with INM on soil quality as indicated by water holding capacity, moisture content, bulk density, water-filled pore space, aggregated properties (aggregates fraction and aggregated N and P content), SOC and their relations with crop yield. We hypothesized that soil properties and crop productivity improved in pulse-based cropping systems with INM compared with maize–wheat monoculture and chemical fertilization.
Materials and methods
Site characteristics
The present long-term experiment started in 2003 at the ICAR-Indian Institute of Pulses Research, Kanpur (26° 27′ N latitude and 80° 14′ E longitude), India. The trial region belongs to subtropical climate having a mean yearly air temperature of 26.0 °C. Mean annual rainfall was around 722 mm during 2004–2017. The least precipitation (510 mm) was in 2015, and the maximum (1225 mm) was in 2013. The soil order of the study site is Inceptisol (Typic Ustochrept). Soil properties (sandy-loam texture) at the start of the experiment at 0–15 cm depth included: pH 8.1 (soil-to-water proportion of 1:2.5), 2.8 g kg−1 SOC, 182 kg ha−1 available nitrogen (N), 18.6 kg ha−1 phosphorus (P) and 159 kg ha−1 potassium (K).
Treatment details and layout
The experimental design was split-plot with three replications every year. The experiment consisted of four cropping systems (main plot): maize–wheat (M–W), maize–wheat–mungbean (M–W–Mb), maize–wheat–maize–chickpea (M–W–M–C) and pigeonpea–wheat (P–W) and three soil fertilization techniques (subplot): no-fertilizer application (CT), recommended chemical fertilizers of the region (RDF), and integrated nutrient management [INM: 50% fertilizer dose of RDF + full crop residues + farmyard manure 5 t ha−1 + biofertilizers]14. Each subplot size was 49 m2 (7 m × 7 m). Thus, we studied total number of 36 plots (3 replications × 4 different crop rotations × 3 fertilization treatments = 36). Farmyard manure was mixed uniformly during land preparation (with tillage) 2 weeks before sowing during the rainy season. The farmyard manure contained 0.56% nitrogen, 0.18% phosphorus, and 0.52% potassium. Biofertilizers (Azotobacter for maize and wheat, Rhizobium for pigeonpea, chickpea, mungbean, and phosphate solubilizing bacteria Bacillus polymyxa for all crops) were applied (107 bacteria g−1 culture) through seed treatment at the time of sowing. Fertilizer doses were 120:60:40 kg ha−1 N:P2O5:K2O (maize and wheat) and 20:60:40 kg ha−1 of N:P2O5:K2O (for pulses) in RDF. Fertilizers were applied in three splits with the one-third amount of N (through urea), the full rate of P (through diammonium phosphate), and K (through muriate of potash) at sowing and remaining at 25 and 45 days after sowing (DAS) in cereals. All fertilizers were applied during sowing in pulse crops.
Crop management
The cultivars were ‘Azad Uttam’ for maize, ‘UPAS 120’ for pigeonpea, ‘PBW 343’ for wheat, ‘DCP 92-3’ for chickpea, and ‘Samrat’ for mungbean. The seasons consisted of June to October as the rainy (maize and pigeonpea), November to March as the winter (wheat and chickpea), and April to June as the summer (mungbean). The seed rates included 20 kg ha−1 for maize, 15 kg ha−1 for pigeonpea, 100 kg ha−1 for wheat, 80 kg ha−1 for chickpea, and 12 kg ha−1 for mungbean. The required irrigations were two for maize/chickpea/pigeonpea, five for wheat, and four for mungbean. On average (average of 14 years), the applied amount of irrigation water was 525 mm in maize, 380 mm in wheat, 225 mm in pigeonpea and chickpea, and 256 mm in mungbean, irrespective of treatments.
The yield data of all component crops in each system was presented for four cropping cycles (2013–2014 to 2016–2017). The yields of 4 years represent the 11th–14th cycles of experimentation. Wheat was the common crop in each rotation in the present study. Hence, we used base–crop (wheat) productivity and sustainable yield index as indicative for the assessment of soil health27. A net plot area of 5 m × 5 m was manually harvested for seed yield estimation in each crop and expressed as t ha−1 at 14% moisture.
Soil sampling, processing and analysis
The soil was collected after 14 years (April 2017) at the harvest of the wheat crop because wheat was the base crop for all systems. Soil samples were collected at the same time for the present study. The requirement of soil sampling and processing differed for various parameters under study. Accordingly, we elaborated the sampling procedure subsequently. For example, the soil was collected from six sites in each plot (~ treatment) from each replication at two depths (0–20 cm and 20–40 cm). Soil sampling was performed with a post-hole auger (having a sharp edge at the end to open the pit at the sampling site) with a core height of 20 cm for analysis of physical indices. A composite sample was prepared by mixing the collected soil from each plot4. We analyzed 36 samples for each parameter based on the design of the experiment (4 cropping systems × 3 fertilization techniques × 3 replications) for affirming the exactness of the results. The composite soil was separated into two sub-sets. One sub-set was air-dried for 72 h and passed through a 2.0 mm sieve and oven-dried at 105 °C for 24 h for examination of soil physical and chemical properties. Another sub-set (field-moist soil) was sieved with a 3 mm screen and kept in packed plastic bags at 4 °C for soil biological properties assessment. It was analyzed within seven days of sampling.
Bulk density, specific volume and total porosity
Soil sampling was performed with three undisturbed soil cores at two depths (0–20 cm and 20–40 cm). A core sampler with a core height of 12.6 cm and a 2.45 cm radius was used for dry bulk density estimation with the method described by Veihmeyer and Hendrickson28. The core was inserted into the soil with a hammer for sampling without disturbing the soil block. The sampled soil blocks were trimmed to the precise rim/volume of the core and oven-dried at 105 °C for 24 h28. A particle density value of 2.65 g cm−3 was considered for porosity calculation28. Dry bulk density was calculated using Eq. (1) below:
where, Md is the weight of dry soil (g), V is the volume of soil (cm3)
Specific volume (Eq. 2) of soil was calculated by formula of Veihmeyer and Hendrickson28:
Subsequently, total porosity was calculated using Eq. (3) below:
Subsequently, different ratios were calculated by using formulas given by Das and Agrawal 29 as given below:
Water holding capacity and volume expansion
Soil water holding capacity was estimated using Keen Raczkowski box by placing soil samples on a porous plate with applying pressure to drain water to field capacity using the method of Saha30. Keen Raczkowski Box (5.6 cm internal diameter and 1.6 cm height) had a perforated base with holes of 0.75 mm diameter and spaced 4 mm apart. The perforated base of the cylinder was covered with filter paper and filled with soil up to the top edge of the Keen Raczkowski box. The box was weighed and placed in a tray. Water was added to the tray and left overnight for equilibrium. At equilibrium, the soil was fully saturated and the box was removed from the tray. The expanded wet soil found above the rim of the box was removed using the straight edge of a spatula. The removed soil was kept in a pre-weighed aluminium moisture box. The Keen Raczkowski box and aluminium moisture box were weighed immediately and then oven-dried at 105 °C for 24 h to dry the soil14. The dry weight of both boxes was recorded. The matric potential at the water holding capacity measurement varied between (−) 90.8 and (−) 114.1 kPa at 0–20 cm and (−) 109.3 to (−) 116.4 kPa at 20–40 cm.
The water holding capacity and volume expansion were calculated using equations given by Reynolds31:
Gravimetric and volumetric moisture content
Gravimetric moisture content was determined by method of Reynolds31 by collecting field moist soils with soil cores at two depths (0–20 cm and 20–40 cm). The collected soil sample was immediately kept in an aluminium moisture box (weighed moisture box) and wrapped with a cotton cloth to protect it from evaporation. The moisture boxes were transferred to the laboratory after sampling and fresh weight was recorded. Subsequently, an aluminium box filled with soil was oven-dried at 105 °C for 72 h. The weight of aluminum boxes was deducted from the fresh and dry weight of the sample in the respective treatment. Finally, gravimetric moisture content and volumetric moisture content were estimated using formulae of 31:
The air-filled porosity and water-filled pore space were calculated using formulas of Das and Agrawal29:
Aggregate stability and aggregate associated N/P estimation
The wet sieving technique was used for soil aggregation following the standards of Yoder's apparatus through a progression of four sieves (2, 0.5, 0.25, and 0.053 mm)1, 4, 8, 32, 33. A 100 g of > 4 mm soil aggregates were put on top of a 2 mm sieve. Yoder's apparatus comprised a water drum that was loaded with deionized water. The sieving process finished by moving the sieves all over 3 cm in deionized water with a frequency of 25 times each minute in this water drum. The soil was moved upward in a water drum for 5 min. The sieving system brought about the development of four total size portions: (1) > 2 mm (coarse macroaggregates), (2) 0.25–2 mm (macroaggregates), (3) 0.053–0.25 mm (coarse macroaggregates), and (4) < 0.053 mm (‘silt + clay’ − size particles). Soil material held on each sieve after wet sieving was moved into a container and dried at 65 °C until a steady weight32.
Mean weight diameter was calculated with Van Bavel and Kirkham method34:
xi is the mean diameter of the i-th size class (mm), and wi is the proportion of the total sample in the corresponding size fraction.
The available N and P content in water-stable macroaggregates and water-stable microaggregates were estimated using the alkaline permanganate procedure for N35 and Olsen method for P36 and expressed in mg kg−1 dry soil.
Soil organic carbon, available nutrients, and biological properties (bulk soil)
The estimation methods were wet oxidation strategy for soil organic carbon37, Alkaline KMnO4 technique for available N35, Olsen's extractant for available P (0.5 N NaHCO3, pH 8.5)36, and 1 N NH4OAc for available K (pH 7.0)38. Soil pH (soil-to-water proportion of 1:2.5) was assessed by techniques of Jackson 38. The chloroform-fumigation extraction technique was used for microbial biomass carbon and communicated as mg kg−1 dry soil39. The extraction efficiency of microbial biomass carbon (kEC) was 0.4539. Alkaline phosphatase was determined using 16 mM para (p)-nitrophenyl phosphate as substrate and reported as µg p-nitrophenol produced g−1 soil h−1 40. The β-glucosidase was assessed utilizing 25 mM p-nitrophenol-β-d-glucopyranoside as the substrate41.
Yield estimation
Yields of each crop were converted to wheat equivalent yield using price of crops6. The sum of wheat yields and wheat equivalent yields of other crops was the system productivity as follows:
Sustainable yield index of wheat was calculated as follows42.
where, Y is the estimated average yield of base-crop across the years; σ is its estimated standard deviation, and Ymax is the observed maximum yield of base-crop.
Statistical analysis
The analysis of data was performed by the analysis of variance (ANOVA) technique for split-plot design utilizing the program OPSTAT43. For mean comparison, Tukey's honest significance test was used at p ≤ 0.05. The bivariate regression among wheat yield and chosen soil parameters were undertaken by Microsoft Excel™ 200744.
Results
Bulk density, void ratio, and air-filled porosity
P–W system decreased bulk density (by 0.06 g cm−3) compared to the M–W system at 0–20 cm (p < 0.05) (Table 1). Notably, M–W–M–C and P–W systems had lower bulk density (mean 4%) than the cereal-cereal system (M–W) (p < 0.05). P–W and M–W–Mb systems significantly increased void ratio and air-filled porosity over M–W (Table 1; Supplementary Table S1). The P–W rotation enhanced 5–19% void ratio and 25–54% air-filled porosity over M–W across depth (p < 0.05). Long-term practice of INM had reduced dry bulk density more than RDF by 3% (p < 0.05).
Water filled pore space, liquid ratio and air ratio
M–W–M–C and P–W had a lower water-filled pore space (by 5–9%) and liquid ratio (4–10%) than M–W across depth (Table 1; Supplementary Table S1). Subsequently, pulse-based systems had a significantly higher air ratio (0.2) over M–W (0.15). Even in lower soil depth (20–40 cm), P–W and M–W–Mb systems decreased water-filled pore space and increased air ratio compared with M–W (p < 0.05). INM minimized 5% liquid ratio and increased 33–53% air ratio (p < 0.05) compared with RDF across depths (Table 1; Supplementary Table S1).
Soil mass-volume relationship
P–W system had the higher water holding capacity and moisture content (gravimetric and volumetric) by 9, 10, and 5% (p < 0.05) higher than the M–W system, respectively in 0–20 cm depth (Table 2). The extent of increase was 6, 7, and 4% under the P–W system at 20–40 cm depth over the M–W. INM increased 6–7% gravimetric moisture content, 6–7% water holding capacity, and 4–5% volumetric moisture content over RDF (Table 2). P–W (INM) and M–W–Mb (INM) increased 6–18% water holding capacity (p < 0.05) and 5–21% gravimetric moisture content over M–W (RDF) at 20–40 cm depth (Supplementary Fig. S1).
Volume expansion, specific volume and porosity
Pulse-based rotation significantly increased these parameters over M–W (Table 2; Supplementary Table S1). Specifically, P–W and M–W–Mb rotation had 9–24% and 7–9% higher (p < 0.05) volume expansion and porosity over the M–W, respectively. INM increased by 5% and 4% porosity than RDF (p < 0.05) across depth. The substitution of wheat with chickpea (M–W–M–C) increased by 9% porosity over M–W (p < 0.05) (Supplementary Table S1).
Soil organic carbon and available nutrients in bulk soil
P–W and M–W–Mb rotations increased soil organic carbon by 10–13% (p < 0.05) and 12–18% (p < 0.05) across depth over M–W rotation after 14 years of cropping (Table 3). P–W, M–W–Mb, and M–W–M–C increased (p < 0.05) available nitrogen (6–11%), available phosphorus (42–73%), and available potassium (8–16%) over the M–W at 0–20 cm depth. The INM resulted in 11–18% (p < 0.05) higher SOC over the RDF across soil depths (Table 3). The INM increased 15% available phosphorus over RDF at 0–20 cm depth.
Aggregate stability and aggregated N and P content
P–W (INM), P–W (RDF), and M–W–Mb (INM) significantly enhanced the water-stable macroaggregates by 83%, 92%, and 78% over M–W (RDF) (Fig. 1). Subsequently, these rotations decreased 64.4–83.8% water-stable microaggregates than M–W (RDF) after 14 years. The pulse-based system with RDF/INM increased mean weight diameter over M–W (RDF) at 0–20 cm soil depth. Macroaggregated nitrogen was higher in M–W–M–C, P–W, and M–W–Mb by 31%, 40%, and 56% (p < 0.05) and microaggregated nitrogen by 13%, 18%, and 36% (p < 0.05) over M–W, respectively (Table 4). Similarly, these systems improved macroaggregated phosphorus (7–11%) and microaggregated phosphorus (4–12%) than M–W. INM increased nitrogen content in macro- and micro-aggregates by 10% over the RDF (p < 0.05). INM enhanced 20–22% macro- and micro-aggregated phosphorus compared with RDF (p < 0.05). Notably, M–W–Mb (INM), P–W (RDF), and P–W (INM) significantly increased nitrogen and phosphorus content in aggregates over M–W (RDF) (Table 4).
Biological properties in the soils
P–W (INM) and M–W–Mb (INM) increased (p < 0.05) soil microbial biomass carbon by 75–113%, alkaline phosphatase by 114–125%, and β-glucosidase by 83% over M–W (RDF) (Fig. 2). Notably, RDF in each crop rotation had a reduced enzymes activity than that in INM (p < 0.05). The CT and RDF under the M–W system had similar alkaline phosphatase content over time.
Crop productivity and sustainable yield index
M–W–Mb increased 26% maize yield and 21% wheat yield over M–W (p < 0.05) in the 13th crop cycle (2015–2016) (Table 5). Similarly, it increased 23% maize yield and 8% wheat yield over M–W (p < 0.05) in the 14th crop cycle (2017–2018). This increasing trend of maize and wheat yields under M–W–Mb has also been observed in the 11th (2013–2014) and 12th (2014–2015) crop cycles. Notably, M–W–Mb increased system yield by 127% (2015–2016) and 80% (2016–2017) over the M–W (p < 0.05) (Table 5; Fig. 3a). Even, M–W–M–C resulted in higher yields of maize (19.4%), wheat (12.5%), and system (9–15%) over M–W (p < 0.05). The P–W system had 23.7% higher system productivity than the M–W system in 2016–17. The mean yield of 14 years (2004–2005 to 2016–2017) revealed higher wheat yield (base crop) under M–W–Mb and higher sustainable yield index of wheat under P–W (Fig. 3b). INM had higher yields of chickpea (14%) and mungbean (12%) over the RDF (Table 5).
Discussion
Role of pulses on soil properties
Soil compaction in tillage-intensive M–W rotation45 could restrict crop/root growth and productivity6, which is an evident/pervasive problem in the IGP33. Reduction in bulk density is essential for enhancing soil health and crop productivity in the regions. Higher macroaggregate in pulse-based cropping systems reduced bulk density and increased porosity, air ratio, and mean weight diameter in the present study. Tillage operations were similar in all crop rotations in the present study. Hence, variable soil properties were because of the inclusion of pulse crops in the cereal-cereal system (M–W), the deep root system of pulse crops, higher root activities, and leaf fall. A previous study indicated that pulse crops (mungbean, chickpea, and pigeonpea) in rotation increased macropores and macroaggregates because of the decomposition of leaf litter fall, root biomass, and rhizodeposition46. The ligno-protein and polysaccharide complexes from fresh leaves and lower carbon:nitrogen ratio of residues of pulse crops increase the soil-aggregate cohesion and aggregate stability (mean weight diameter), thereby reducing the bulk density in the long run33. The low molecular weight organic acids and root exudates secreted from pulse rhizosphere could play a crucial role in soil aggregation21. In this regard, the pigeonpea crop under the P–W system had higher leaf fall in combination with deep root system and bioturbation (biological tillage) activities that resulted in reduced bulk density and higher physical properties such as soil aggregation, porosity, and air ratio8. Added organic matter through leaf fall and root biomass in pulse-based systems could build-up humus that restored soil porosity and aeration in compacted soil47. The intensification of the maize–wheat system with mungbean resulted in added crop residue (3 crops year−1) and belowground biomass and increased soil aggregation and porosity over maize–wheat8.
Higher moisture (gravimetric and volumetric) content and water holding capacity with the inclusion of pulses and INM than chemical fertilization in M–W [M–W (RDF)] could be due to higher SOC that retained soil moisture in these systems. Water-filled pore space was reduced under pulse-based systems than under M–W. The ecological significance of lower water filled pore space is the reduced greenhouse gas emission (specifically carbon dioxide). Microbial respiration, which returns carbon to the atmosphere, can be higher with higher water-filled pore space48. The higher water-filled pore space creates anaerobic conditions in the root zone, which generates nitrous oxide emissions. The higher soil aggregation and porosity create aerobic conditions and release nitrous oxide into the atmosphere47. In this regard, pulse-based rotations could minimize nitrous oxide emission over the maize–wheat, which had a higher water-filled pore space. Besides, higher air-filled porosity and lower water filled porosity in pulse-based systems can stabilize microbial carbon and minimize CO2 emissions44.
Pulse-based cropping systems increased aggregated N and P content and available nutrients in the present study. It is because of added carbon and nitrogen through crop residues and rhizospheric alteration by pulse crops. The higher aggregated N and P content and available nutrients are the results of increased nutrient stock in aggregates (N and P) and better solubility of nutrients in M–W–Mb (INM), P–W (INM) and M–W–M–C (INM) over M–W (RDF). The acidification in the root zone during biological nitrogen fixation and mineralization of added organic matter increased the nutrient availability in the mineral fraction of soil with alkaline soil pH (pH 8.1 in the present studied soil)9. The average nutrient concentrations in crop residues were 1.03% N, 0.21% P, 1.12% K in rice, 1.48% N, 0.23% P, 0.87% K in wheat, 1.80% N, 0.27% P, 0.99% K in chickpea and 2.14% N, 0.22% P, 0.52% K in mungbean. The higher nutrients inputs through crop residues in pulse-based systems (P–W, M–W–Mb, M–W–M–C) resulted in higher aggregated and available nutrients over time. A lower C/N ratio of pulse crop residues (pigeonpea, chickpea, and mungbean), the additional residue of mungbean crop (under M–W–Mb), higher leaf fall (under P–W system) and acidification in root zone had significant contributions in nutrients availability/solubility15. Soil water content and temperature have a crucial role in the sequestration of nutrients in the cropping system47. The higher water-holding capacity and soil moisture content in P–W, M–W–Mb, and M–W–M–C systems resulted in higher aggregated nutrient content5.
P–W (INM) and M–W–Mb (INM) increased SOC and soil microbial biomass carbon over the M–W (RDF) because of higher carbon input through organic amendments (farmyard manure and biomass of above-ground crop residues returning into the soil)8. Long-term inclusion of pulses in the cropping system increased SOC and soil microbial biomass carbon over the M–W system due to the enhanced crop growth, higher crop residue addition, rejuvenation of rhizosphere with diversified microbes, and carbon-rich substrates addition into the soil44. The increased substrate availability in a pulse-based system increased microbe abundance (bacteria, fungi, and actinomycetes)15. Pulse crops enhanced the SOC and SMBC, which acted as substrates for microbial proliferation, thereby enriching the soil enzymes activity such as alkaline phosphatase and β-glucosidase. The increased activities of soil enzymes are a good indicator of soil health and components for sustainable ecology. Hence, crop diversification with pigeonpea and mungbean as P–W (INM) and M–W–Mb (INM) can be a good management practice for higher soil physical health, SOC sequestration, and enzyme activity in long run.
Impact of fertilization practices
Added organic amendments in INM under all pulse-based cropping systems reduced bulk density to a greater extent than chemical fertilization in the M–W system. The reduced bulk density in INM practice was because of differential densities of added organic amendments (crop residues and farmyard manure). The dilution effect from mixing of added organic matter reduced bulk density in the mineral fraction of soil having alkaline soil pH. Besides, added organic amendments and their decomposition products could increase microbial activity that favors more aggregation and thus reduce bulk density. Soinne et al. highlighted the higher improvement of bulk density under farm-yard manure/biochar over chemical fertilization49. In the present study, macroaggregate was increased under INM in pulse-based systems because of better soil flocculation, and chelating agents that bind the soil50. The regulating factors of soil water holding capacities as porosity and specific surface area were regulated by long-term fertilization21. Increased SOC under INM also enhanced macroaggregates and mean weight diameter8. Total carbon input through organic amendments (farmyard manure and biomass of above-ground crop residues returning to soil) was 88.8 t ha−1 in INM (average of crop rotations) in the present study (Fig. 4). On average farmyard manure contained 0.56% nitrogen, 0.18% phosphorus and 0.52% potassium in the present study. It resulted in the addition of 392 kg N, 126 kg P, and 364 kg K ha−1 through farmyard manure in 14 years in INM treatment. The total carbon input (FYM + crop residue) in thirteen years in different crop rotations followed the order of P–W (96.0 Mg carbon ha−1) > M–W–Mb (95.3 Mg carbon ha−1) > M–W–M–C (91.3 Mg carbon ha−1) > M–W (72.4 Mg carbon ha−1) (Fig. 4). The variable biomass production of crops under study created the difference in added organic amendments (FYM and crop residue). The added carbon into the soil increased aggregate stability and SOC, and resulted in higher gravimetric and volumetric moisture content. Possibly, a reduced soil enzyme activity (β-glucosidase and soil phosphatase) and microbial biomass carbon under RDF minimized aggregate stability15. Thus, INM consisting of farmyard manure and crop residues could increase soil aggregation, physical properties, and available nutrients over chemical fertilization in the long run.
Benefit of carbon and water in soils/aggregation processes
The increased aggregate stability and reduced bulk density in pulse-based systems may be due to the higher SOC concentration51. The better pore size distribution and aggregation increased water holding capacity at higher tension under INM treatment than RDF. The higher specific surface area of organic amendments in INM increased water holding capacity and soil moisture content than RDF52. P–W and M–W–Mb had an increasing trend of carbon over the year. The increased SOC in P–W and M–W–Mb systems resulted in higher soil biogeochemical properties. Pulses contributed to carbon sequestration with more root biomass than cereals47. Thus, higher water holding capacity, gravimetric/volumetric moisture content, and volume expansion in pulses systems contributed to the high carbon input through root biomass that ultimately increased the carbon sequestration potential. The reduced SOC, restricted root zone at the soil surface of cereal crops, low microbial activity, and reduced biomass input in soil over time resulted in the disintegration of soil aggregates in tillage intensive M–W system under RDF. Microbial biomass carbon acted as a chelating agent in the soil aggregation processes and soil moisture retention47. Thus, a higher carbon could stabilize soil aggregates and soil water holding capacity in pulse-based systems under INM.
Implications of pulse-based system for yield sustainability in the region
The present study deciphered that M–W system intensification with mungbean and diversification with pigeonpea/chickpea could restore soil physical health and enhance the yield of crops. The M–W–Mb increased base crop yield (wheat) over the year. It is vital for yield sustainability and food security in the IGP8, 53. Wheat is a predominating crop in these regions, where the yield decline of crops is a concern. Further, M–W–Mb and P–W increased system productivity (wheat equivalent yield) over M–W. The increased system productivity could be due additional yield of mungbean in M–W–Mb and the higher price of pigeonpea in the P–W system. Although inorganic fertilizer had a similar grain yield to cereal component crops (maize, wheat) with INM, RDF resulted in a limited effect on soil physical properties. The present study deciphered that intensification of the maize–wheat system with mungbean and diversification with pigeonpea and chickpea could restore the soil's physical, chemical, and biological health in the long run. The higher SOC, soil microbial biomass carbon, β-glucosidase, water holding capacity, available nutrients, and aggregated P could contribute towards yield maximization over time. The advantage of pulse crops in cropping system along with INM [P–W (INM) and M–W–Mb (INM)] can be related to more mineralizable N in pulse crop residues, more residual water in the subsoil, and general rotational advantages of having different preceding crop type27. Another benefit of pulses is that they fix atmospheric N2 by root nodule symbiosis, and slow release of N from pulse residues and roots favors the growth of succeeding crops and yields44.
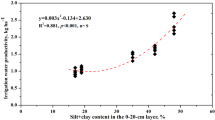
It is evident that volume expansion, gravimetric moisture content, water-stable macroaggregate, macroaggregated P, air ratio, and soil porosity (%) significantly correlated with wheat yield after 14 years of cropping (Table 6). It indicated that not all parameters could equally contribute to crop yield maximization. Aggregate stability, porosity, and soil moisture content had the higher impact on yield. Hence, management practices that increase soil porosity and aggregate stability could be adopted in tillage-intensive systems. M–W system might increase micro-porosity and soil compaction, restricted root growth, and lower yield. Reversibly, the pulse-based system with INM could rejuvenate aggregate formation, porosity, soil moisture availability, and aggregated nutrient concentration which are vital for crop yield maximization.
Conclusions
The present study highlighted that the mechanical disintegration of soil under the conventional tilled maize–wheat system of IGP could be ameliorated by pulse inclusion and INM practice in a cropping system. P–W (INM) and M–W–Mb (INM) enhanced soil physical properties: aggregate stability, gravimetric and volumetric moisture content, porosity, air ratio, and chemical properties: soil organic carbon and available nutrients, and soil enzymes activity over time than M–W (RDF). P–W (INM) and M–W–Mb (INM) reduced bulk density and water-filled pore space over M–W after 14 years and could increase soil organic carbon sequestration. Also, these systems increased aggregated N/P content and available nutrients resulting in enhanced soil fertility. The higher soil physical, chemical, and biological properties under pulse-based systems with INM could resulting in higher crop and system productivity over the M–W (RDF). Pre-dominating chemical fertilization proved detrimental to physical and aggregate properties of soil. Notably, P–W (INM) and M–W–Mb (INM) provide carbon substrate into the soil, which enhanced soil aggregate stability and SOC over time. Thus, the present study highlights that sustainable cropping intensification must consist of pulse crops in the cereal dominating agroecologies to minimize soil degradation.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Bhattacharyya, R. et al. Aggregate-associated N and global warming potential of conservation agriculture-based cropping of maize–wheat system in the north-western Indo-Gangetic Plains. Soil Tillage Res. 182, 66–77 (2018).
Lichter, K. et al. Aggregation and C and N contents of soil organic matter fractions in a permanent raised-bed planting system in the Highlands of Central Mexico. Plant Soil 305(1), 237–252 (2008).
Burrell, L. D., Zehetner, F., Rampazzo, N., Wimmer, B. & Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 282, 96–102 (2016).
Nath, C. P. et al. Including grain legume in rice–wheat cropping system improves soil organic carbon pools over time. Ecol. Eng. 129, 144–153 (2019).
Bhattacharyya, R. et al. Impacts of conservation agriculture on soil aggregation and aggregate-associated N under an irrigated agroecosystem of the Indo-Gangetic Plains. Nutr. Cycl. Agroecosyst. 96(2), 185–202 (2013).
Das, T. K. et al. Conservation Agriculture in rice-mustard cropping system for five years: Impacts on crop productivity, profitability, water-use efficiency, and soil properties. Field Crop Res. 250, 107781 (2020).
Sepat, S. & Rana, D. S. Effect of double no-till and permanent raised beds on productivity, profitability and physical properties of soil in maize (Zea mays)–wheat (Triticum aestivum) cropping system under Indo-Gangetic plains of India. Indian J. Agron. 58(4), 469–473 (2013).
Hazra, K. K. et al. Diversification of maize–wheat cropping system with legumes and integrated nutrient management increases soil aggregation and carbon sequestration. Geoderma 353, 308–319 (2019).
Nath, C. P. et al. Pulse crop and organic amendments in cropping system improve soil quality in rice ecology: Evidence from a long–term experiment of 16 years. Geoderma 430, 116334 (2023).
Meena, B. P. et al. Long-term sustaining crop productivity and soil health in maize–chickpea system through integrated nutrient management practices in Vertisols of central India. Field Crop Res. 232, 62–76 (2019).
Minasny, B. & McBratney, A. B. Limited effect of organic matter on soil available water capacity. Eur. J. Soil Sci. 69(1), 39–47 (2018).
Khaleel, R., Reddy, K. R. & Overcash, M. R. Changes in soil physical properties due to organic waste applications: a review. J. Environ. Qual. 10(2), 133–141 (1981).
Mtyobile, M., Muzangwa, L. & Mnkeni, P. N. S. Tillage and crop rotation effects on soil carbon and selected soil physical properties in a Haplic Cambisol in Eastern Cape, South Africa. Soil Water Res. 15(1), 47–54 (2019).
Nath, C. P. et al. Long-term impact of legume-based cropping with chemical and integrated fertilisation on viable weed seed density, diversity and community structure. Weed Res. 61, 360–374 (2021).
Borase, D. N. et al. Long-term impact of diversified crop rotations and nutrient management practices on soil microbial functions and soil enzymes activity. Ecol. Indic. 114, 106322 (2020).
Rahman, M. M., Alam, M. S., Islam, M. M., Kamal, M. Z. U., Rahman, G. M., Haque, M. M., Miah, M. G. & Biswas, J. C. Potential of legume-based cropping systems for climate change adaptation and mitigation. in Advances in Legumes for Sustainable Intensification, 381–402. Academic Press (2022).
Liu, K., Bandara, M., Hamel, C., Knight, J. D. & Gan, Y. Intensifying crop rotations with pulse crops enhances system productivity and soil organic carbon in semi-arid environments. Field Crops Res. 248, 107657 (2020).
Kumar, S., Meena, R. S., Lal, R., Singh Yadav, G., Mitran, T., Meena, B. L. & EL-Sabagh, A. Role of legumes in soil carbon sequestration. Legumes for soil health and sustainable management, 109–138 (2018).
Pernes-Debuyser, A. & Tessier, D. Soil physical properties affected by long-term fertilization. Eur. J. Soil Sci. 55(3), 505–512 (2004).
Mulumba, L. N. & Lal, R. Mulching effects on selected soil physical properties. Soil Tillage Res. 98(1), 106–111 (2008).
Lehmann, J. et al. Biochar effects on soil biota—A review. Soil Biol. Biochem. 43(9), 1812–1836 (2011).
Anderson, S. H., Gantzer, C. J. & Brown, J. R. Soil physical properties after 100 years of continuous cultivation. J. Soil Water Conser. 45(1), 117–121 (1990).
Das, B. et al. Effect of integrated nutrient management practice on soil aggregate properties, its stability and aggregate-associated carbon content in an intensive rice–wheat system. Soil Tillage Res. 136, 9–18 (2014).
Celik, I., Ortas, I. & Kilic, S. Effects of compost, mycorrhiza, manure and fertilizer on some physical properties of a Chromoxerert soil. Soil Tillage Res. 78(1), 59–67 (2004).
Liu, X. et al. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 373, 583–594 (2013).
Lal, R. Improving soil health and human protein nutrition by pulses-based cropping systems. Adv. Agron. 145, 167–204 (2017).
Ghosh, P. K. et al. Grain legume inclusion in cereal–cereal rotation increased base crop productivity in the long run. Exper. Agric. 56(1), 142–158 (2020).
Veihmeyer, F. J. & Hendrickson, A. Soil density and root penetration. Soil Sci. 65(6), 487–494 (1948).
Das, D. K., Agrawal, R. P. Fundamentals of Soil Science. In: Goswami, N.N., Rattan, R.K., Dev, G., Narayanasamy, G., Das, D.K., Sanyal., S.K., Pal., D.K., Rao, D.L.N. (2nd Eds.). Indian Soc. Soil Sci. 91 (2009).
Saha, A. K. Methods of Physical and Chemical Analysis of Soil 112 (Kalyani Publishers, 2014).
Reynolds, S. G. The gravimetric method of soil moisture determination Part IA study of equipment, and methodological problems. J. Hydrol. 11(3), 258–273 (1970).
Elliott, E. T. & Cambardella, C. A. Physical separation of soil organic matter. Agric. Ecosyst. Environ. 34(1–4), 407–419 (1991).
Nath, C. P. et al. Nitrogen effects on productivity and soil properties in conventional and zero tilled wheat with different residue management. Proc. Nat. Acad. Sci. India Sect. B Biol. Sci. 89(1), 123–135 (2019).
Van Bavel, C. H. M. & Kirkham, D. Field measurement of soil permeability using auger holes. Soil. Sci. Soc. Am. J. 13(C), 90–96 (1949).
Subbiah, B. V. A rapid procedure for the determination of available nitrogen in soils. Curr. Sci. 25, 259–260 (1956).
Olsen, S. R. Estimation of available phosphorus in soils by extraction with sodium bicarbonate (No. 939). US Department of Agriculture (1954)
Walkley, A. & Black, I. A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37(1), 29–38 (1934).
Jackson, M. L. Soil Chemical Analysis. Prentice Hall—Of India (Pvt.) Ltd., New Delhi (1973).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987).
Tabatabai, M. A. & Bremner, J. M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1, 301–307 (1969).
Eivazi, F. & Tabatabai, M. A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 20, 601–606 (1988).
Ray, D. K., Gerber, J. S., MacDonald, G. K. & West, P. C. Climate variation explains a third of global crop yield variability. Nat. Comm. 6(1), 5989 (2015).
Sheoran, O. P., Tonk, D. S., Kaushik, L. S., Hasija, R. C. & Pannu, R. S. Statistical software package for agricultural research workers. Recent Advances in information theory, Statistics & Computer Applications by D.S. Hooda & R.C. Hasija Department of Mathematics Statistics, CCS HAU, Hisar (139–143) (1998).
Borase, D. N. et al. Long-term impact of grain legumes and nutrient management practices on soil microbial activity and biochemical properties. Arch. Agron. Soil Sci. 67(14), 2015–2032 (2021).
Ladha, J. K., Yadvinder-Singh, Erenstein, O. & Hardy, B. Integrated crop and resource management in the rice-wheat system of South Asia. International Rice Research Institute, Los Baños, Philippines 395 (2009).
Kavdir, Y. A. S. E. M. İN., Hellebrand, H. J. & Kern, J. Seasonal variations of nitrous oxide emission in relation to nitrogen fertilization and energy crop types in sandy soil. Soil Tillage Res. 98(2), 175–186 (2008).
Mathew, I., Shimelis, H., Mutema, M. & Chaplot, V. What crop type for atmospheric carbon sequestration: Results from a global data analysis. Agric. Ecosyst. Environ. 243, 34–46 (2017).
Bateman, E. J. & Baggs, E. M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fert. Soil. 41, 379–388 (2005).
Soinne, H., Hovi, J., Tammeorg, P. & Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 219, 162–167 (2014).
Kumar, N. et al. Impact of zero-till residue management and crop diversification with legumes on soil aggregation and carbon sequestration. Soil Tillage Res. 189, 158–167 (2019).
Lal, R. Soil erosion and carbon dynamics. Soil Tillage Res. 81(2), 137–142 (2005).
Doran, J. W. & Zeiss, M. R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 15(1), 3–11 (2000).
Kumar, N. et al. Long-term impact of zero-till residue management in post-rainy seasons after puddled rice and cropping intensification on weed seedbank, above-ground weed flora and crop productivity. Ecol. Eng. 176, 106540 (2022).
Acknowledgements
Authors sincerely acknowledge all the persons associated with long-term experiment since inception. We acknowledge the previous authors who published research papers from present long-term experiments and for description of soil sampling and processing. We have cited previous studies for methodologies and formulas for calculating various soil physical and chemical properties in the present study. We sincerely acknowledge all these authors whose methodologies and formulas (bulk density, specific volume, total porosity, void ratio, air ratio, liquid ratio, water holding capacity, volume expansion, gravimetric, volumetric moisture content, air-filled porosity, water-filled pore space, water stable macroaggregates, and water stable microaggregates) are mentioned in present study.
Funding
Authors gratefully acknowledge the ICAR-Indian Institute of Pulses Research, Kanpur, India for financial support in this study.
Author information
Authors and Affiliations
Contributions
C.P.N.: Conceptualization; Investigation; Methodology; Data Formal analysis; Writing—original draft; & editing. A.D.: Supervision; Monitoring, Experimental materials, Data curation; K.K.H.: Writing, Editing and Review; C.S.P.: Writing—review & editing; N.K.: Conceptualization; Investigation; Methodology; S.S.S.: Supervision; Monitoring, U.S.; Statistical analysis; K.D.: Supervision; Monitoring, Experimental materials.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nath, C.P., Dutta, A., Hazra, K.K. et al. Long-term impact of pulses and organic amendments inclusion in cropping system on soil physical and chemical properties. Sci Rep 13, 6508 (2023). https://doi.org/10.1038/s41598-023-33255-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33255-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.