Abstract
Individualize treatment after cardiac arrest could potentiate future clinical trials selecting patients most likely to benefit from interventions. We assessed the Cardiac Arrest Hospital Prognosis (CAHP) score for predicting reason for death to improve patient selection. Consecutive patients in two cardiac arrest databases were studied between 2007 and 2017. Reasons for death were categorised as refractory post-resuscitation shock (RPRS), hypoxic-ischaemic brain injury (HIBI) and other. We computed the CAHP score, which relies on age, location at OHCA, initial cardiac rhythm, no-flow and low-flow times, arterial pH, and epinephrine dose. We performed survival analyses using the Kaplan–Meier failure function and competing-risks regression. Of 1543 included patients, 987 (64%) died in the ICU, 447 (45%) from HIBI, 291 (30%) from RPRS, and 247 (25%) from other reasons. The proportion of deaths from RPRS increased with CAHP score deciles; the sub-hazard ratio for the tenth decile was 30.8 (9.8–96.5; p < 0.0001). The sub-hazard ratio of the CAHP score for predicting death from HIBI was below 5. Higher CAHP score values were associated with a higher proportion of deaths due to RPRS. This score may help to constitute uniform patient populations likely to benefit from interventions assessed in future randomised controlled trials.
Similar content being viewed by others
Introduction
Mortality after cardiac arrest (CA) is very high and chiefly due to refractory post-resuscitation shock (RPRS), hypoxic-ischaemic brain injury (HIBI), and brain death1. Preventing HIBI is considered crucial, since withdrawal of life-sustaining treatments (WLST) warranted by a poor neurological prognosis is the most common reason for death2. Despite decades of research, few treatments have been shown to improve outcomes after CA3,4,5,6,7,8. Targeted temperature management (TTM) has long been considered the only effective neuroprotective treatment. In the HYPERION randomised trial, TTM at 33 °C improved outcomes in patients with an initial non-shockable rhythm compared to normothermia9. However, TTM failed to provide benefits in other studies, including the TTM2 randomised controlled trial, which also compared 33 °C to normothermia8. One possibility is that specific treatments may benefit some patient sub-groups but not others, and that trials have included patients with heterogeneous phenotypes associated with different reasons for death. For instance, in the TTM2 trial8, a substantial proportion of patients died from RPRS with multiorgan failure within 48 h after intensive-care-unit (ICU) admission. Such patients are unlikely to benefit from neuroprotective interventions, and their inclusion in the trial is therefore criticisable10. The ability to predict the most likely reason for death might improve patient selection for specific treatment strategies.
We hypothesised that the severity of illness at ICU admission might be associated with the reason for death in the ICU. The Cardiac Arrest Hospital Prognosis (CAHP) score has been proven effective in predicting the risk of death based on seven variables readily available at ICU admission (age, CA in a public place vs. at home, initial rhythm, no-flow and low-flow times, admission pH, and total epinephrine dose), with higher scores being associated with worse outcomes11,12,13. The objective of this study was to assess whether the CAHP score at ICU admission also predicted the reason for death, notably RPRS and HIBI.
Methods
We used prospectively established databases from two tertiary referral centres for CA in the Paris area, France (#NCT03594318). Data collection was approved by the Ethics Committee of the French Intensive Care Society [#CESRLF_12-384 (November, 14, 2012) and 20–41 (May, 5, 2020)] which waived the requirement for written consent in accordance with French law on retrospective studies of anonymized data. The study was conducted according to French health authorities’ regulations (French Data Protection Authority #MR004_2209691. All procedures involving the patients complied with the ethical standards of the institutional and national research committees and with the 1964 Declaration of Helsinki and its later amendments. The study is reported according to the STROBE statement.
Study population and objectives
Inclusion criteria were non-traumatic out-of-hospital cardiac arrest (OHCA) between 2007 and 2017, ICU admission with sustained return of spontaneous circulation (ROSC), age older than 18 years, and availability of the data needed to compute the CAHP score.
The primary study objective was to assess potential associations linking the CAHP score to the reason for death after OHCA. The secondary objectives were to determine levels of CAHP scores associated with death from RPRS and from HIBI.
Post-resuscitation care
The management protocol for patients admitted to ICU after OHCA has been described elsewhere14,15,16. The only changes during the study period involved the sedation and neuromuscular blockade protocols (Electronic Supplementary Material [ESM]). In the event of persistent coma 72 h after the ROSC, sedation was discontinued and multimodal neuroprognostication carried out (ESM)17,18,19. In patients with preserved N20 peaks, preserved cranial reflexes, and a motor Glasgow Coma Scale score above 2, life-sustaining treatments were continued to allow investigations for a confounding factor such as sepsis, residual sedation, or a concomitant disease. A WLST decision was made collegially if no treatable factor was identified.
Data collection
In each of the two prospective databases, the Utstein style20 was followed to collect demographic data and data related to the OHCA including age, sex, location at OHCA, first recorded rhythm, no-flow time, low-flow time, presence of a witness, cardiopulmonary resuscitation by a bystander, number of defibrillations, and epinephrine administration. The following variables were also collected in the ICU: use of TTM, development of post-resuscitation shock, admission blood lactate, and cause of OHCA. The CAHP score was computed for each patient.
To investigate whether the CAHP score predicted the reason for death, we retrospectively reviewed the ICU records to determine the reason for death categorised as RPRS, HIBI, or other (e.g., brain death; recurrent CA and WLST warranted by comorbidities) (ESM). Death from RPRS was defined as progressive, refractory haemodynamic failure despite aggressive critical care, with or without WLST. Death from HIBI was defined as WLST warranted by the results of multimodal neuroprognostication indicating a very low likelihood of neurological recovery1. Classification of death reason was performed by 2 authors (MP and SB), blinded from each other and a third (SA) in case of disagreement.
Statistical analysis
Quantitative parameters were described as median [interquartile range] and qualitative parameters as proportion (percentage). For comparisons between categorical variables, we applied Pearson’s or Fisher’s test, as appropriate.
We divided the CAHP scores into deciles, instead of the usual 3 grades, to potentiate CAHP discrimination for prediction of mode of death. For each decile, we determined the outcome with the distribution of the three reasons for death (RPRS, HIBI, other). We plotted cumulative incidence curves for each reason for death over time and in terms of CAHP decile and compared them using the Fine-and-Gray method21,22. We then built competing-risks regression models for reasons for death in the ICU, with computation of the sub-hazard ratio and of its 95% confidence interval (95% CI) for each CAHP score decile. A sensitive analysis was performed, restricted to cardiac cause of cardiac arrest and a second restricted to initial shockable rhythm.
All tests were two-sided, with p values below 0.05 considered statistically significant. The frequency of missing data was less than 5% and we therefore conducted a complete-case analysis. For the statistical analyses, we used STATA/SE 14.0 (College Station, TX, USA).
Results
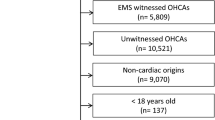
Figure 1 is the patient flow chart. Among the 1543 included patients, 985 (64%) died during the ICU stay, including 291 (30%) who died of RPRS and 447 (45%) of HIBI. Table 1 reports the main patient characteristics.
Outcomes in each CAHP score decile
CAHP scores ranged from 44.4 to 362.2. Figure 2 shows patient outcomes at ICU discharge in each CAHP decile. Higher CAHP scores were significantly associated with worse outcomes, with a 99% mortality rate in the tenth decile (p < 0.0001).
Starting at the fifth CAHP decile, the proportion of deaths due to RPRS increased from one decile to the next. In the tenth decile, 55.2% of patients died of RPRS and 18.9% of HIBI.
Median time to death in the overall population was 5 days [2–8 days] (Fig. 3). The cause of death differed according to time since ICU admission. Thus, of the 412 patients who died during the first 3 days, 241 (58%) died of RPRS. In contrast, of the 573 patients who died on day 4 or later, 426 (74%) died of HIBI.
The cumulative incidence curves showed that, between CAHP deciles 5 and 10, the risk of RPRS as expressed by the sub-hazard ratio increased from one CAHP decile to the next (Fig. 4). In the tenth decile, the sub-hazard ratio for RPRS was 30.8 (95% CI 9.8–96.5; p < 0.0001) (ESM).
In contrast, the CAHP score did not seem to predict death from HIBI. The sub-hazard ratios for death from HIBI were not exponential between the fourth and ninth deciles and decreased for the tenth decile. The results did not differ in the sensitive analysis restricted to CA from cardiac cause and to initial shockable rhythm (ESM).
Discussion
This study of two prospectively established population-based databases showed that CAHP score shows a discriminant power of this score to identify patients at risk of death. Indeed, higher CAHP scores at ICU admission after OHCA were associated with a higher risk of death due to RPRS. In contrast, the CAHP score did not predict death from HIBI.
The mortality rate in our study is consistent with the results of other studies done in Western countries1,23,24. Although guidelines issued in 2021 recommend collecting the reason for death as an Utstein variable17,23,25,26,27, such data remain sparse. One explanation may be the absence of a consensus about defining reasons for death. A 2019 retrospective study of 408 patients identified five reasons for death, namely, WLST warranted by HIBI, WLST warranted by co-morbidities, RPRS, recurrent CA, and respiratory failure1. In our study, HIBI was the most common reason for death, although the 45% proportion was lower than in earlier studies (65% and 73%)1,24, perhaps because these considered brain death a form of HIBI-related death, whereas we did not. Our finding that RPRS was the main cause of death in the first 3 days and HIBI later on is also in keeping with other data24.
The CAHP score was developed in France to predict outcomes of patients admitted to the ICU with ROSC after OHCA11,12,13,29. The predictive performance of the CAHP score has received robust external validation12,13,28. Scores fall into three groups, at low, moderate, and high risk for poor outcomes, respectively, with higher scores indicating worse outcomes. Poor outcome includes cerebral performance category (CPC) 3–4–5 without information of cause of death neither awakening before death. Instead, we distinguished CAHP score deciles to potentiate CAHP discrimination. In keeping with previous studies, we found that the mortality rate was 95% in patients with CAHP scores above 200.
The proportion of deaths due to RPRS was higher in patients with high CAHP scores in our competing-risks analysis. This finding is consistent with the reported association with post-resuscitation shock of three of the seven variables in the CAHP score, namely, low-flow time, arterial pH, and epinephrine dose29. Death by HIBI was not associated with the CAHP score. The seven variables are collected early, before or at ICU admission, whereas brain damage develops over time, with the possible participation of secondary insults30,31. Our competing-risks approach took into account the high early mortality from RPRS in patients with high CAHP scores.
Our findings suggest that the CAHP score may be useful to constitute uniform populations for future trials focus on post CA selecting the patients most likely to benefit from interventions aimed at preventing either RPRS or HIBI. Thus, patients with high CAHP scores at ICU admission may be unlikely to derive benefit from neuroprotective treatments such as TTM, given their high risk of early death due to RPRS. According to Sunde et al., patients at highest risk for poor outcomes should not be included in trials, as interventions may be beneficial only in low- and moderate-risk patients32. Using CAHP score deciles to conduct post hoc sub-group analyses of data from the recent trials of TTM might identify reasons for their divergent results. The efficacy of TTM has been found to vary according to the severity of post-CA syndrome, supporting the possibility that patient selection might improve outcomes33. In a population-based study, early coronary angiography was associated with higher survival in the low-risk CAHP-score group but not in the moderate- or high-risk groups34. An advantage of the CAHP score as an aid to treatment decisions is that the seven variables are available at ICU admission.
Several randomised controlled trials of interventions aimed at improving neurological outcomes are currently recruiting patients. To the best of our knowledge, none selects patients based on the risk of early death from RPRS, before neuroprotective interventions can show benefits. Our study indicates that the CAHP score might help to establish appropriate populations for evaluating neuroprotective treatments, excluding patients with a high risk of death from RPRS. A score specifically designed to predict neurological outcomes might also be useful. Moreover, neuroprotective treatment may be of no benefit in patients with minimal brain damage. Thus, the patient group most likely to benefit from TTM and other neuroprotective interventions may be the group at moderate risk.
Our study has several limitations. First, the design was retrospective, however, most of these data were collected prospectively. The medical resources and practices of the two recruiting centres in France may differ from those in other countries. Second, the reason for death was determined by at least two investigators, based on data available during the file review. For each reason for death, competition occurred with other reasons, but we tried to compensate for this by using a competing-risks regression models for reasons for death. Therefore, we don’t know the extent to which this competitive risk might contribute to the observed result that the CAHP score did not predict death from HIBI. The CAHP was designed for OHCA and we therefore confined our study to this sub-set, similar to most other studies of CA. The no-flow time, which is used to determine the CAHP score, can be difficult to estimate. Another variable used for CAHP-score determination is arterial pH, which may be modified by on-scene sodium bicarbonate administration35. We did not compare our results based on CAHP score to other existing scores using other prognostic variables. It would be interesting to develop a new score to answer the question of the best population to include in future CA trials. Given these limitations, our findings should be interpreted as preliminary and hypothesis-generating and should be tested in future studies.
Conclusions
In conclusion, among reasons for death after OHCA, RPRS was more common in patients with high CAHP scores. This score may help to constitute uniform patient populations likely to benefit from interventions assessed in future randomised controlled trials. Further studies are warranted to assess this possibility.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CA:
-
Cardiac arrest
- CAHP:
-
Cardiac Arrest Hospital Prognosis
- CI:
-
Confidence interval
- CPC:
-
Cerebral performance category
- CPR:
-
Cardio pulmonary resuscitation
- CT:
-
Computerized tomography
- ESM:
-
Electronic supplementary material
- HIBI:
-
Hypoxic–ischemic brain injury
- GCS:
-
Glasgow Coma Scale
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile-range
- OHCA:
-
Out-of-hospital cardiac arrest
- ROSC:
-
Return of spontaneous circulation
- RPRS:
-
Refractory post-resuscitation shock
- SHR:
-
Sub-hazard ratio
- TTM:
-
Targeted temperature management
- WLST:
-
Withdrawal of life-sustaining treatments
References
Witten, L. et al. Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation 136, 93–99 (2019).
Nolan, J. P. et al. Intensive care medicine research agenda on cardiac arrest. Intensive Care Med. 43, 1282–1293 (2017).
Lemkes, J. S. et al. Coronary angiography after cardiac arrest without ST-segment elevation. N. Engl. J. Med. 380, 1397–1407 (2019).
Geri, G. et al. Hemodynamic efficiency of hemodialysis treatment with high cut-off membrane during the early period of post-resuscitation shock: The HYPERDIA trial. Resuscitation 140, 170–177 (2019).
Cariou, A. et al. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: A matched control study. Resuscitation 76, 397–404 (2008).
Stöckl, M. et al. Continuous versus intermittent neuromuscular blockade in patients during targeted temperature management after resuscitation from cardiac arrest-A randomized, double blinded, double dummy, clinical trial. Resuscitation 120, 14–19 (2017).
Jakkula, P. et al. Targeting low-normal or high-normal mean arterial pressure after cardiac arrest and resuscitation: A randomised pilot trial. Intensive Care Med. 44, 2091–2101 (2018).
Dankiewicz, J. et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N. Engl. J. Med. 384, 2283–2294 (2021).
Lascarrou, J.-B. et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N. Engl. J. Med. 381, 2327–2337 (2019).
Nielsen, N. et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N. Engl. J. Med. 369, 2197–2206 (2013).
Maupain, C. et al. The CAHP (Cardiac Arrest Hospital Prognosis) score: A tool for risk stratification after out-of-hospital cardiac arrest. Eur. Heart J. 37, 3222–3228 (2016).
Wang, C.-H. et al. Prognostic performance of simplified out-of-hospital cardiac arrest (OHCA) and cardiac arrest hospital prognosis (CAHP) scores in an East Asian population: A prospective cohort study. Resuscitation 137, 133–139 (2019).
Isenschmid, C. et al. Performance of clinical risk scores to predict mortality and neurological outcome in cardiac arrest patients. Resuscitation 136, 21–29 (2019).
Paul, M. et al. Comparison of two sedation regimens during targeted temperature management after cardiac arrest. Resuscitation https://doi.org/10.1016/j.resuscitation.2018.03.025 (2018).
Legriel, S. et al. Prognostic value of electrographic postanoxic status epilepticus in comatose cardiac-arrest survivors in the therapeutic hypothermia era. Resuscitation 84, 343–350 (2013).
Benghanem, S. et al. Value of EEG reactivity for prediction of neurologic outcome after cardiac arrest: Insights from the Parisian registry. Resuscitation 142, 168–174 (2019).
Monsieurs, K. G. et al. European resuscitation council guidelines for resuscitation 2015: Section 1. Executive summary. Resuscitation. https://doi.org/10.1016/j.resuscitation.2015.07.038.
Koster, R. W. et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 81, 1277–1292 (2010).
Nolan, J. & European Resuscitation Council. European Resuscitation Council guidelines for resuscitation 2005. Section 1. Introduction. Resuscitation. 67(Suppl 1), S3–6 (2005).
Perkins, G. D. et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: A Statement for Healthcare Professionals From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 96, 328–340 (2015).
Resche-Rigon, M., Azoulay, E. & Chevret, S. Evaluating mortality in intensive care units: Contribution of competing risks analyses. Crit. Care Lond. Engl. 10, R5 (2006).
Austin, P. C. & Fine, J. P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat. Med. 36, 4391–4400 (2017).
Laver, S., Farrow, C., Turner, D. & Nolan, J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 30, 2126–2128 (2004).
Lemiale, V. et al. Intensive care unit mortality after cardiac arrest: The relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 39, 1972–1980 (2013).
Dragancea, I., Rundgren, M., Englund, E., Friberg, H. & Cronberg, T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation 84, 337–342 (2013).
Bougouin, W. & Cariou, A. Mode of death after cardiac arrest: We need to know. Resuscitation 138, 282–283 (2019).
Nolan, J. P. et al. European resuscitation council and European society of intensive care medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. https://doi.org/10.1007/s00134-021-06368-4 (2021).
Sauneuf, B. et al. The CAHP (cardiac arrest hospital prognosis) score: A tool for risk stratification after out-of-hospital cardiac arrest in elderly patients. Resuscitation 148, 200–206 (2020).
Laurent, I. et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 40, 2110–2116 (2002).
Fontaine, C., Jacq, G., Perier, F., Holleville, M. & Legriel, S. The role of secondary brain insults in status epilepticus: a systematic Review. J. Clin. Med. 9, 2521 (2020).
Siesjö, B. K. & Siesjö, P. Mechanisms of secondary brain injury. Eur. J. Anaesthesiol. 13, 247–268 (1996).
Sunde, K. & Andersen, G. Ø. Prediction of outcome after out-of-hospital cardiac arrest already on hospital admission-not reliable enough to be true!. Eur. Heart J. 37, 3229–3231 (2016).
Kikutani, K. et al. Differential effectiveness of hypothermic targeted temperature management according to the severity of post-cardiac arrest syndrome. J. Clin. Med. 10, 5643 (2021).
Bougouin, W. et al. Should we perform an immediate coronary angiogram in all patients after cardiac arrest? Insights from a large French registry. JACC Cardiovasc. Interv. 11, 249–256 (2018).
Jamme, M. et al. Severe metabolic acidosis after out-of-hospital cardiac arrest: Risk factors and association with outcome. Ann. Intensive Care 8, (2018).
Acknowledgements
We thank the Centre Hospitalier de Versailles for editorial assistance.
Author information
Authors and Affiliations
Contributions
M.P.: investigation, data curation, conceptualization, writing-original draft preparation, visualization, writing-reviewing and editing. SL: data curation, writing-reviewing and editing. S.B.: data curation, writing-reviewing and editing. S.A.: data curation, writing-reviewing and editing. A.F.: data curation, reviewing. G.L.: data curation, reviewing. O.R.: reviewing. F.D.: investigation, data curation, conceptualization, methodology, formal analysis, software, writing-reviewing and editing, A.C.: conceptualization, supervision, writing-reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paul, M., Legriel, S., Benghanem, S. et al. Association between the Cardiac Arrest Hospital Prognosis (CAHP) score and reason for death after successfully resuscitated cardiac arrest. Sci Rep 13, 6033 (2023). https://doi.org/10.1038/s41598-023-33129-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33129-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.