Abstract
T-follicular helper (TFH) markers are expressed in the microenvironnement of marginal zone B-cell lymphoma (MZL), and in lymphomas arising from TFH-cells, sometimes making the differential diagnosis difficult. In the skin, the “TFH-spectrum” is poorly defined, going from primary cutaneous lymphoproliferative disorder with small/medium CD4+ T-cells (SMLPD) to cutaneous localizations of systemic angioimmunoblastic T-cell lymphoma (cAITL), and may pass through intermediate forms (primary cutaneous T-follicular helper derived lymphoma, not otherwise specified (PCTFHL,NOS)). We retrospectively analyzed 20 MZL, 13 SMLPD, 5 PCTFHL, and 11 cAITL clinically, histologically, and molecularly, to define tools to differentiate them. Characteristics that might favor the diagnosis of MZL over SMLPD are: multiple skin nodules (p < 0.001), nodular architecture (p < 0.01), residual germinal centers with follicular dendritic cell network (p < 0.001), monotypic plasma cells (p < 0.001), and few staining with PD1 (p = 0.016) or CXCL13 (p = 0.03). PCTFHL and cAITL presented as multiple (p < 0.01) lesions, in older patients (p < 0.01), with systemic symptoms and/or biological alterations (p < 0.01). Immunophenotypic loss of T-cell markers (p < 0.001), BCL6 (p = 0.023) and/or CD10 staining (p = 0.08), and a higher proliferative index (≥ 30%, p = 0.039) favoured these diagnoses over SMLPD. Pathogenic variants were observed by genomic sequencing in 47% of MZL (TNFAIP3 (32%), EP300 (21%), NOTCH2 (16%), KMT2D (16%), CARD11 (10.5%)), 8% of SMLPD (TET2), 40% of PCTFHL (SOCS1 (20%), ARID1A (20%)) and 64% of cAITL (TET2 (63.6%), RHOA (36.4%), NOTCH1 (9%)). This study characterizes the various clinical and histological features between cutaneous lymphomas expressing TFH markers and highlights the value of the interest of screening for genomic mutations in difficult cases.
Similar content being viewed by others
Introduction
Since their arrival in the panel of immunohistochemistry, T-follicular helper (TFH) markers have helped improving diagnostic accuracy in many subtypes of lymphomas. Their expression is mainly observed in T-cell lymphomas arising from TFH-cells. In the skin, lymphoma deriving from TFH-cells are poorly defined. Some authors suggest that there is probably a spectrum of different lymphoproliferative diseases arising from TFH cells, with different clinical presentations and prognosis. Primary cutaneous lymphoproliferative disorder with small/medium CD4+ T-cells (SMLPD)1 is described, as the beginning of this spectrum, as an asymptomatic and indolent entity2. On the other end of the spectrum, cutaneous localizations of angioimmunoblastic T lymphoma3 (cAITL) are generally associated with generalized lymphadenopathy and systemic symptoms. Halfway, some authors claim the existence of an emerging entity4,5,6 called primary cutaneous T-follicular helper derived lymphoma, not otherwise specified (PCTFHL, NOS). The clinical presentation of PCTFHL is intermediate between SMLPD and AITL: unique or multiple plaques/nodules with lymphadenopathies or biological alterations (increase LDH, cytopenia, eosinophilia)6. The presence of multiple lesions have been associated with an unfavorable clinical course7. It seems crucial to find histological or molecular tools to better differentiate those entities which present a distinct aggressiveness and evolution. Furthermore, molecular alterations of PCTFHL are unknown.
TFH-cells are also find in the microenvironnement of B-cell lymphomas, especially marginal zone lymphomas (MZL)6,8,9,10. The clinicopathological characteristics of cutaneous MZL are close to some cutaneous T-cell lymphomas with a TFH phenotype, in particular primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder (SMLPD)10. The distinction between MZL and SMLPD is sometimes difficult and clonality study is not always discriminatory due to cases without monoclonal rearrangement or with both B (BCR) and T-cell receptor (TCR) rearrangements7.
Targeted Next Generation Sequencing (TNGS) has proven its interest in daily practice11, but few studies have analyzed the molecular profile of cutaneous lymphomas. Concerning AITL, most studies focus on nodal locations, in which mutations of TET2 (52–76%), IDH2 (20–45%), DNMT3A (30–40%) and RHOA G17V (28–70%) are typical11,12,13,14,15,16. The only study currently published in cutaneous localizations of AITL is the one of Leclaire Alirkilicarslan et al., which included 41 patients and found IDH2 R172K/S and RHOA G17V mutations in 19% and 78% of cases respectively17. A recent study also showed a mutation of DNMT3A18 in one case of SMLPD.
We retrospectively described a series of 49 cutaneous lymphomas with TFH-markers expression, including cases with TFH expression in tumoral cells and cases with TFH hyperplasia in the microenvironnement, to characterize them at a clinicopathological and molecular level and highlight tools to differentiate them.
Methods
Patient selection
TFH-cells arising lymphomas (SMLPD and cutaneous localizations of systemic AITL) and cutaneous MZL diagnosed from March 2017 to March 2019 in agreement to the 2016 WHO classification of hematologic malignancies were retrieved from the pathological department of the Lyon Sud University Hospital, France. Intermediate forms between SMLPD and AITL, so-called PCTFHL, were also retrieved.
Inclusion criteria for MZL, PCTFHL and SMLPD were the presence of a monoclonal B- or T-cell population, to limit the inclusion of reactive lymphoid hyperplasia, and enough FFPE material available. All cases had to have benefited from an extensive assessment including either a CT-scan or chest X-ray and abdominal and pelvic ultrasound, to exclude systemic lymphoma with secondary involvement of the skin. MF and/or Sezary syndrome were ruled out by clinicopathological correlation and expert opinion from the French cutaneous lymphomas study group (GFELC). Concerning cutaneous localizations of AITL, cases were included only if they had a known history of systemic AITL, proved by a lymph node biopsy.
Clinical, biological, and radiological data
Clinical data were retrospectively collected. It included: age at diagnosis, sex, medical history, clinical presentation (number of lesions, localization, systemic symptoms, and lymphadenopathy), biological data (blood count, borreliosis serology), imaging data, received treatments and evolution. Patients were divided into two groups: (i) “indolent” (spontaneous disappearance and/or no relapse after local treatment); or (ii) “refractory/relapsing” (relapse/persistence after initial local treatment requiring chemotherapy or radiotherapy).
Histological review
Experts pathologists of the French cutaneous lymphomas study group (GFELC)18,19,20 (BB, OH) or of the Lymphopath Network21 (ATG, MD) reviewed cases. They recorded: localization (superficial, mid or deep-dermis, hypodermis), architecture (lichenoid (sub-epidermal band), nodular, diffuse, or interstitial (scattered cells in the dermis) pattern), size of cells, epidermotropism (in these cases, results of the blood immunophenotyping were retrieved to exclude cases with a doubtful diagnosis with Sezary syndrome), germinal centers, and associated cells.
Immunochemistry study
Immunohistochemistry for CD2, CD3, CD4, CD5, CD7, CD8, TFH markers, CD20, CD21, CD23, CD30, CD138, kappa, lambda, and Ki67/Mib1 was performed using standard procedures (Leica BOND-MAX, Leica Microsystems SA, Nanterre, France). In situ hybridization for Epstein-Barr virus (EBV) encoded RNAs (EBER) was performed using a Ventana BenchMark XT automated immunostainer. Protocol and antibodies are detailed in Suppl. Methods and Suppl. Table S1. The proportion of B and T-cells was estimated as the ratio of cells to the total lymphocytic infiltrate. The expression of CD138 was scored semi quantitatively on the basis of the proportion of positive cells in the whole tissue: 0: no staining, 1+: < 10%, 2+: 10 to 25%, 3+: ≥ 25%. Immunochemistry of TFH markers was performed using the following antibodies: PD1 (NAT105; Roche Diagnostics; Meylan, France), CXCL13 (BCA-1; R&D System, Minneapolis, USA), BCL6 (GI191E/A8; Roche Diagnostics; Meylan; France), CD10 (SP67; Roche Diagnostics; Meylan; France), and ICOS (AB105227; Abcam, Cambridge, UK). Their expression was scored quantitatively on the basis of the proportion of positive cells in the whole T-cells infiltrate. They are presented with their standard deviation. The proliferative index was evaluated as the percentage of Ki67-positive cells relative to the total lymphocyte population.
Clonality study and targeted next generation sequencing (TNGS)
Clonality study was performed according to the EuroClonality/BIOMED-2 protocol22. IGH and IGK assays were used for BCR study, with the following primers: FR1, FR2, FR3, DH–JH, Ig kappa, and Ig lambda. TCR Clonality was assessed using the BIOMED-2 primer sets for TCR gamma (TCRG). TNGS have been performed as previously reported11,23. The applied panel of 47 genes is detailed in Suppl. Table S2. Only pathogenic variants with a minimal depth of 700× were retained. Supplemental quality data are available in Suppl. Table S4.
Statistical analysis
Statistical analysis has been performed using the Medistica pvalue.io software24, performing univariate analysis for descriptive variables. Kruskal–Wallis test was used to study ordinal qualitative ordinal variables (immunohistochemistry results) and quantitative variables (age) in the 4 independent groups (CMZL, SMLPD, PCTFHL, and cAITL). Fisher’s exact test or χ2 test were used to study nominal qualitative variables (clinical and histological characteristics) in the different groups.
Compliance with ethical standards in research
This study was performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all subjects and/or their legal guardian(s) according to the guidelines of the French Bioethics Law. The protocol has received the validation of the local ethics committee (Ethics committee of the Hospices Civils de Lyon, number 21_5588).
Results
Sixty-seven cases corresponding to 27 MZL, 21 SMLPD, 8 PCTFHL and 11 cAITL were retrieved. Seven MZL were excluded due to the presence of a systemic MZL. Eight SMLPD were excluded: 3 because the material was exhausted, 4 due to the absence of any monoclonal T-cell population, and one because further evaluation led to reclassification as MF. Three PCTFHL were excluded, two due to the absence of TCR clonal rearrangement, and one due to the presence of Sezary cells in the blood, leading to reclassification as Sezary syndrome. Twenty MZL, 13 SMLPD, 5 PCTFHL, and 11 cAITL were finally included.
Clinical presentation (Table 1)
MZL presented as erythematous papules or nodules (16/20, 80%) or erythematous-squamous plaque (4/20, 20%). Lesions were more often multiple (11/20, 55%) with, on average, 3 lesions per patient [range: 2–5]. Median age was of 56-years-old [27–84]. Locations were upper limbs (40%), trunk and abdomen (40%), lower limbs (10%), and head and neck (10%). Borrelia serology was positive in 2 cases. None had lymphadenopathy. Blood counts were normal.
SMLPD presented as unique (100%) erythematous nodule (9/13, 69%) or erythematous-squamous plaque (4/13, 31%), mainly located on the trunk (55%) or head and neck (30%), with a median age of 56-years-old [25–78]. Borrelia serology was negative in all cases. Blood counts were normal.
PCTFHL presented as multiples (5/5, 100%) erythematous-squamous plaques (2/5, 40%) or erythematous nodules (2/5, 40%), or papules (1/5, 20%). One case (PCTFHL no 5) presented a monoclonal circulating CD4+ T-cell population without immunophenotyping feature of Sezary syndrome. The median age was 74-years-old [47–91]. Two PCTFHL presented biological alterations (anemia: Haemoglobin 11.2 g/dl and increased LDH level: 352 U/L).
Most cAITL presented as a maculopapular rash (6/11, 55%), with a median age of 74-years-old [56–92]. Other cases presented as multiple (100%) erythematous papules (2/11, 18%) or erythematous-squamous plaques (3/11, 27%). In AITL no 11 the disease appeared as a unique, infiltrated, necrotic plaque of the abdomen. In six cases, biopsies were performed at the onset of the disease (at diagnosis). For the five other cases, cutaneous lesions occurred during a relapse of the disease (after a first line of chemotherapy). All patients described systemic symptoms (deterioration in the general condition: n = 11/11, pruritus: n = 3/11, fever: n = 4/11) or biological alterations (bi/pancytopenia: n = 7/11, increase in LDH [338–600]: n = 4/11).
Histological review and immunohistochemistry study (Table 2)
MZL (Figs. 1, 2) presented a nodular architecture (19/20, 95%), with residual germinal centers (GC) (12/20, 60%) and a CD23+ follicular dendritic cell (FDC) network (15/20, 75%). One case presented a lichenoid architecture. Hypodermis involvement was obseved in 7/20 cases (35%). MZL were composed of more B (55% (± 17.2)) than T-cells (43.5% (± 16.1)), but 4 had more T-cells than B-cells, and 2 had equal proportions of both. CD138+ plasma cells were present in almost all cases (n = 19/20, 95%), representing more than 10% of cells in 65% of cases, and being monotypic in 75% of cases. Eosinophils were present in 4/20 cases (20%). TFH markers were not constantly expressed in MZL cases; PD1: n = 18/20; CXCL13: n = 15/20; BCL6: n = 5/20; no CD10 expression, and average of stained were low; PD1: 19% (± 17.6); CXCL13: 8% (± 8.01); BCL6: 2.5% (± 4.44). ICOS was expressed in 6 cases (< 1% of stained cells). When positive, PD1 was expressed with a high intensity on the T-cells of the germinal centers, and, with a weak or moderate staining in small T-cells of the background. Ki67 was evaluated at 18% (± 6.1).
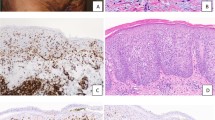
Histological presentation of a primary cutaneous marginal zone lymphoma (MZL, case no 6). (A) Clinical presentation as 3 erythematous nodules of the face; (B) (Hematoxylin–Eosin-Saffron (HES), ×2.5): Nodular and diffuse architecture involving all the dermis; (C) (HES, ×20): small hyperchromatic cells associated with histiocytes; (D) (CD20, ×20) and (E) (CD4, ×20): Association of B and T-cells in equal proportions (among 50% of each); (F) (CD23, ×10): Residual germinal centers, underlined by a network of CD23+ follicular dendritic cells; (G–I) (CD138 × 10, kappa/lambda, ×20); Association to numerous monotypic kappa plasma cells located outside of the B-cell nodules; (J) (PD1, ×10), (K) (CXCL13, ×20), and (L) (BCL6, ×10): small T-cells of the background harbor a TFH phenotype (PD1 30%, CXCL13 15%, BCL6 10%, CD10 negative). Proliferative index using Ki67/Mib1 was evaluated a 20% (picture not shown). The diagnosis of MZL was confirmed thanks to the presence of a monoclonal B-cell population. This case displayed no pathogenic variant in TNGS.
Histological presentation of a primary cutaneous marginal zone lymphoma (MZL, case no 2) with significant TFH hyperplasia, illustrating the diagnostic difficulty between SMLPD and MZL. (A) (Hematoxylin–Eosin-Saffron (HES), ×10): Nodular and diffuse architecture involving all the dermis, well separated from the epidermis by a “grenz-zone”; (B) (HES, ×20): Small hyperchromatic cells; (C) (CD20, ×5) and (D) (CD4, × 5): The infiltrate is composed of admixed B-cells (50%) and CD4+ T-cells (50%) ; (E) (PD1, ×10) and (F) (CXCL13, ×20): Strong expression of TFH markers in the microenvironnement (PD1: 80%, CXCL13: 25%); (G) (CD23, ×20): Absence of residual CD23+ follicular dendritic cells network; (H,I) (kappa and lambda, ×20); Plasma cells were few (< 10%) but presented a kappa monotypia. The diagnosis of MZL was confirmed thanks to molecular data; the presence of a monoclonal B-cell population, and pathogenic variants of ITPKB, NOTCH2, TNFAIP3, and KMT2D genes using TNGS.
SMLPD (Fig. 3) presented a nodular (7/13, 54%), diffuse (2/13, 15%), or lichenoid (4/13, 31%) pattern, without residual GC nor FDC network, and with hypodermis involvement in 6/13 cases (46%). Eosinophils were present in 6/13 cases (46%). Infiltrate was composed of a majority of T (61.5% (± 11.4)) rather than B-cells (35% (± 12)), except in 4 cases in which T and B-cells were equally distributed. There was no loss of T-cell markers. Plasma cells were present (n = 12/13, 92%), but represented less than 10% of cells in most cases (n = 11/12, 85%) and were always polytypic. PD1 and CXCL13 were seen in all cases, BCL6 in 6 cases and ICOS in 4 cases. Average of stained T-cells were; PD1: 49% (± 33.7), CXCL13: 20% (± 20.0), BCL6: 11% (± 20.7), ICOS: < 1%. There was no CD10 expression. PD1 showed a higher intensity in medium/large cells, and some PD1+ cells tended to form clusters or “rosettes” around large lymphocytes (Fig. 3H). Ki67 was evaluated at 15% (± 6.6).
Histological presentation of a primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder (SMLPD, case no 7). (A) Clinical presentation as an infiltrated erythematous-squamous plaque of the left breast; (B) (Hematoxylin–Eosin-Saffron (HES), ×1.5), (C,D) (HES, ×20): lichenoid pattern constituted of atypical cells, small-medium sized; (E) (CD4, ×10) and (F) (CD20, ×10): slight predominance of T-cells (60%), with a CD2+ CD5+ CD7+ phenotype, and a significant predominance of CD4+ T-cells (75%) instead of CD8+ T-cells (25%). (G) (CD138, ×10): very few associated plasma cells (black arrow), which were polytypic for kappa and lambda, and located outside of the proliferation in this particular case, but scattered among T-cells in most SMLPD cases; (H) (PD1, ×20), (I) (CXCL13, ×20), (J) (BCL6, ×20) and (K) (CD10, ×20): huge expression of TFH markers except for CD10 which was negative (PD1 90%, CXCL13 40%, BCL6 25%). As illustrated, PD1 expression shows a higher intensity in medium/large cells, and some PD1+ cells also tended to form “rosettes” around large lymphocytes (insert). Proliferative index using Ki67/Mib1 was evaluated a 15%, and no follicular dendritic cells network (pictures not shown). The diagnosis of SMLPD was confirmed thanks to molecular data; the presence of a monoclonal T-cell population. This case displayed an isolated TET2 pathogenic variant using TNGS.
In PCTFHL (Fig. 4), architecture was lichenoid (3/5, 60%), interstitial (1/5, 20%) or nodular (1/5, 20%). There were no epidermotropism, excepted in PCTFHL no 3. Hypodermis involvement was rare (1/5, 20%). Eosinophils were seen in 3 PCTFHL (60%). There were no residual GC nor FDC network. Plasma cells were rare (n = 2/5, 40), representing less than 10% of the cells without monotypia. T-cell markers were lost in 80% of PCTFHL (CD7: 3/5, CD5: 1/5). PD1, CXCL13 and BCL6 were expressed in all cases, and CD10 expression was observed in 2/5 PCTFHL. Average of stained T-cells in PCTFHL were; PD1: 71% (± 21.3); CXCL13: 30% (± 20.0); BCL6: 20% (± 12.2), and CD10: 4% (± 5.48). ICOS had not been performed. PD1 was expressed with a moderate to high intensity on the T-cells, with a diffuse pattern. Ki67 was evaluated at 32% (± 27.7).
Histological presentation of a primary cutaneous T-follicular helper lymphoma (PCTFHL, case no 3). (A) clinical presentation as pruriginous annular, erythematous and infiltrated lesions, located in the back, the right preauricular region, and the chin; (B) (Hematoxylin–Eosin-Saffron (HES), ×1.5) and (C) (HES, ×20): lichenoid pattern with epidermotropism. Infiltrate was constituted of atypical cells, small-medium sized, associated with eosinophilic polynuclear (black arrow); (D,E) (CD3, ×10, ×20) and (F,G (CD20, ×10, ×20): slight predominance of T-cells (60%), with a partial loss of CD7, associated to numerous B-cells (without CD30 expression or RNA EBER positivity); (H) (PD1, ×20), (I) (CXCL13, ×20), (J) (BCL6, ×20) and (K) (CD10, ×20): huge expression of TFH markers (PD1 80%, CXCL13 50%, BCL6 20% CD10 10%). Proliferative index using Ki67/Mib1 was evaluated a 35% (picture not shown). The diagnosis of PCTFHL was confirmed thanks to molecular data; the presence of a monoclonal T-cell population. This case displayed a pathogenic variant of SOCS1 using TNGS.
In cAITL (Fig. 5), infiltrates were sparse; architecture was mostly interstitial (9/11, 82%) than nodular (2/11, 18%). Plasma cells were rare (n = 4/11, 36%), representing less than 10% of the cells without monotypia. Eosinophils were seen in 7 cAITL (64%). There were no residual GC nor FDC network. Hypodermis involvement was never seen. CD30+ B-cells were seen in 8/11 cAITL, with overexpression of ARN EBERs using in situ hybridization in 1/11 case. T-cell markers were lost in 45.5% of cAITL (CD7: 4/11, CD5: 1/11). PD1, CXCL13 and BCL6 were expressed in all cases, and CD10 expression was observed in 3/11 cAITL (28%). Average of stained T-cells in cAITL were; PD1: 40.5% (± 19.7); CXCL13: 16.5% (± 16.7); BCL6: 21% (± 17.7), and CD10: 4% (± 6.74). ICOS had not been performed. PD1 was expressed with a moderate to high intensity on the T-cells, with a diffuse pattern. Ki67 was evaluated at 35% (± 22).
Histological presentation of a cutaneous localization of angioimmunoblastic T-cell lymphoma (cAITL, case no 2). (A) Clinical presentation as maculopapular rash reaching 50% of the skin surface; (B) (Hematoxylin–Eosin-Saffron (HES), ×1.5) and (C) (HES, ×20): nodular and diffuse architecture, constituted of atypical cells, small-medium sized, associated to large immunoblastic cells; (D) (CD3, ×20): Atypical cells presented a T-cell phenotype, with loss of the CD7; (E) (CD20, ×10) and (F) (CD30, ×10): large immunoblastic B-cells, which intensely expressed CD30. (K) (hybridization in situ (HIS) RNA EBER, ×1.5): CD30+ B-cells also presented an overexpression of RNA EBER of the Epstein Barr Virus; (G) (PD1, ×20), (H) (CD10, ×20), (I) (BCL6, ×20) and (J) (CXCL13, ×20): Expression of TFH markers (PD1 40%, CD10 25%, BCL6 10%, and CXCL13 10%). Proliferative index using Ki67/Mib1 was evaluated a 70% (picture not shown). The diagnosis of cAITL was confirmed thanks to molecular data; the presence of a monoclonal T-cell population associated with a minor monoclonal B-cell population. This case did not display any pathogenic variant using TNGS.
Clinical and histological comparison between CMZL and SMLPD
Both CMZL and SMLPD presented as erythematous nodules (80% vs. 69%, p = 0.68), but lesions were more often multiples in MZL (55% vs. 0%, p < 0.001). Histologically, nodular architecture (95% vs. 54%, p < 0.01), presence of residual germinal centers with FDC network (75% vs. 8%, p < 0.001), and monotypic plasma cells (75% vs. 0%, p < 0.001) were in favor of MZL versus SMLPD. Lichenoid architecture was not significantly different in these two subgroups (p = 0.06), nor was the presence of eosinophils (p = 0.14). B-cells were more abundant in MZL (55% vs. 35%, p < 0.01), whereas T-cells were more abundant in SMLPD (61.5% vs. 43.5%, p < 0.001). PD1 expression was lower in MZL compared to SMLPD (19% vs. 49%, p = 0.016). CXCL13 (8% vs. 20%, p = 0.03) and BCL6 (2.5% vs. 11%, p = 0.27) were less expressed in CMZL than SMLPD.
Comparison between lymphomas of the TFH spectrum
PCTFHL and cAITL arised in older patients (p < 0.01) than SMLPD. Clinically, presentation as maculopapular rash was caracteristical of cAITL (p < 0.001). Presence of multiple lesions (nodules or papules) may guide the diagnosis toward these subtypes rather than SMLPD (p < 0.001), nodules being more characteristics of SMLPD or PCTFHL than cAITL (p < 0.01). Histologically, lichenoid architecture was suggestive of PCTFHL or SMLPD instead of cAITL (p = 0.023), in which interstitial architecture was more frequent (p < 0.001). Eosinophils were more evocative of a lesion of the TFH spectrum (CMZL: 20% vs. SMLPD 46%, PCTFHL 80%, cAITL 64%, p < 0.01) but were not statistically different within the spectrum of TFH lymphomas (p = 0.71). Losses of T-cell markers were seen only in PCTFHL (80%) and cAITL (45%) (< 0.001). PD1 was more often expressed in PCTFHL compared to other subtypes, without significant statistical differences (SMLPD: 49%, PCTFHL: 71%, cAITL: 40.5%, p = 0.12). There were no statistical differences between the expressions of CXCL13 in lymphomas of the TFH spectrum (SMLPD: 20%, PCTFHL: 30%, cAITL: 16.5%, p = 0.79). BCL6 was significantly more frequent in PCTFHL and cAITL than SMLPD (SMLPD: 11%, PCTFHL: 20%, cAITL: 21%, p = 0.024). CD10 was expressed only in PCTFHL and cAITL (p = 0.083). Proliferative index was slightly more elevated in intermediate forms and systemic lymphomas (SMLPD: 15% (± 6.60), PCTFHL: 32% (± 27.7) and cAITL: 35% (± 22.0), p = 0.039).
Treatments and evolution (Tables 1, 4)
MZL treatments consisted of topical corticosteroids (11/20, 55%), doxycycline (10/20, 50%), often associated with surgical excision (15/20, 75%). Nine cases suffered local relapses, treated by topical corticosteroids (6/9), radiotherapy (3/9).
SMLPD treatments consisted in topical corticosteroids (betamethasone dipropionate or clobetasol propionate, 3/13, 23%), antifungal cream (1/13, 8%), antibiotic therapy (21 days of doxycycline, 3/13, 23%), alone or associated with surgical excision (10/13, 77%). One case presented a spontaneous disappearance after biopsy. All cases presented an indolent evolution. Among the three cases which did not undergo surgery, two suffered a relapse after dermocorticoids disruption.
Among PCTFHL, the median follow-up was of 20 months. The case no 5 with a monoclonal circulating T-cell population responded completely under topical corticosteroids and was considered indolent. Three were refractory/relapsing patients; one received topical corticosteroids with a remitting relapsing course (median follow-up of 50 months), one received radio-chemotherapy (gemcitabine), and the last received methotrexate. The fifth was lost to follow-up.
All cAITL were treated by chemotherapy (9 CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and 2 GEMOx: gemcitabine, oxaliplatin).
Clonality study (Tables 2, 3, 4)
With the presence of a monoclonal population being inclusion criteria, BCR rearrangements were present in all MZL and TCR rearrangements in all SMLPD and PCTFHL. The same clone was found in the skin biopsy for the PCTFHL case with a circulating T monoclonal population.
Concerning cAITL, 5 presented a monoclonal T-cell population, 2 presented both BCR and TCR rearrangement, results were undetermined in 3 cases, and one did not display any monoclonal population (AITL no 9).
Two SMLPD and one MZL presented both BCR and TCR rearrangements. The 2 SMLPD presented characteristics typical of SMLPD: unique nodules, 66–80% of T-cells, absence of FDC network, 5–20% polytypic plasma cells, medium PD1/CXCL13 staining (10–30%). The MZL case presented characteristics typical of MZL: multiple lesions, majority of B-cells (60%), nodular pattern, residual germinal centers, 25% of plasma cells with lambda monotypia, low PD1/CXCL13 staining (10%).
TNGS analysis (Tables 3, 4)
The TNGS analysis (Table 3) was performed in all cases except for 1 MZL and 1 SMLPD due to a poor DNA quality. Pathogenic variants were found in 9 MZL (47%), 1 SMLPD (8%), 2 PCTFHL (40%) and 7 cAITL (64%). Pathogenic variants found in MZL were: TNFAIP3 (6/19, 32%), EP300 (4/19, 21%), NOTCH2 (3/19, 16%), KMT2D (3/19, 16%), CARD11 (2/19, 10.5%), PLCG2 (1/19, 5%), CREBBP (1/19, 5%), ITPKB (1/19, 5%), CIITA (1/19, 5%), GNA13 (1/19, 5%), and FBXW7 (1/19, 5%). Despite good quality NGS data, few mutations were identified in SMLPD and PCTFHL. Pathogenic variant found in SMLPD was TET2 (1/12, 8.5%). Pathogenic variants found in PCTFHL were SOCS1 (1/5, 20%) and ARID1A (1/5, 20%). Patient with the SOCS1 variant presented the following characteristics: presentation as multiple erythematous and infiltrated plaques without Sezary cells or circulating population, lichenoid pattern with epidermotropism, 60% of T-cells (CD7 lost) and 40% of B-cells, high expression of TFH markers (PD1 80%, CXCL13 50%, BCL6 20% CD10 10%). Patient with the ARID1A variant presented the following characteristics: multiple papules without Sezary cells or circulating population, lichenoid pattern separated from the epidermis by a grenz-zone and without epidermotropism, 80% of T-cells (CD7 lost) and 20% of B-cells, variable expression of TFH markers (PD1 95%, CXCL13 20%, BCL6 10%, no CD10 expression). Pathogenic variants found in cAITL were: TET2 (n = 7, 64%), RHOA (n = 4, 36%; 3 RHOAG17V and 1 RHOAG17L), and NOTCH1 (1/11, 9%). Five cAITL displayed at least two pathogenic variant (TET2 ± RHOA or NOTCH1), and two displayed only variants of TET2. Patients with TET2 mutations had two TET2 Single Nucleotide Variant (SNV) except one, with a single TET2 SNV.
Molecular analysis had also been performed in addition to the skin tissue in lymph node biopsies of 4 AITL (Table 4). Cases no 3, 7 and 10 presented a similar monoclonal T population both samples, case no 9 had had a TCR gamma chain rearrangement with an undetermined ratio in the skin biopsy, and a true T monoclonal population in the lymph node. Cases no 7, 9 and 10, presented the same pathogenic variants in both samples, including TET2 and/or RHOAG17V hotspot.
Discussion
The differential diagnosis of cutaneous lymphomas with TFH expression and/or hyperplasia is frequently a diagnostic challenge in daily diagnosis work. The spectrum of cutaneous T-cell lymphomas arising from TFH-cells extends from SMLPD to AITL6. Hyperplasia of TFH reactive T-cells is also frequently observe in cutaneous MZL25. The objective of the present study was to better characterize these different lesions (20 MZL, 13 SMLPD, 5 PCTFHL, and 11 cAITL) at the clinicopathological and molecular levels and to highlight tools to differentiate them.
The clinical presentation and histological appearance of the 49 cases described in this study were consistent with the literature3,6,18,26. In particular, MZL corresponded to erythematous papules/nodules (80%), or plaques (20%), which were most frequently located on the limbs (50%) and trunk (40%)26. Infiltrates in SMLPD could be separated into two patterns, as described by Beltzung et al.; (i) "Pattern 1" (n = 9/13, 69%): erythematous nodules, nodular/diffuse architecture, located on the head and neck (n = 4/9, 44%), followed by the trunk (n = 3/9, 33%) and upper extremities (n = 2/9, 23%); and (ii) "Pattern 2" (n = 4/13, 31%): erythematous-squamous plaques, lichenoid architecture, all located on the trunk18. Interestingly, loss of CD7 is described in the literature in 24% of SMLPD18. However, in this serie, none of the SMLPD cases showed CD7 loss, even partially. Although these data need to be confirmed in a larger cohort, it could be a tool to differentiate more aggressive forms of SMLPD, or even PCTFHL. Clinical presentation of PCTFHL was similar as described (multiple papules, plaques, and nodules of trunk/head)5,6, excepted that only one case presented a circulating monoclonal population (vs. 4/5 for Battistella et al.)6. Maculo-papular rash seems to be the classical presentation of cutaneous locations of AITL3.
MZL appears to differ clinically from SMLPD in their presentation as multiple skin nodules (p < 0.001). Histologically, nodular architecture (p < 0.01), presence of an FDC network (p < 0.001), and presence of monotypic plasma cells (p < 0.001) are characteristics that should favor the diagnosis of MZL rather than SMLPD. PD1 and CXCL13 were expressed in all SMLPD, but inconstantly in MZL (90% and 75% respectively), and with fewer positive cells in MZL than SMLPD: PD1 (19% in MZL vs. 49% in SMLPD, p = 0.016), CXCL13 (8% in MZL vs. 20% in SMLPD, p = 0.03), or BCL6 (2.5% in MZL vs. 11% in SMLPD, p = 0.27). PD1 expression profile was also different in MZL and SMLPD ; PD1 was expressed on medium to large and frequently clustered cells in SMLPD18, whereas, in MZL, PD1 was expressed by the small T-cells of the germinal centers.
Concerning T-cell lymphoproliferations/lymphomas, features in favor of a more aggressive disease such as PCTFHL or AITL are: elderly patients (p < 0.01), presentation as multiple (p < 0.001) erythematous and scaly patches or as a maculopapular rash for cAITL (p < 0.001), association with systemic symptoms and/or biological alterations (p < 0.001), interstitial architecture (p < 0.001), loss of T-cell markers (p < 0.001), and an elevated proliferative index (p < 0.01). Concerning TFH markers, there was no difference in the amount of stained cells with PD1 (p = 0.12) or CXCL13 (p = 0.79) across these subtypes, but BCL6 (p = 0.024) and CD10 (p = 0.08) seemed more often positive in cAITL and PCTFHL. Extension assessment, including a CT scan and immunophenotyping of the circulating blood, is particularly important to differentiate PCTFHL from cAITL.
In this study, the main objective was to compare these entities. That’s why it was decided to include only cases with a monoclonal population, to try to include only "typical" cases and to limit the possibility of including inflammatory diseases, as, in the skin, dominant clones may also be found in a variety of benign dermatoses. Literature has already proved the interest of clonality assessment in the diagnosis of cutaneous lymphomas, the finding of a monoclonal population orienting the diagnosis in more than 80% of MZL26, more than 65% of SMLPD18,27, and all PCTFHL6. This study further emphasizes the importance of an integrated histomolecular diagnosis. Indeed, 4 MZL presented a PD1 expression with more than 30% of stained cells, making the differential diagnosis with SMLPD particularly difficult without molecular data. In these patients, a monoclonal B-cell population was found, without monoclonal T-cell population, and the diagnosis of MZL could be made. The study of clonality may therefore be helpful in these cases of MZL with TFH hyperplasia of the microenvironnement10. Moreover, this study also highlights the value of comparing molecular techniques in cases of suspected cutaneous localization of systemic lymphoma. The discovery of the same monoclonal population (AITL no 3) or same NGS pathogenic variants (AITL no 7, 9 and 10), in skin biopsy and lymph node can, indeed, allow to link two locations of the same disease.
Taken individually, TNGS also identified pathogenic variants helpful for the diagnosis in 42% of MZL and 64% of cAITL. Even if these cases do not represent a large majority, they are not negligible considering the importance of an optimal classification of these lesions for the management and prognosis.
In the present study, 47% of MZL presented pathogenic variants, involving TNFAIP3 (n = 6, 32%), KMT2D (n = 3, 16%), EP300 (n = 4, 21%), NOTCH2 (n = 3, 16%), and CARD11 (n = 2, 10.5%). According to the Cosmic database, NOTCH2 (19%), KMT2D (15%), and TNFAIP3 (11%) are the top 3 most frequent mutated genes observed in mucosa-associated lymphoid tissue (MALT) lymphomas28 and nodal MZL29. EP300 and CARD11 are also frequently described in MZL30. FBW7 has not been considered as helpful31. Mutations do not seem correlated to prognosis in MZL: 44% of indolent cases among the mutated cases and 60% of relapses among the non-mutated cases. However, the panel targeted the most relevant genes for lymphoma diagnosis but did not cover all exons for some genes (e.g., KMT2D) and lacked a few select genes (e.g., FAS, SLAMF1, SPEN, and NCOR2), which are described in cutaneous MZL10,32. This could explain the negativity of some cases and the lack of correlation with the clinical course.
Concerning AITL, most studies concern nodal locations, in which mutations of TET2 (52–76%), IDH2 (20–45%), DNMT3A (30–40%) and RHOA G17V (28–70%) seem frequent11,12,13,14,15,16. The only study currently published in cutaneous localizations is the one of Leclaire Alirkilicarslan et al., which included 41 patients and found IDH2 R172K/S and RHOA G17V mutations in 19% and 78% of cases respectively using PCR17. This study is the only one to study mutations using TNGS on a cohort of cutaneous localizations of AITL. Among them, 64% presented pathogenic variants involving TET2 (n = 7, 64%), RHOA (n = 4, 36%), and NOTCH1 (n = 1, 9%). Leclaire Alirkilicarslan et al. found 78% of RHOA G17V and 19% of cases with IDH2 R172 substitutions using PCR in a cohort of cAITL. In nodal AITL, mutations are much more frequent11,12,15; TET2 SNV (76%), RHOA G17V (60–78%), and IDH2 R172 substitutions (19.5%). In the present study, the percentage of mutated cases was lower than described in the literature, and no IDH2 pathogenic variants were found. This may be explained by the small number of cases in the study or by the low density of tumoral cells in the samples. However, these molecular findings were confirmative of the diagnosis in 64% of cases, as in 4/5 cases with an atypical presentation (slight perivascular infiltrates, no identifiable atypical lymphocytes) in the study of Leclaire Alirkilicarslan et al. These data suggest that TNGS may represent a useful tool for the diagnosis of cAITL. The VAF of the SNV were lower in cAITL (average = 6.7%, median = 3.1%, ranging from 1.2 to 22%), compared to 14.2% in MZL and 45.2% in PCTFHL. It can be explained by the low cell density in these biopsies (less than 25% of the surface) or by clonal tumor heterogeneity, as already described in these entities13,33. Nevertheless, the sequencing quality remains optimal; the described variants are robust due to a minimal depth of 700× (Suppl. Table 3).
The second objective of this study was to decipher the spectrum of TFH lymphomas, including also lymphoproliferations and controversial intermediate forms. Concerning SMLPD, only one case (8%) presented a pathogenic variant of TET2, which may correspond to clonal hematopoiesis. Beltzung et al. reported a unique pathogenic variant of DNMT3A among 13 SMLPD, but this gene was not part of our panel18. Concerning PCTFHL, two cases (40%) presented isolated pathogenic variants of ARID1A and SOCS1. These two mutated cases required aggressive treatment, whereas the two cases with an indolent disease did not present any detectable variant. ARID1A mutations are reported in numerous cancers and lymphomas and therefore does not seem really helpful in isolation15,34. SOCS1 was a class 3 of uncertain clinical significance35,36. This variant has already been described in TFH lymphomas34, but also in MF37. This finding was arguable, PCTFHL currently not being a recognized entity, and of difficult differential diagnosis with MF expressing TFH markers or Sezary syndrome38,39. In PCTFHL no 3 in particular, the clinical presentation as erythematosquamous plaques, the presence of epidermotropism, and the SOCS1 mutation may have suggested the diagnosis of MF or Sezary syndrome. Nevertheless, the absence of Sezary cells in the blood, the presence of interface dermatitis lesions, the polymorphism of the infiltrate (presence of 50% of B-cells and numerous plasma cells), and the intense expression of all TFH markers (PD1: 80%, CXCL13: 50%, BCL6: 20%, CD10: 10%) pleaded against this diagnosis. Within the panel studied, there does not appear to be a common or recurrent molecular profile between SMLPD, PCTFHL, or cAITL. The molecular abnormalities usually present in AITL were not found in SMLPD or PCTFHL. These data remain debatable and future studies will undoubtedly allow a better classification of these case. Indeed, one of the raised hypotheses was that the spectrum of TFH-lesions was potentially underpinned by a mutational spectrum. The frequency of mutations found in TNGS (8% in SMLPD, 40% in PCTFHL and 64% in cAITL) seems to go in the same direction as the prognosis of these lesions. As already described in nodal AITL, these data may suggest that RHOA mutation may be a secondary event in lymphomatous cells after other critical molecular events such as TET2 mutations that first occur in "premalignant" lymphocyte precursors13,17,40,41,42. This hypothesis may agrees with the distribution of pathogenic variants in the present study and reinforces the theory of a spectrum of lymphoma derived from a TFH lymphocyte. These data will need to be confirmed in a larger cohort, gathering a larger number of PCTFHL cases. If accepted, the provisional PCTFHL entity will also need to be defined, in particular its exact terminology (lymphoproliferation or lymphoma), and diagnosis criteria. The controversial nature of these intermediate forms makes it difficult to include these patients in studies.
This study highlights some histological features to distinguish the main subtypes of cutaneous lymphomas expressing TFH markers, that might reasonably be considered in a differential diagnosis. It also underlines the interest of integrated histomolecular diagnosis, using clonality and NGS, to classify these pathologies and strength the hypothesis of a spectrum of cutaneous lymphomas arising from a T-follicular helper lymphocyte is, even if further studies on a more significant number of patients are required to draw firm conclusions. In particular, the provisional entity so-called PCTFHL will need to be defined thanks to larger cohorts, from clinical, histological, and molecular points of view, in order to determine if it related to lymphoproliferations (such as SMLPD), or to real lymphomas with TFH phenotype, by analogy to nodal lymphomas.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Willemze, R. et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 133, 1703–1714 (2019).
Garcia-Herrera, A. et al. Primary cutaneous small/medium CD4+ T-cell lymphomas: A heterogeneous group of tumors with different clinicopathologic features and outcome. J. Clin. Oncol. 26, 3364–3371 (2008).
Oishi, N. et al. Cutaneous lesions of angioimmunoblastic T-cell lymphoma: Clinical, pathological, and immunophenotypic features. J. Cutan. Pathol. 46, 637–644 (2019).
Le Tourneau, A. et al. Primary cutaneous follicular variant of peripheral T-cell lymphoma NOS. A report of two cases. Histopathology 56, 548–551 (2010).
Ortonne, N. et al. Lymphomes T cutanés non classables à différenciation TFH: à propos de six cas illustrant de nouveaux aspects anatomocliniques. Ann. Dermatol. Vénéréologie 139, B70–B71 (2012).
Battistella, M. et al. Primary cutaneous follicular helper T-cell lymphoma: A new subtype of cutaneous T-cell lymphoma reported in a series of 5 cases. Arch. Dermatol. 148, 832–839 (2012).
Grogg, K. L., Jung, S., Erickson, L. A., McClure, R. F. & Dogan, A. Primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma: A clonal T-cell lymphoproliferative disorder with indolent behavior. Mod. Pathol. 21, 708–715 (2008).
Kaffenberger, B. et al. Extranodal marginal zone lymphoma-like presentations of angioimmunoblastic T-cell lymphoma: A T-cell lymphoma masquerading as a B-cell lymphoproliferative disorder. Am. J. Dermatopathol. 37, 604–613 (2015).
Park, J.-H., Han, J. H., Kang, H. Y., Lee, E.-S. & Kim, Y. C. Expression of follicular helper T-cell markers in primary cutaneous T-cell lymphoma. Am. J. Dermatopathol. 36, 465–470 (2014).
Obiorah, I. E. et al. Overlapping features of primary cutaneous marginal zone lymphoproliferative disorder and primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder: A diagnostic challenge examined by genomic analysis. Am. J. Surg. Pathol. 47, 344–353 (2023).
Bommier, C. et al. Real-life targeted next-generation sequencing for lymphoma diagnosis over 1 year from the French Lymphoma Network. Br. J. Haematol. https://doi.org/10.1111/bjh.17395 (2021).
Vallois, D. et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell–derived lymphomas. Blood 128, 1490–1502 (2016).
Chiba, S. & Sakata-Yanagimoto, M. Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia 34, 2592–2606 (2020).
Steinhilber, J. et al. The pathological features of angioimmunoblastic T-cell lymphomas with IDH2R172 mutations. Mod. Pathol. 32, 1123–1134 (2019).
Odejide, O. et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood 123, 1293–1296 (2014).
Fukumoto, K., Nguyen, T. B., Chiba, S. & Sakata-Yanagimoto, M. Review of the biologic and clinical significance of genetic mutations in angioimmunoblastic T-cell lymphoma. Cancer Sci. 109, 490–496 (2018).
LeclaireAlirkilicarslan, A. et al. Expression of TFH markers and detection of RHOA pG17V and IDH2 pR172K/S mutations in cutaneous localizations of angioimmunoblastic T-cell lymphomas. Am. J. Surg. Pathol. 41, 1581–1592 (2017).
Beltzung, F. et al. Primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorders: A clinical, pathologic, and molecular study of 60 cases presenting with a single lesion. Am. J. Surg. Pathol. https://doi.org/10.1097/PAS.0000000000001470 (2020).
Bergqvist, C. et al. Lymphomatoid papulosis types D and E: A multicentre series of the French Cutaneous Lymphomas Study Group. Clin. Exp. Dermatol. https://doi.org/10.1111/ced.14730 (2021).
Dequidt, L. et al. Cutaneous lymphomas appearing during treatment with biologics: 44 cases from the French Study Group on Cutaneous Lymphomas and French Pharmacovigilance Database. Br. J. Dermatol. 181, 616–618 (2019).
Laurent, C. et al. Impact of expert pathologic review of lymphoma diagnosis: Study of patients from the French Lymphopath Network. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 35, 2008–2017 (2017).
van Dongen, J. J. M. et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17, 2257–2317 (2003).
Trecourt, A. et al. Plasticity of mature B cells between follicular and classic hodgkin lymphomas: A series of 22 cases expanding the spectrum of transdifferentiation. Am. J. Surg. Pathol. Publish Ahead of Print, (2021).
Medistica. pvalue.io, a Graphic User Interface to the R statistical analysis software for scientific medical publications https://www.pvalue.io/fr (2020) .
Edinger, J. T., Kant, J. A. & Swerdlow, S. H. Cutaneous marginal zone lymphomas have distinctive features and include 2 subsets. Am. J. Surg. Pathol. 34, 1830–1841 (2010).
Gibson, S. E. & Swerdlow, S. H. How I diagnose primary cutaneous marginal zone lymphoma. Am. J. Clin. Pathol. 154, 428–449 (2020).
Shi, H. et al. Clinicopathological analysis of primary cutaneous CD4-positive small/medium pleomorphic T-cell lymphoproliferative disorder: A retrospective study of 22 patients. Int. J. Dermatol. https://doi.org/10.1111/ijd.15372 (2020).
Cascione, L. et al. Novel insights into the genetics and epigenetics of MALT lymphoma unveiled by next generation sequencing analyses. Haematologica 104, e558–e561 (2019).
Spina, V. et al. The genetics of nodal marginal zone lymphoma. Blood 128, 1362–1373 (2016).
Spina, V., Mensah, A. A. & Arribas, A. J. Biology of splenic and nodal marginal zone lymphomas. Ann. Lymphoma 5, 6–6 (2021).
Zhu, Q. et al. FBW7 in hematological tumors (review). Oncol. Lett. https://doi.org/10.3892/ol.2020.11264 (2020).
Maurus, K. et al. Panel sequencing shows recurrent genetic FAS alterations in primary cutaneous marginal zone lymphoma. J. Investig. Dermatol. 138, 1573–1581 (2018).
Jacoby, M. A., Duncavage, E. J. & Walter, M. J. Implications of tumor clonal heterogeneity in the era of next-generation sequencing. Trends Cancer 1, 231–241 (2015).
Watatani, Y. et al. Molecular heterogeneity in peripheral T-cell lymphoma, not otherwise specified revealed by comprehensive genetic profiling. Leukemia 33, 2867–2883 (2019).
Tate, J. G. et al. COSMIC: The catalogue of somatic mutations in cancer. Nucleic Acids Res. 47, D941–D947 (2019).
Kopanos, C. et al. VarSome: The human genomic variant search engine. Bioinformatics 35, 1978–1980 (2019).
Bastidas Torres, A. N. et al. Genomic analysis reveals recurrent deletion of JAK-STAT signaling inhibitors HNRNPK and SOCS1 in mycosis fungoides. Genes. Chromosomes Cancer 57, 653–664 (2018).
Meyerson, H. J. et al. Follicular center helper T-cell (TFH) marker positive mycosis fungoides/Sezary syndrome. Mod. Pathol. 26, 32–43 (2013).
Follicular center helper T-cell (TFH) marker positive mycosis fungoides/Sezary syndrome|Modern Pathology. https://www.nature.com/articles/modpathol2012124?draft=collection.
Quivoron, C. et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell 20, 25–38 (2011).
Tsiouplis, N. J., Bailey, D. W., Chiou, L. F., Wissink, F. J. & Tsagaratou, A. TET-mediated epigenetic regulation in immune cell development and disease. Front. Cell Dev. Biol. 8, 623948 (2021).
Cortes, J. R. et al. RHOA G17V induces T follicular helper cell specification and promotes lymphomagenesis. Cancer Cell 33, 259-273.e7 (2018).
Acknowledgements
Authors would thank Laurianne Brand, Blandine Grangier, Aurélie Gaultier, and Mathilde Bardou (Centre de Pathologie et de Biologie Moléculaire de Lyon Sud, Hospices Civils de Lyon) for their excellent technical support.
Author information
Authors and Affiliations
Contributions
M.D., A.T.G. and B.B. performed study concept and design; M.D., and A.T.G. performed development of methodology and writing ; S.D. and M.P. provided acquisition of clinical data (dermatology); E.B. and H.G. provided acquisition of clinical data (hematology); C.M. and A.T. provided acquisition, analysis and interpretation of molecular data, M.D. performed statistical analysis; J.F. and N.O. performed review and revision of the paper. All authors performed review and revision of the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Donzel, M., Trecourt, A., Balme, B. et al. Deciphering the spectrum of cutaneous lymphomas expressing TFH markers. Sci Rep 13, 6500 (2023). https://doi.org/10.1038/s41598-023-33031-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33031-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.