Abstract
Axon guidance cues direct the growth and steering of neuronal growth cones, thus guiding the axons to their targets during development. Nonetheless, after axons have reached their targets and established functional circuits, many mature neurons continue to express these developmental cues. The role of axon guidance cues in the adult nervous system has not been fully elucidated. Using the expression pattern data available on FlyBase, we found that more than 96% of the guidance genes that are expressed in the Drosophila melanogaster embryo continue to be expressed in adults. We utilized the GeneSwitch and TARGET systems to spatiotemporally knockdown the expression of these guidance genes selectively in the adult neurons, once the development was completed. We performed an RNA interference (RNAi) screen against 44 guidance genes in the adult Drosophila nervous system and identified 14 genes that are required for adult survival and normal motility. Additionally, we show that adult expression of Semaphorins and Plexins in motor neurons is necessary for neuronal survival, indicating that guidance genes have critical functions in the mature nervous system.
Similar content being viewed by others
Introduction
An extraordinary characteristic of the nervous system is the complexity of its wiring. Although there are multiple mechanisms involved in establishing these connections, a key event is the guidance of axons to their specific targets. During the development of the nervous system, axons navigate to their targets in response to various attractive and repulsive guidance cues1. Recently, it has become clear that many neurons continue to express developmental guidance genes after functional circuits have been established. Indeed, gene expression studies in the adult rodent brain have identified brain regions that are enriched for genes involved in neuronal development and axon guidance2.
The expression of guidance genes in the adult indicates that they likely have additional roles beyond the initial phase of axonal outgrowth and synaptogenesis3. For example, blocking the function of Ephrins and class 3 Semaphorins promotes axonal sprouting and regeneration after nerve injury4,5,6,7. Guidance cues are also known to regulate adult synaptic plasticity. Conditional deletion of the receptor for Netrin-1 (deleted in colorectal cancer/DCC) in adult mice forebrain neurons results in shorter dendritic spines, loss of long-term potentiation, and impaired spatial and recognition memory8. Semaphorin 5B has been shown to regulate the elimination of synaptic connections in cultured hippocampal neurons9 and Semaphorin 2b- PlexinB signalling was shown to drive homeostatic synaptic plasticity in Drosophila10,11. Axon guidance genes have also been linked to neurodegenerative diseases. Given their role in axonal guidance and synapse regulation, it has been proposed that changes in guidance protein expression or function may trigger defects in neuronal networks leading to neuronal dysfunction and loss12. Thus, based on their known functions during development, their expression in the adult brain and possible implications in neurodegenerative diseases, we examined whether guidance genes are critical for the maintenance and survival of neurons in the mature nervous system.
Results
Knockdown of a subset of axon guidance genes reduces adult survival
Using the expression pattern data13 available on FlyBase14,15, we compared the expression of axon guidance genes in the embryonic (10–24 h) and adult (1–20 days) Drosophila melanogaster. We found that more than 96% of the guidance genes expressed in the embryo continue to be expressed in adults. Query results from the RNA-Seq Expression Profile search tool showed that 162 genes expressed in the adult Drosophila nervous system were categorized under the biological process Gene Ontology (GO) term ‘axon guidance’ and 122 genes under ‘dendrite morphogenesis’ (Supplementary Tables S1, S2). After excluding transcription factors, 44 genes were prioritized based on their previously known roles in axonal guidance and the availability of genetic tools (Supplementary Tables S3, S4). We refer to these genes collectively as ‘axon guidance genes’. Based on the molecular function GO terms provided on FlyBase, these 44 axon guidance genes were categorized as ‘actin/ microtubule-binding’, ‘cell adhesion’, ‘guidance ligand/ protein binding’ and ‘receptor activity’ (Fig. 1a).
Knockdown of a subset of axon guidance genes reduces adult survival rate. (a) A summary of the axon guidance genes that were tested for the impact of knockdown on adult survival rate using the GeneSwitch screen. The axon guidance cues are categorized into four groups based on the molecular function GO terms. (b, c) Survival analysis curves of axon guidance genes that showed a significant effect on survival from day 9 post eclosion. We note that Sema2a is not significantly different from the control on day 10 (c), however we included these flies in the graph to group the analysis of the Semaphorin and Plexin genes together. (d, e) Survival analysis curves of axon guidance genes that showed a significant effect from day 14 post eclosion. Data shown are mean ± SEM (p < 0.05, two-way ANOVA and Tukey’s multiple comparison tests; n = 30). ‘*’ indicates the time points when the survival of knockdowns was significantly different from that of the controls.
In an initial screen to identify whether any genes were essential for viability, we used the GeneSwitch (elav-GeneSwitch-Gal4) and TARGET (elav-Gal4 with tub-Gal80ts) systems16 (see Supplementary Table S5) to knockdown the expression of all 44 genes (using multiple dsRNA and shRNA lines; see Supplementary Table S6) selectively in the adult neurons. Since all the flies used in this study have UAS-Dicer2 on the X chromosome and since Drosophila males have a hyperactive X chromosome, we picked male flies for all the experiments. The hyperactivation of a single X chromosome in male flies increases the transcription of genes to match that of both X chromosomes in females17,18. This dosage compensation in Drosophila males is due to the increased acetylation at the K16 residue of histone H4 which, in this case, causes transcriptional activation and upregulation of the UAS-Dicer2 on the X chromosome which in turn enhances the potency of RNAi19. In the GeneSwitch screen, the adult-specific knockdown of 15 of the 44 genes showed a significant reduction in the adult survival rate (Fig. 1, Supplementary Fig. S1). The TARGET system screen confirmed these data for 14 of the 15 genes (Fig. 2). For each gene, we used multiple UAS-dsRNA and -shRNA lines as listed in Supplementary Table S6. In Figs. 1, 2 and Supplementary Fig. S1, we report survival data from only the most effective lines. For each UAS-dsRNA and -shRNA line, we employed two controls- an injection strain control and a UAS-dsRNA-GFP control. All controls used in this study are listed in Supplementary Table S7. Since all the controls exhibited similar survival rates in the adults, we report the data from only one control in each survival analysis curve. In the control flies, 97% survive to day 20 post eclosion, whereas the percentage survival post knockdown for the 14 genes was significantly reduced to 27% (chic), 30% (Sema1a), 33% (fra, Sema1b), 37% (Sema5c), 40% (PlexB), 53% (Abl, Dg, msps), 57% (Fas3), 60% (Apc), 67% (PlexA, robo1), and 77% (Sema2a) (Fig. 1b–e).
The TARGET system screen confirmed a reduced adult survival rate for 14 axon guidance genes knocked down. (a–d) Knockdown of Sema1a, Sema1b, PlexA, chic, Sema5c, Sema2a, PlexB, Abl, Apc, Fas3, robo1, msps, Dg and fra exhibited a significant reduction in the adult survival rate. (e) Knockdown of Lis-1 did not show a significant effect on the adult survival rate in the TARGET screen. Data shown are mean ± SEM (p < 0.05, two-way ANOVA and Tukey’s multiple comparison tests; n = 30). ‘*’ indicates the time points when the survival of knockdowns was significantly different from that of the controls.
Motility is impacted by the knockdown of specific axon guidance genes
To examine adult fly behaviour in the period before their survival declines, we focused on these 14 genes and divided them into two groups based on their survival analysis graphs: (1) those that started to show a reduction in adult survival from approximately day 9 post eclosion (Sema1a, Sema1b, Sema2a, Sema5c, PlexA, PlexB, chic), and (2) those that started to show a reduction from approximately day 14 post eclosion (Abl, Apc, Dg, Fas3, fra, msps, robo1). Using these two time points, we conducted motility assays with group one at 9 days and group two at 14 days, after the initiation of shRNA and dsRNA expression. Here, we used the UAS-dsRNA and -shRNA lines that were most effective in the survival assays and employed two controls (an injection strain control and a UAS-dsRNA-GFP control) for each line. There was no significant difference between the controls on day 9 and day 14. As an initial examination of motility, we tested whether pan-neuronal knockdown (elav-GeneSwitch-Gal4) of the genes impacted adult climbing behaviour. We found that flies that had axon guidance genes knocked down showed a significant climbing defect. Flies that failed to reach the target line were either very slow or did not start climbing even after tapping the vials. In the control flies, the success of climbing ranged from 87 to 94%. We observed the following climbing success rates in knockdowns of Sema1a (72%), Sema2a (63%), PlexA (38%) and PlexB (37%). The climbing success rates ranged from 10 to 59% in the knockdowns of the other 10 genes (Fig. 3a).
Motility is impacted by the knockdown of specific axon guidance genes (a) Effect of pan-neuronal knockdown of axon guidance genes (using elav-GeneSwitch-Gal4) on adult climbing behaviour. A significant decrease in adult climbing ability was observed after the knockdown. (b) Similar observations were made when each gene was knocked down specifically in glutamatergic motor neurons using the OK371-Gal4. Data shown are mean ± SEM (‘*’ denotes a significant difference from the control and dsRNA-GFP; p < 0.05, one-way ANOVA and Tukey’s multiple comparison tests; n = 90). (c) Effect of pan-neuronal knockdown of axon guidance genes (using elav-GeneSwitch-Gal4) on adult activity (measured using DAM). The bar graph compares the normalized average activity counts per 12 h for each gene from multiple flies over a period of 3 days in 12 h light and 12 h dark cycle. The black portion (0–12 h) represents the dark cycle, and the white portion (12–24 h) represents the light cycle. Data shown are mean ± SEM (‘*’ denotes a significant difference from the control and dsRNA-GFP; p < 0.05, one-way ANOVA and Tukey’s multiple comparison tests; n = 32).
Since a significant decline in adult climbing behaviour was observed after pan-neuronal knockdown, we tested whether knocking down genes primarily in motor neurons would induce a similar phenotype using a glutamatergic neuron-specific Gal4 driver (OK371-Gal4). Again, flies with knocked-down guidance genes showed defects in adult climbing behaviour (Fig. 3b). Comparing the severity of climbing defects caused by the knockdown of each gene using the two drivers, only chic and Apc showed less severity following the knockdown in motor neurons compared to the pan-neuronal knockdown. The remaining 12 genes showed no significant difference in the severity of the climbing phenotype between the two Gal4 drivers (Fig. 3a, b).
We further examined the motility of adult flies using a Drosophila Activity Monitor (DAM) by continuously monitoring individual flies for 3 days. Fas3 and chic knockdown flies showed significantly lower activity counts than the controls throughout the day (both light and dark cycles). Whereas robo1 knockdown flies exhibited significantly higher activity counts in both cycles. During the dark cycle, the Sema1a knockdown flies exhibited significantly lower activity counts whereas Sema2a, PlexA and PlexB knockdown flies exhibited significantly higher activity counts. Flies also showed aberrant activity counts following the knockdown of Abl and msps (Fig. 3c).
Knockdown of Semaphorins and Plexins results in motor neuron death
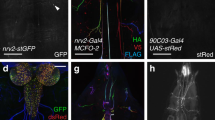
To investigate why motility was impacted after knockdown, we examined the number of leg motor neurons in the thoracic clusters of adult ventral nerve cords in a subset of the 14 genes using the OK371-Gal4 driver. Knockdown of Sema1a, Sema2a, PlexA and PlexB resulted in a significant loss of motor neurons in each thoracic cluster (T1, T2, T3) (Fig. 4). In T1, total neurons were reduced by 32%, 33%, 30% and 38% in Sema1a, Sema2a, PlexA and PlexB knockdowns respectively. The following reductions were observed in T2 and T3 segments respectively: 44% and 42% (Sema1a), 41% and 49% (Sema2a), 32% and 36% (PlexA) and 30% and 34% (PlexB) (Fig. 4c–e). The number of motor neurons in all knockdowns was significantly lower than their injection strain control and the mCherry-control. This was repeated using additional UAS-dsRNA lines and controls which corroborated the reduction in leg motor neuron numbers (Supplementary Fig. S2).
Knockdown of Semaphorins and Plexins results in motor neuron death. (a) Adult ventral nerve cord showing neuronal GFP expression driven by OK371-Gal4. White arrows point to the location of leg motor neuron cell bodies in T1, T2, and T3 thoracic segments. The scale bar represents 100 µm. (b) Effect of glutamatergic knockdown of Semaphorins and Plexins (using OK371-Gal4) on adult leg motor neuron survival. A decrease in the number of leg motor neurons was observed after the knockdown of Semaphorins and Plexins genes. The scale bar represents 20 µm. (c–e) Quantification of adult leg motor neurons in T1, T2, and T3 respectively, following 9 days of axon guidance gene knockdown. Data shown are mean ± SEM (‘*’ denotes a significant difference from the control and mCherry; p < 0.05, one-way ANOVA and Tukey’s multiple comparison tests; n = 20).
Discussion
Among the 44 axon guidance genes screened, we identified 14 genes (chic, Sema1a, Sema1b, Sema2a, Sema5c, PlexA, PlexB, Abl, Apc, Fas3, robo1, msps, Dg, fra) that are essential for adult survival. Mutations in chic20,21,22,23,24,25,26,27,28,29,30, Sema1a19,24,31, Sema2a23,24,32, Abl24,31,33,34,35,36,37,38, Apc39, Fas327,40, robo141,42, msps27,43,44, Dg45,46, and fra47 were previously known to cause lethality during development. This implies that many axon guidance genes are essential throughout the lifespan of Drosophila, from development through adulthood. Since we used the GeneSwitch and TARGET systems to restrict the knockdown to adult flies, the observed phenotypes are not a result of developmental defects but are due to defects in the adult nervous system. One important consideration while using RNAi is that it could produce off-target effects where multiple gene expressions will be altered. To address this issue, we picked UAS-dsRNA and -shRNA lines that were used in previous studies where no off-target effects were reported48. In addition, we used multiple UAS-dsRNA and -shRNA lines to confirm each of the observed phenotypes. It is important to note that UAS-dsRNA and -shRNA lines that use the phiC31a/AttP site-specific integration system to insert the transgene in a well-characterized location on the chromosome ensure high levels of expression and an insertion in a position that does not affect other genes49. We have employed the use of such lines in most of the experiments performed.
The reduction in the survival rate could result from the dysfunction of any number of neuronal populations in the adult nervous system. It is presumed that the functions of the affected neurons are compromised before the flies die. To narrow down the range of neuron populations that could underlie this premature death and to better understand the cellular impact following guidance gene knockdown, we used motility assays and focussed our examination on motor neural circuits. Employing a climbing assay and the DAM system, we found a significant impact on motility in knockdown flies. Defective climbing behavior was observed when the knockdown was mediated either pan-neuronally (using elav-GeneSwitch-Gal4) or restricted to motor neurons (using OK371-Gal4). One potential limitation of using OK371-Gal4 is that although it drives expression predominantly in the motor neurons in the adult ventral nerve cord it is also expressed in a few glutamatergic interneurons in the brain50. As a result, it is unclear whether axon guidance gene knockdown in non-motor glutamatergic neurons would contribute to the defective climbing behavior. Further analysis of these additional neuronal populations may be required to understand this better. It is also interesting that while axon guidance gene knockdown always resulted in a decreased climbing behavior, the same resulted in either an increased or decreased activity using the DAM system. This may be due to the fact that both of these experiments measure different kinds of motility behaviors. The climbing assay measures the negative geotaxis behavior in a short span (8 s) whereas the DAM system measures locomotion as reflected by the ‘activity counts’ over an extended period of time (3 days on a 12 h dark/12 h light cycle). Defects in the circadian rhythm and sleep may also contribute to the differences in the activity observed using DAM. Since it is beyond the scope of our current study, we did not examine the neural circuits underlying the circadian rhythm.
As pointed out earlier, since the knockdown was restricted to adult flies, these locomotor phenotypes are not the result of developmental defects but are likely due to defects in the adult motor neuron circuit. This was confirmed when the knockdown of Sema1a, Sema2a, PlexA and PlexB resulted in a significant loss of adult motor neurons, suggesting a role for these guidance cues in maintaining neuronal survival during adulthood. The loss of motor neurons could explain the motility defects demonstrated in the behavioural assays. It is noteworthy that while the adult-specific knockdown of Semaphorins and Plexins resulted in a similar loss of motor neurons, their effects on locomotor activity appeared quite different. Knockdown of Sema1a decreased the average activity counts, whereas that of Sema2a, PlexA and PlexB increased the average activity counts. This may be due to the differential effect of knockdown on the neuronal populations controlling circadian rhythm and sleep. Further analysis on the inactive time duration/ sleep of knockdown flies and examination of the neural circuits underlying the circadian rhythm may be required to fully understand this phenomenon.
Although the neuroprotective function of Semaphorins has been previously demonstrated in human51 and mouse spinal motor neurons52, cultured cerebellar granule cells53, and non-neuronal cells54,55,56,57 during development, our study is the first to show that adult-specific expression of Semaphorins and Plexins is essential for maintaining motor neuron survival in the mature nervous system. In addition to promoting cell survival, Semaphorins and Plexins are also known to evoke responses such as cell proliferation and differentiation58. An alternative interpretation of the loss of adult motor neurons is that the lack of Semaphorin-Plexin signalling may be inhibiting proliferation and differentiation into motor neurons. This possibility was ruled out since there is no evidence for adult neurogenesis in the Drosophila motor neuron circuits.
Semaphorin/Plexin signalling has been studied throughout the development of embryonic and post-embryonic motor neurons59,60,61. At the Drosophila larval neuromuscular junctions, Sema2b- PlexB signalling has been demonstrated to control presynaptic neurotransmitter release to drive homeostatic plasticity10,11. In addition, previous work has shown that Sema1a, Sema2a, PlexA and PlexB play a range of roles in establishing the circuitry of adult leg motor neurons in Drosophila62. Here, we show that these very same proteins are essential for maintaining motor neuron survival during adulthood. Thus, Semaphorins and Plexins are important equally for the formation as well as the maintenance of adult neural circuits. Semaphorins and Plexins may act cell autonomously on motor neurons or non-cell autonomously on neighbouring motor neurons to maintain their survival. Further studies on Semaphorin/ Plexin signalling downstream events will be required to better understand the mechanism underlying this phenotype.
The main goal of our study was to examine whether axon guidance genes have an important function in the adult. We identified 14 axon guidance genes that are essential for survival and normal motility. From our database search, we found that many of the embryonic axon guidance genes continue to be expressed in the adult. Gene expression studies in adult mouse brains suggest that although the expression patterns of many genes change dramatically during development, the brain retains a degree of embryological gene expression that is important for the maintenance of established units in the adult brain63. Similarly, studies in rhesus monkeys (Macaca mulatta) have identified sets of genes that are important equally for the formation as well as the maintenance and plasticity of connections in the adult thalamus64. These imply that the expression of axon guidance genes in the adult is not just a “residue” of the developmental pattern that reflects processes occurring when connectivity is established, but is functionally relevant with respect to maintaining the connectivity. Indeed, our results show that the expression of axon guidance genes in adulthood is required for organism viability and neuronal survival.
Methods
Prioritizing axon guidance genes for the screen
The high throughput expression pattern data11 accessed through the RNA-Seq Expression Profile search tool from FlyBase12,13 (www.flybase.org) version FB2013_05 (released September 2013) was used to search for genes expressed in Drosophila melanogaster in the embryo (stage-wise expression, 10–24 h) and in the adult (tissue-wise expression in head, 1–20 days post eclosion). An ‘expression on’ filter was used to search for genes expressed above a certain expression profile threshold, namely very low (1–3 RPKM), low (4–10 RPKM), moderate (11–25 RPKM), moderately high (26–50 RPKM), high (51–100 RPKM), very high (101–1000 RPKM) and extremely high (> 1000 RPKM). The resulting dataset was further refined using biological process GO terms ‘axon guidance’ and ‘dendrite morphogenesis’. Transcription factors were excluded using the cellular component GO term ‘nucleus’. Gene list Venn diagram (http://genevenn.sourceforge.net/) was used to categorize the genes into discrete expression levels, ranging from very low to extremely high, and to compare the genes expressed in the embryo and adult Drosophila. These data combined with their previously known roles in receptor activity and signalling during neuronal pathfinding, and the availability of genetic tools were used to prioritize genes.
Fly genetics
Reducing axon guidance gene expression by RNAi in the adult nervous system was carried out using two well-characterized systems: (1) GeneSwitch, (2) TARGET. Gal4 driver line virgin females (see Supplementary Table S5) were crossed with UAS-dsRNA/ shRNA males (see Supplementary Table S6) or the appropriate negative control line males (see Supplementary Table S7). In case of the GeneSwitch screen (using elav-GeneSwitch-Gal4), crosses were set up at 25 °C. The progeny (F1) was fed with food containing 6.5 µg/mL mifepristone (RU486) from day 1 post eclosion to activate the Gal4-UAS system. In the case of the TARGET screen (using elav-Gal4 OR OK371-Gal4 with tub-Gal80ts), crosses were set up at 18 °C and the progeny (F1) was switched to 29 °C immediately after eclosion to repress Gal80ts and activate the Gal4-UAS system.
Fly stocks
Drosophila melanogaster stocks used in this study were maintained on standard cornmeal food and at 18, 25, or 29 °C in environment rooms set at 70% humidity. The stocks used in this study are listed in Supplementary Tables S5, S6 and S7.
Survival assay
F1 progeny males were collected immediately after eclosion. They were maintained in vials of 10 and their survival was recorded every day for the next 20 days. Flies were flipped into fresh vials every week. Survival analysis for the GeneSwitch screen was carried out at 25 °C and that for the TARGET screen was carried out at 29 °C. In both cases, multiple UAS-dsRNA/ shRNA lines were tested for each axon guidance gene. For each line tested, 3 vials containing 10 flies were assayed. The results were analyzed using two-way ANOVA and Tukey’s multiple comparison tests using GraphPad Prism.
Climbing assay
To identify adult climbing defects, F1 male flies were collected immediately after eclosion, maintained in groups of 10 flies per vial and assayed at the desired age. During the climbing assay, flies were transferred to a clean, empty vial with a line drawn 7.5 cm from the bottom. Flies were allowed to acclimatize for 1 min and then tapped to the bottom to induce an innate climbing response. The number of flies that successfully reached the 7.5 cm line in 8 s was recorded. For each genotype, 3 vials were assayed and 3 replicates per vial were performed to ensure an accurate reading for each vial. The results were analyzed using one-way ANOVA and Tukey’s multiple comparison tests using GraphPad Prism.
Drosophila Activity Monitor (DAM) system
The DAM system from TriKinetics was used to monitor the activities of individual flies for 3 days on 12 h light and 12 h dark cycle. F1 male flies were collected immediately after eclosion, maintained in groups of 10 flies per vial and assayed at the desired age at 25 °C. Flies were placed inside the activity monitor tubes for at least 24 h before each experiment to acclimatize to the experimental setup. 32 flies were placed in 5 mm diameter tubes, with one end containing 1 cm worth of food. Drosophila activity was recorded every 5 min. The raw data was processed using the actmon R package publicly available online at https://github.com/kazi11/actmon (kindly provided by Jeff Stafford). Data from flies that died during the experiment were discarded. The processed results were analyzed using one-way ANOVA and Tukey’s multiple comparison tests using GraphPad Prism.
Image acquisition and leg motor neuron quantification
To examine the impact of knockdown at a neuronal level, adult male Drosophila ventral nerve cords (VNCs) were dissected in ice cold 1 × PBS, over a period of 30 min. The VNCs were fixed, on poly-Lysine coated slides, in 4% PFA at room temperature for 30 min followed by 3 × 5 min washes with 0.1% PBT. They were mounted in Vectashield mounting media (Vector Laboratories Inc.), coverslipped and sealed with nail-polish. All images were acquired on a 20X objective at a 1.0-fold magnification using the Fast Airyscan feature on a Zeiss LSM 880 inverted confocal microscope. Images were taken in a Z-stack and the leg motor neurons were quantified in a 3D model (using Zen-blue image processing software) separately for the T1, T2 and T3 segments. The results were analysed using one-way ANOVA and Tukey’s multiple comparison tests using GraphPad Prism.
Data availability
The datasets generated during and/ or analyzed during the current study are available from the corresponding author on request.
References
Tessier-Lavigne, M. & Goodman, C. S. The molecular biology of axon guidance. Science 274, 1123–1133 (1996).
French, L. & Pavlidis, P. Relationships between gene expression and brain wiring in the Adult Rodent Brain. PLoS Comput. Biol. 7, e1001049. https://doi.org/10.1371/journal.pcbi.1001049 (2011).
Giger, R. J., Hollis, E. R. & Tuszynski, M. H. Guidance molecules in axon regeneration. Cold Spring Harbor Perspect. Biol. 2, a001867. https://doi.org/10.1101/cshperspect.a001867 (2010).
Tanelian, D. L., Barry, M. A., Johnston, S. A., Le, T. & Smith, G. M. Semaphorin III can repulse and inhibit adult sensory afferents in vivo. Nat. Med. 3, 1398–1401 (1997).
Kikuchi, K. et al. In vitro and in vivo characterization of a novel Semaphorin 3A inhibitor, SM-216289 or Xanthofulvin. J. Biol. Chem. 278, 42985–42991 (2003).
Pasterkamp, R. J. & Verhaagen, J. Semaphorins in axon regeneration: Developmental guidance molecules gone wrong?. Philos. Trans. R. Soc. B: Biol. Sci. 361, 1499–1511 (2006).
Fabes, J., Anderson, P., Brennan, C. & Bolsover, S. Regeneration-enhancing effects of EPHA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur. J. Neurosci. 26, 2496–2505 (2007).
Horn, K. E. et al. DCC expression by neurons regulates synaptic plasticity in the adult brain. Cell Rep. 3, 173–185 (2013).
O’Connor, T. P. et al. Semaphorin 5b mediates synapse elimination in hippocampal neurons. Neural Dev. 4, 18. https://doi.org/10.1186/1749-8104-4-18 (2009).
Orr, B. O., Fetter, R. D. & Davis, G. W. Retrograde semaphorin–plexin signalling drives homeostatic synaptic plasticity. Nature 550, 109–113 (2017).
Orr, B. O., Fetter, R. D. & Davis, G. W. Activation and expansion of presynaptic signaling foci drives presynaptic homeostatic plasticity. Neuron 110, 3743–3759 (2022).
Van Battum, E. Y., Brignani, S. & Pasterkamp, R. J. Axon guidance proteins in neurological disorders. Lancet Neurol. 14, 532–546 (2015).
Graveley, B. et al. The developmental transcriptome of Drosophila melanogaster. Nature 471, 473–479 (2011).
Gramates, L. S. et al. FlyBase: A guided tour of highlighted features. Genetics 220, iyac035. https://doi.org/10.1093/genetics/iyac035 (2022).
Gelbart, W. M. & Emmert, D. B. Flybase high throughput expression pattern data. FlyBase Anal. 2013, FBrf0021009 (2013).
McGuire, S. E., Mao, Z. & Davis, R. L. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, pl6. https://doi.org/10.1126/stke.2202004pl6 (2004).
Philip, P. & Stenberg, P. Male X-linked genes in drosophila melanogaster are compensated independently of the male-specific lethal complex. Epigenet. Chromatin 6, 1–8 (2013).
Lucchesi, J. C. & Kuroda, M. I. Dosage compensation in Drosophila. Cold Spring Harbor Perspect. Boil. 7, a019398. https://doi.org/10.1101/cshperspect.a019398 (2015).
Dietzl, G. et al. A genome-wide transgenic rnai library for conditional gene inactivation in Drosophila. Nature 448, 151–156 (2007).
Van Vactor, D., Sink, H., Fambrough, D., Tsoo, R. & Goodman, C. S. Genes that control neuromuscular specificity in Drosophila. Cell 73, 1137–1153 (1993).
Verheyen, E. M. & Cooley, L. Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development 120, 717–728 (1994).
Manseau, L., Calley, J. & Phan, H. Profilin is required for posterior patterning of the Drosophila oocyte. Development 122, 2109–2116 (1996).
Roch, F. et al. Screening of larval/pupal p-element induced lethals on the second chromosome in Drosophila melanogaster: Clonal analysis and morphology of Imaginal Discs. Mol. Gen. Genet. MGG 257, 103–112 (1998).
Spradling, A. C. et al. The berkeley drosophila genome project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153, 135–177 (1999).
Wills, Z., Marr, L., Zinn, K., Goodman, C. S. & Van Vactor, D. Profilin and the ABL tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron 22, 291–299 (1999).
Schnorr, J. D., Holdcraft, R., Chevalier, B. & Berg, C. A. ras1 interacts with multiple new signaling and cytoskeletal loci in Drosophila eggshell patterning and morphogenesis. Genetics 159, 609–622 (2001).
Schnorrer, F. et al. Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature 464, 287–291 (2010).
Wang, D. et al. Drosophila twinfilin is required for cell migration and synaptic endocytosis. J. Cell Sci. 123, 1546–1556 (2010).
Kooij, V. et al. Profilin modulates sarcomeric organization and mediates cardiomyocyte hypertrophy. Cardiovasc. Res. 110, 238–248 (2016).
Wu, C.-H. et al. A Drosophila model of ALS reveals a partial loss of function of causative human PFN1 mutants. Hum. Mol. Genet. 26, 2146–2155 (2017).
Nagarkar-Jaiswal, S. et al. A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. Elife 4, e05338. https://doi.org/10.7554/eLife.05338 (2015).
Kolodkin, A. L., Matthes, D. J. & Goodman, C. S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75, 1389–1399 (1993).
Gertler, F. B., Bennett, R. L., Clark, M. J. & Hoffmann, F. M. Drosophila Abl tyrosine kinase in embryonic CNS axons: A role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell 58, 103–113 (1989).
Hamilton, B. A., Ho, A. & Zinn, K. Targeted mutagenesis and genetic analysis of a Drosophila receptor-linked protein tyrosine phosphatase gene. Rouxs Arch. Dev. Biol. 204, 187–192 (1995).
Hill, K. K., Bedian, V., Juang, J. L. & Hoffmann, F. M. Genetic interactions between the drosophila abelson (Abl) tyrosine kinase and failed axon connections (fax), a novel protein in axon bundles. Genetics 141, 595–606 (1995).
Harvie, P. D., Filippova, M. & Bryant, P. J. Genes expressed in the ring gland, the major endocrine organ of drosophila melanogaster. Genetics 149, 217–231 (1998).
Grevengoed, E. E., Loureiro, J. J., Jesse, T. L. & Peifer, M. Abelson kinase regulates epithelial morphogenesis in drosophila. J. Cell Biol. 155, 1185–1198 (2001).
Rogers, E. M., Allred, S. C. & Peifer, M. Abelson kinase’s intrinsically disordered region plays essential roles in protein function and protein stability. Cell Commun. Signal. 19, 1–26 (2021).
Ahmed, Y., Hayashi, S., Levine, A. & Wieschaus, E. Regulation of armadillo by a drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93, 1171–1182 (1998).
Mummery-Widmer, J. L. et al. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature 458, 987–992 (2009).
Bhat, K. M., Gaziova, I. & Krishnan, S. Regulation of axon guidance by slit and netrin signaling in the Drosophila ventral nerve cord. Genetics 176, 2235–2246 (2007).
Ito, H., Sato, K., Kondo, S., Ueda, R. & Yamamoto, D. Fruitless represses robo1 transcription to shape male-specific neural morphology and behavior in Drosophila. Curr. Biol. 26, 1532–1542 (2016).
Cullen, C. F., Deák, P., Glover, D. M. & Ohkura, H. mini spindles: A gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila. J. Cell Biol. 146, 1005–1018 (1999).
Brittle, A. L. & Ohkura, H. Mini spindles, the XMAP215 homologue, suppresses pausing of interphase microtubules in drosophila. EMBO J. 24, 1387–1396 (2005).
Haines, N., Seabrooke, S. & Stewart, B. A. Dystroglycan and protein O-mannosyltransferases 1 and 2 are required to maintain integrity of Drosophila larval muscles. Mol. Biol. Cell 18, 4721–4730 (2007).
Yatsenko, A. S., Marrone, A. K. & Shcherbata, H. R. Mirna-based buffering of the cobblestone–lissencephaly-associated extracellular matrix receptor dystroglycan via its alternative 3′-UTR. Nat. Commun. 5, 1–17 (2014).
Kolodziej, P. A. et al. Frazzled encodes a drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87, 197–204 (1996).
Hu, Y. et al. UP-TORR: Online tool for accurate and Up-to-Date annotation of RNAi Reagents. Genetics 195, 37–45 (2013).
Heigwer, F., Port, F. & Boutros, M. RNA interference (RNAi) screening in Drosophila. Genetics 208, 853–874 (2018).
Mahr, A. & Aberle, H. The expression pattern of the drosophila vesicular glutamate transporter: A marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns 6, 299–309 (2006).
Birger, A., Ottolenghi, M., Perez, L., Reubinoff, B. & Behar, O. ALS-related human cortical and motor neurons survival is differentially affected by Sema3A. Cell Death Dis. 9, 1–9 (2018).
Molofsky, A. V. et al. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature 509, 189–194 (2014).
Moreno-Flores, M. T. et al. Semaphorin 3C preserves survival and induces neuritogenesis of cerebellar granule neurons in culture. J. Neurochem. 87, 879–890 (2004).
Hall, K. T. et al. Human CD100, A novel Leukocyte Semaphorin that promotes B-cell aggregation and differentiation. Proc. Natl. Acad. Sci. 93, 11780–11785 (1996).
Granziero, L. et al. CD100/plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood 101, 1962–1969 (2003).
Luchino, J. et al. Semaphorin 3e suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell 24, 673–685 (2013).
Eissa, N. et al. Semaphorin 3e regulates apoptosis in the intestinal epithelium during the development of colitis. Biochem. Pharmacol. 166, 264–273 (2019).
Roth, L. et al. The many faces of semaphorins: From development to pathology. Cell. Mol. Life Sci. 66, 649–666 (2008).
Winberg, M. L. et al. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell 95, 903–916 (1998).
Yu, H.-H., Araj, H. H., Ralls, S. A. & Kolodkin, A. L. The transmembrane Semaphorin Sema I is required in drosophila for embryonic motor and CNS axon guidance. Neuron 20, 207–220 (1998).
Ayoob, J. C., Terman, J. R. & Kolodkin, A. L. Drosophila plexin B is a sema-2a receptor required for axon guidance. Development 133, 2125–2135 (2006).
Syed, D. S., Gowda, S. B., Reddy, O. V., Reichert, H. & Vijay-Raghavan, K. Glial and neuronal Semaphorin signaling instruct the development of a functional myotopic map for Drosophila walking. Elife 5, e11572. https://doi.org/10.7554/eLife.11572 (2015).
Zapala, M. A. et al. Adult mouse brain gene expression patterns bear an embryologic imprint. Proc. Natl. Acad. Sci. 102, 10357–10362 (2005).
Murray, K. D., Choudary, P. V. & Jones, E. G. Nucleus- and cell-specific gene expression in monkey thalamus. Proc. Natl. Acad. Sci. 104, 1989–1994 (2007).
Funding
This work was supported by Natural Sciences and Engineering Research Council of Canada (Grant no. DG 20R23899).
Author information
Authors and Affiliations
Contributions
A.V.C. conducted all the experiments, and analyzed and interpreted the data under the supervision of D.A. and T.O.C. All authors contributed to conceptualizing the study and design of experiments. The manuscript was written by A.V.C., which was reviewed, edited and approved by D.A. and T.O.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vaikakkara Chithran, A., Allan, D.W. & O’Connor, T.P. Adult expression of Semaphorins and Plexins is essential for motor neuron survival. Sci Rep 13, 5894 (2023). https://doi.org/10.1038/s41598-023-32943-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32943-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.