Abstract
Homoarginine (hArg) is a non-essential cationic amino acid which inhibits hepatic alkaline phosphatases to exert inhibitory effects on bile secretion by targeting intrahepatic biliary epithelium. We analyzed (1) the relationship between hArg and liver biomarkers in two large population-based studies and (2) the impact of hArg supplementation on liver biomarkers. We assessed the relationship between alanine transaminase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), alkaline phosphatases (AP), albumin, total bilirubin, cholinesterase, Quick’s value, liver fat, and Model for End-stage Liver Disease (MELD) and hArg in appropriately adjusted linear regression models. We analyzed the effect of L-hArg supplemention (125 mg L-hArg daily for 4 weeks) on these liver biomarkers. We included 7638 individuals (men: 3705; premenopausal women: 1866, postmenopausal women: 2067). We found positive associations for hArg and ALT (β 0.38 µkatal/L 95% confidence interval (CI): 0.29; 0.48), AST (β 0.29 µkatal/L 95% CI 0.17; 0.41), GGT (β 0.033 µkatal/L 95% CI 0.014; 0.053), Fib-4 score (β 0.08 95% CI 0.03; 0.13), liver fat content (β 0.016% 95% CI 0.006; 0.026), albumin (β 0.030 g/L 95% CI 0.019; 0.040), and cholinesterase (β 0.003 µkatal/L 95% CI 0.002; 0.004) in males. In premenopausal women hArg was positively related with liver fat content (β 0.047% 95%CI 0.013; 0.080) and inversely with albumin (β − 0.057 g/L 95% CI − 0.073; − 0.041). In postmenopausal women hARG was positively associated with AST (β 0.26 µkatal/L 95% CI 0.11; 0.42). hArg supplementation did not affect liver biomarkers. We summarize that hArg may be a marker of liver dysfunction and should be explored further.
Similar content being viewed by others
Introduction
Homoarginine (hArg), a homologue of L-arginine, is a non-essential cationic amino acid, and is synthesized from arginine and lysine by arginine:glycine amidinotransferase (AGAT) and possibly enzymes of the urea cycle1,2. This amino acid is synthesized mainly in the liver, but also in the kidneys, brain, and small intestine, organs which also express AGAT1,3. AGAT is involved in energy metabolism by catalyzing the first and rate-limiting step in creatine synthesis4. Being a weak substrate for nitric oxide (NO) synthase hArg competes with L-arginine for NO synthesis5 thereby contributing to vascular homeostasis6. Experimental7 and clinical studies8,9 have shown that low endogenous hArg levels in the circulation are associated with cardio- and cerebrovascular events and all-cause mortality10.
Of particular note, hArg is a non-competitive inhibitor of alkaline phosphatases (APs), a group of enzymes that hydrolyze orthophosphoric-monoester in alkaline pH and consist of different subtypes expressed in several mammalian tissues, among them liver, bone, kidney, intestine, and placenta11,12. The human bone and liver APs are most strongly inhibited by hArg11. AP exerts inhibitory effects on bile secretion in the liver by targeting intrahepatic biliary epithelium thus having a protective effect on the liver tissue as a further increase of bile pressure is avoided13. The relationship between liver function and hArg release is currently unclear. Low serum hArg concentrations predicted higher long-term mortality in patients with liver cirrhosis and correlated with model of end-stage liver disease (MELD), a score that assesses urgency of liver transplantation14. The relevance of arginine metabolism on liver function is further supported by the observation, that other metabolites such as asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) are related to acute and chronic liver disorders15,16,17.
Here we analyzed (1) the relationship between liver biomarkers and hArg in the large population based epidemiological Study of Health In Pomerania (SHIP) and (2) the impact of hArg supplementation on liver biomarkers in healthy individuals.
Material and methods
Study population
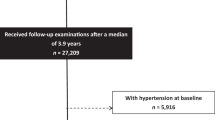
For the analyses, we used data from two independent cohorts of the Study of Health In Pomerania (SHIP)18 and from a cross-over trial with L-hArg supplementation19. The SHIP project includes several large population-based studies, which were all conducted in the Northeast of Germany. In the first SHIP cohort; SHIP-START, 6265 individuals aged 20–79 years were selected from population registries, of which 4308 individuals (response 68.8%) participated between 1997 and 200120. Between 2008 and 2012 baseline examinations of a second cohort (SHIP-TREND) were conducted. For SHIP-TREND a separate stratified random sample of 8826 adults aged 20–79 years was drawn and 4420 subjects participated (response 50.1%). For the present analyses, we used data from the baseline examinations of SHIP-START-0 and SHIP-TREND-0. In both studies all participants gave written informed consent. The studies were approved by the Local Ethics Committee of the University of Greifswald and comply with Declaration of Helsinki. Of the 8728 individuals, who participated in SHIP-START-0 or SHIP-TREND-0, we excluded 1008 individuals with missing data in any of the considered variables and 74 individuals with hArg concentrations higher than 6 µmol/L resulting in a final study population of 7646 individuals.
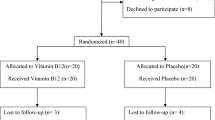
The cross-over trial was conducted in healthy individuals, which were recruited from the staff of the University Medical Centre Hamburg-Eppendorf, as described previously19. In brief, after inclusion (baseline), participants were randomized to supplement or placebo, i.e. 125 mg L-hArg or lactose once daily for 4 weeks, respectively. After a wash-out phase of 28 days, participants were switched to the other treatment. Finally, participants were examined 28 days after the discontinuation of the second treatment (follow-up). Alanine transaminase (ALT), aspartate aminotransferase (AST), and AP were determined with routine laboratory assays. The study was conducted as a non-drug study and the study protocol has been approved by the Ethics Committee of the Hamburg board of Physicians (PV4038) accordingly. The investigation was conducted in accordance with the Declaration of Helsinki and registered at clinicaltrials.gov (NCT02675660).
Assessments in SHIP
Socio-demographic characteristics and menopause status were assessed by computer-assisted personal interviews. Mean daily beverage-specific alcohol (beer, wine, and distilled spirits) consumption was determined from alcohol intake during the last thirty days preceding the examination. Subjects who participated in exercise training during summer or winter for at least 1 h per week were classified as being physically active. Height and weight were measured to calculate the body mass index (BMI = weight (kg)/height2 (m2)). Waist circumference was measured to the nearest 0.1 cm using an inelastic tape midway between the lower rib margin and the iliac crest in the horizontal plane with the subject standing comfortably with weight evenly distributed on both feet.
Blood samples were taken non-fasting in SHIP-START-0. In SHIP-Trend 75% of the blood samples were taken in the fasted state. All samples were collected between 7 a.m. and 2 p.m. and analysed in the Institute of Clinical Chemistry and Laboratory Medicine of the University Medicine Greifswald. Alanine transaminase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), triglycerides, creatinine, AP, albumin, total bilirubin, and cholinesterase were measured in serum on the Dimension Vista 1500 analytical system (Siemens Healthcare GmbH, Eschborn, Germany). The fibrosis (FIB-4) score was calculated using the formula: age*AST/(platelets*sqrt(ALT))21. Model for end-stage liver disease (MELD) was calculated using the following formula: 3.8*loge(serum bilirubin [mg/dL]) + 11.2*loge(INR) + 9.6*loge(serum creatinine [mg/dL]) + 6.4. High and low biomarker levels were defined according to the reference limits provided by our laboratory (ALT: > 0.77 µkatal/L in males and > 0.60 µkatal/L in females; AST: > 0.59 µkatal/L; GGT: > 0.96 µkatal/L in males and > 0.65 µkatal/L in females; AP: > 2.26 µkatal/L; albumin: < 34 g/L; bilirubin: > 17 µmol/L; cholinesterase: > 316 µkatal/L in males and women > 40 years; > 262 µkatal/L in women ≤ 40 years).
HArg was determined at the University Medical Centre Hamburg-Eppendorf by LC–MS/MS22. In brief, 25 µL of serum were diluted with stable isotope labeled internal standard ([13C6]-homoarginine). Subsequently, proteins were precipitated with methanol and guanidine compounds were converted to their butyl esters. Concentrations were calculated with calibration curves (four levels, triplicates), and platewise quality controls were run (two levels, duplicates). Intra- and interassay coefficients of variation was ≤ 7.5%.
Transabdominal ultrasound of the liver was performed by examiners using a B-mode ultrasound device (vivid I; GE-Healthcare, Waukesha, WI, USA) with a 2.5 MHz ultrasonic transducer. The examiners used a 2-point scale to assess the presence of hepatic steatosis: (0) no steatosis and (1) steatosis. Hepatic steatosis was defined as a hyperechogenic liver pattern.
In SHIP-TREND-0 liver MRI was performed without intravenous contrast using a 1.5-Tesla MRI system (Magnetom Avanto, VB15; Siemens Healthineers, Erlangen, Germany) with a 12-channel-phased-array surface coil23. Three-dimensional chemical shift encoded gradient-echo data with three echoes and flyback readout gradient were acquired from an axial slab during a single 19-s breath hold. Offline reconstructions of a PDFF map (including correction for T1 bias and T2* decay) and a R2* map (based on T2* decay measurement of PDFF) were performed24. Parametric maps of PDFF and R2* were used for further analyses. Mean PDFF was determined at operator-defined regions of interest placed at the center of the liver, by using Osirix (v3.8.1; Pixmec Sarl, Bernex, Switzerland)23.
Statistical methods
Stratified by AST levels continuous data were reported as median, 25th, and 75th percentile, while categorical data were reported as percentage. All multivariable analyses were conducted stratified by sex or menopausal status for the female study participants. Associations of liver biomarkers and AP as exposure variables with hArg levels as outcome were analysed by linear regression models for each exposure separately adjusted for age, waist circumference, alcohol consumption, physical activity, triglycerides, HDL-C, serum creatinine, and study. Assumptions of the linear regression model were visually inspected by QQ and residual plots. In none of the models the assumptions for the linear regression model were violated. To compare the effect sizes of the different biomarkers on hArg we repeated the regression analyses using percentile values of the biomarkers.
In the cross-over trial we plotted the individual time courses of ALT and AST concentrations during the study at the visits baseline, after L-hARG supplementation, after placebo supplementation, and at follow-up. Differences in AST and ALT concentrations between different time points were compared by signed rank tests. All analyses were conducted with Stata 17.0 (Stata Corporation, College Station, TX, USA).
Results
There were 396 individuals (5.2%) with high AST levels (Table 1). Individuals with high AST were older, more often males, drank more alcohol and had a higher BMI than individuals with normal AST. hArg levels were in median 0.21 µmol/L higher in the group with high AST. The median of AST, ALT, GGT, AP, fibrosis score, and liver fat content were in median higher in the high AST group.
In the adjusted multivariable analyses, we found positive associations of ALT, AST, GGT, Fib-4 score, liver fat content, albumin, bilirubin, and cholinesterase with hArg concentrations in males (Table 2). Associations were strongest for AST and cholinesterase concentrations (Fig. 1). In premenopausal women we observed positive associations of high GGT and liver fat content with hArg concentrations, whereas albumin was inversely associated with hArg. In postmenopausal women only ALT was positively associated with hArg concentrations.
In men and postmenopausal women, we identified inverse associations between hArg and AP concentrations (men: β = − 0.020, 95% confidence interval [CI] − 0.032 to − 0.007, p = 0.002; postmenopausal women: β = − 0.035, 95% CI − 0.056 to − 0.015, p = 0.001). In premenopausal women we could not show such an association (β = 0.003, 95% CI − 0.010 to 0.017, p = 0.614). Quick’s value was positively associated with hArg in premenopausal women only (β = 0.004, 95% CI 0.001 to 0.009, p = 0.042). MELD was inversely related with hArg in men only (β = − 0.019, 95% CI − 0.034 to − 0.005, p = 0.002).
In the cross-over trial we found no increase of mean ALT or AST concentrations after supplementation with L-hArg (Fig. 2). Compared to baseline ALT and AST concentrations were not significantly higher at the end of the L-hArg supplementation phase (p = 0.588 for ALT and p = 0.269 for AST). Likewise, values differed not significantly between end of the L-hArg supplementation phase and end of the placebo phase (p = 0.685 for ALT and p = 0.618 for AST). AP was not influenced by hArg supplementation (Fig. 2).
Discussion
Here we report that abnormal liver biomarkers are related to greater circulating hArg concentrations in the general population. We further report that hArg supplementation did not alter liver markers in healthy individuals. We believe that these two results are not contradictive but rather imply a potentially causal relationship between hArg measured in the circulation and liver function. Classical studies from the 1970s already highlighted the important role for hArg as a potent inhibitor of human hepatic AP11,25. While we acknowledge that much greater concentrations of hArg are required to inhibit AP in hepatocytes, we could not measure hArg in hepatocytes in an epidemiological setting. We also report that greater hArg concentrations were related to lower liver AP in men and post-menopausal women. While one may consider our finding that lower AP concentrations are related to higher hArg as potentially counterintuitive, it is important to distinguish between hepatocellular disease and cholestatic patterns, although the latter might also co-occur in advanced liver disorders. Hepatocellular disease is characterized by greater ALT and AST out of proportion of AP and bilirubin. Cholestatic patterns are characterized by higher AP and bilirubin compared to ALT and AST26. Taken together, the greater hArg concentrations may inhibit hepatic AP. Hence, hArg may be a marker for subclinical liver damage. Since hArg supplementation which increased circulating hArg levels did not induce liver abnormalities, we speculate that greater hepatic hArg synthesis by the liver may be a compensatory mechanism for liver damage.
Assessment of hepatic function in clinical routine is done by several ways but each of them bears their own limitations. They consist of measurements of single liver parameters including ALT or AST, markers of cholestasis, albumin, and prothrombin time or combinations of blood parameters in form of various indices such as the FIB-4 score27. Imaging-based assessment of the liver, i.e. by ultrasound or MRI can provide additional information on liver pathologies and functional volume. The patterns of abnormalities in liver function tests enable the differentiation into hepatocellular defects or biliary tract pathologies or combinations of them. Elevations of ALT and AST, two enzymes responsible for amino acid catabolism and ATP-production, indicate hepatocellular damage, while AST is less specific due to a considerable expression in other organs besides the liver28. Moreover, a disproportionate increase of ALT and AST is often observed, which results from their different intracellular concentrations. In contrast to ALT, exclusively localized in the cytoplasm, AST is also found inside mitochondria. A rise in AST indicates a more severe damage to hepatocytes such as alcohol-induced toxicities29. On the other hand, elevations in bilirubin and GGT are indicative of cholestatic diseases although the latter one may also be enhanced following cytochrome-inducing drugs or alcohol abuse28.
Our results show a positive correlation of hArg with both transaminases, GGT, and bilirubin in males while no significant associations were observed in females. In addition, hArg was related with liver fat content estimated by MRI in both men and women but this association only remained significant in males in conditions with higher liver fat content exceeding 5.1%. These findings lead to the assumption that in men higher endogenous serum hArg concentrations seem to correlate with both parenchymal, i.e. hepatocellular, or biliary damage and contradict investigations done in patients with advanced liver diseases where low hArg levels indicated impaired liver function14. However, our study population did not include individuals with progressed liver disorder and liver enzyme variations might rather indicate subclinical changes of liver function. One may hypothesize that hArg elevations result from a counter-regulative effect leading to an induction of synthesis of the vasodilator NO. This gas subsequently improves perfusion of intestinal organs, such as the liver. Hepatoprotective effects and improvements of vascular perfusion by NO have been demonstrated in experimental studies30,31. In addition, the identified diverse correlations between sexes with regards to liver markers and hArg deserves further research. However, albumin is a known binding partner of female sex steroid hormones and its synthesis is altered by estradiol32 indicating that indirect effects of estrogens and other female sex hormones might co-exist. In this respect additional investigations are necessary.
Oral supplementation of healthy humans with 120 mg L-hArg once daily for 4 weeks increased the plasma concentration several fold in the cross-over trial19. In contrast to the positive association of hArg with liver enzymes in the SHIP cohort, we did not observe an increase of liver enzymes after oral supplementation in the cross-over trial. This observation further substantiates the hypothesis that hArg elevations are rather a consequence than a cause of liver enzyme elevations in SHIP. hArg is a constituent of diet including some pulses33,34. We believe that participants of SHIP unlikely have adapted their dietary habits to a more hArg-rich food depending on liver enzyme status. Unfortunately, we do not have sufficient dietary records from our SHIP participants. hArg is extensively metabolized in the liver, kidney and other organs1. Catabolism of hArg is catalyzed by AGAT and degradation of hArg is catalyzed by AGXT235. Moreover, hArg is a substrate and competitive inhibitor of arginases1. In particular, the expression of these enzymes in the liver might be regulated in dependence of the liver function. Therefore, it seems likely that either the synthesis of hArg in the liver is elevated or the degradation of hArg in the liver is impaired with increasing circulating concentrations of liver enzymes.
The FIB-4 score integrates the patient’s age, AST, ALT, and platelets and was initially developed for prediction of fibrosis in HCV/HIV co-infected patients36. However, unintentionally this score was useful for fibrotic liver diseases of other origin as well37. Albeit, liver biopsies remain the gold standard for assessment of severity of liver disease. We found a positive correlation of hArg with FIB-4 only for male study participants. The non-significant association between the majority of standard liver functions tests and hArg remains elusive and data are sparse. Sex-dependent differences of metabolites, among them hArg, were observed in sedentary hearts of mice with higher basal levels in female hearts that were altered after physical exercise38.
We acknowledge several limitations in our analysis. For example, SHIP study participants are from a rural area in northeast Germany of mostly Caucasian descent. In addition, the analysis of the observational data from the SHIP cohorts cannot determine causality. In addition, albeit we tried to correct for a multitude of potentially confounding factors, residual confounding cannot be ruled out. Nonetheless, a strength of our analysis is the combination of epidemiological and clinical data which suggests the direction of the observed association. We would like to highlight the large sample size available for the cross-sectional analysis between hArg and liver biomarkers.
We summarize that hArg may be a marker of liver dysfunction and should be explored further. Especially the observed sex specific results warrant further investigation.
Data availability
SHIP data are publicly available for scientific and quality control purpose. Data usage can be applied for via www.community-medicine.de18.
References
Adams, S. et al. Novel biosynthesis, metabolism and physiological functions of L-Homoarginine. Curr. Protein Pept. Sci. 20(2), 184–193 (2019).
Malle, O. et al. NO synthesis markers are not significantly associated with blood pressure and endothelial dysfunction in patients with arterial hypertension: A cross-sectional study. J. Clin. Med. 9(12), 3895 (2020).
Hannemann, J. et al. Arginine: Glycine amidinotransferase is essential for creatine supply in mice during chronic hypoxia. Front. Physiol. 12, 703069 (2021).
Pilz, S. et al. Homoarginine in the renal and cardiovascular systems. Amino Acids 47(9), 1703–1713 (2015).
Atzler, D., Schwedhelm, E. & Choe, C. U. L-homoarginine and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 18(1), 83–88 (2015).
Radomski, M. W., Palmer, R. M. & Moncada, S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. U. S. A. 87(13), 5193–5197 (1990).
Choe, C. U. et al. Homoarginine levels are regulated by L-arginine:glycine amidinotransferase and affect stroke outcome: Results from human and murine studies. Circulation 128(13), 1451–1461 (2013).
Marz, W. et al. Homoarginine, cardiovascular risk, and mortality. Circulation 122(10), 967–975 (2010).
Tomaschitz, A. et al. Homoarginine in patients with primary hyperparathyroidism. Am. J. Med. Sci. 349(4), 306–311 (2015).
Niekamp, C. et al. Cross-sectional associations between homoarginine, intermediate phenotypes, and atrial fibrillation in the community-The Gutenberg health study. Biomolecules 8(3), 86 (2018).
Fishman, W. & Sie, H. G. L-homoarginine; an inhibitor of serum “bone and liver” alkaline phosphatase. Clin. Chim. Acta 29(2), 339–341 (1970).
Suzuki, K., Yoshimura, Y., Hisada, Y. & Matsumoto, A. Sensitivity of intestinal alkaline phosphatase to L-homoarginine and its regulation by subunit-subunit interaction. Jpn. J. Pharmacol. 64(2), 97–102 (1994).
Alvaro, D. et al. The function of alkaline phosphatase in the liver: Regulation of intrahepatic biliary epithelium secretory activities in the rat. Hepatology 32(2), 174–184 (2000).
Pilz, S. et al. Association of homoarginine and methylarginines with liver dysfunction and mortality in chronic liver disease. Amino Acids 47(9), 1817–1826 (2015).
Lluch, P. et al. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J. Hepatol. 41(1), 55–59 (2004).
Laleman, W. et al. A role for asymmetric dimethylarginine in the pathophysiology of portal hypertension in rats with biliary cirrhosis. Hepatology 42(6), 1382–1390 (2005).
Mookerjee, R. P. et al. Inflammation is an important determinant of levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) in acute liver failure. Liver Transpl. 13(3), 400–405 (2007).
Volzke, H. et al. Cohort profile update: The study of health in pomerania (SHIP). Int. J. Epidemiol. https://doi.org/10.1093/ije/dyac034 (2022).
Atzler, D. et al. Oral supplementation with L-homoarginine in young volunteers. Br. J. Clin. Pharmacol. 82(6), 1477–1485 (2016).
Khattak, R. M., Ittermann, T., Nauck, M., Below, H. & Völzke, H. Monitoring the prevalence of thyroid disorders in the adult population of Northeast Germany. Popul. Health Metrics 14(1), 39 (2016).
Vallet-Pichard, A. et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 46(1), 32–36 (2007).
Atzler, D. et al. Serum reference intervals of homoarginine, ADMA, and SDMA in the study of health in Pomerania. Clin. Chem. Lab. Med. 52(12), 1835–1842 (2014).
Kühn, J. P. et al. Quantitative chemical shift-encoded MRI is an accurate method to quantify hepatic steatosis. J. Magn. Reson. Imaging 39(6), 1494–1501 (2014).
Kühn, J.-P. et al. Effect of multipeak spectral modeling of fat for liver iron and fat quantification: Correlation of biopsy with MR imaging results. Radiology 265(1), 133–142 (2012).
Lin, C. W. & Fishman, W. H. L-Homoarginine. An organ-specific, uncompetitive inhibitor of human liver and bone alkaline phosphohydrolases. J. Biol. Chem. 247(10), 3082–3087 (1972).
Vagvala, S. H. & O’Connor, S. D. Imaging of abnormal liver function tests. Clin. Liver Dis. (Hoboken) 11(5), 128–134 (2018).
Sharma, P. Value of liver function tests in cirrhosis. J. Clin. Exp. Hepatol. 12(3), 948–964 (2022).
Kalas, M. A., Chavez, L., Leon, M., Taweesedt, P. T. & Surani, S. Abnormal liver enzymes: A review for clinicians. World J. Hepatol. 13(11), 1688–1698 (2021).
Green, R. M. & Flamm, S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 123(4), 1367–1384 (2002).
Khaneki, S., Jensen, A. R., Drucker, N. A. & Markel, T. A. Direct peritoneal resuscitation improves mesenteric perfusion by nitric oxide dependent pathways. J. Surg. Res. 1(213), 274–280 (2017).
Brenner, T. et al. Reactive metabolites and AGE-RAGE-mediated inflammation in patients following liver transplantation. Mediat. Inflamm. 2013, 501430 (2013).
May, F., Ryffel, G., Weber, R. & Westley, B. Estrogen dramatically decreases albumin mRNA levels and albumin synthesis in Xenopus laevis liver. J. Biol. Chem. 257(23), 13919–13923 (1982).
Sacristán, M. et al. Determination of β-N-oxalyl-L-α, β-diaminopropionic acid and homoarginine in Lathyrus sativus and Lathyrus cicera by capillary zone electrophoresis. J. Sci. Food Agric. 95(7), 1414–1420 (2015).
Onar, A. N., Erdoğan, B. Y., Ayan, I. & Acar, Z. Homoarginine, β-ODAP, and asparagine contents of grass pea landraces cultivated in Turkey. Food Chem. 15(143), 277–281 (2014).
Jarzebska, N. et al. Kidney and liver are the main organs of expression of a key metabolic enzyme alanine:glyoxylate aminotransferase 2 in humans. Atheroscler. Suppl. 40, 106–112 (2019).
Sterling, R. K. et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43(6), 1317–1325 (2006).
McPherson, S., Stewart, S. F., Henderson, E., Burt, A. D. & Day, C. P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 59(9), 1265–1269 (2010).
Fulghum, K., Collins, H. E., Jones, S. P. & Hill, B. G. Influence of biological sex and exercise on murine cardiac metabolism. J. Sport Health Sci. https://doi.org/10.1016/j.jshs.2022.06.001 (2022).
Acknowledgements
Guarantor of the article: Martin Bahls is acting as the submission's guarantor (i.e. the person who takes responsibility for the integrity of the work as a whole, from inception to published article). A statement indicating that all authors approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Study of Health in Pomerania (SHIP) is part of the Community Medicine Research network (CMR) (http://www.medizin.uni-greifswald.de/icm) of the University Medicine Greifswald, which is supported by the German Federal Ministry of Education and Research (BMBF, grant number: 01ZZ96030 and 01ZZ0701) and by the German Competence Network Heart Failure. This study was carried out in collaboration with the German Centre for Cardiovascular Research (DZHK) and the German Center for Diabetes Research (DZD), which are funded by the German Federal Ministry of Education and Research (BMBF).
Author information
Authors and Affiliations
Contributions
A.A., E.S., T.I. and M.B. wrote the manuscript, D.A., M.N., J.K., M.L.K., H.V., S.B.F., M.D. provided critical feedback to the manuscript. T.I., M.B. performed the analysis of the epidemiological data. E.S. performed the analysis of the clinical trial. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aghdassi, A., Schwedhelm, E., Atzler, D. et al. The relationship between homoarginine and liver biomarkers: a combination of epidemiological and clinical studies. Sci Rep 13, 5230 (2023). https://doi.org/10.1038/s41598-023-32363-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32363-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.